User login
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
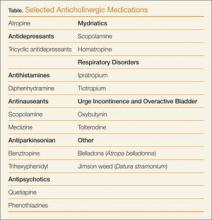
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.