User login
Case Studies in Toxicology: Hot as a Hare and Red as a Beet
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
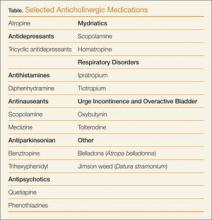
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
A previously healthy 11-month-old boy was brought to the ED after his parents discovered him with an open bottle of nonprescription diphenhydramine. On initial presentation, the child was irritable with diffuse skin redness and dry mucous membranes. He was tremulous and making nonpurposeful reaching movements with his arms. He had roving eye movements and markedly dilated pupils that were minimally reactive. Initial vital signs were: blood pressure, 140/95 mm Hg; heart rate, 220 beats/minute; respiratory rate, 30 breaths/minute; temperature, 100.6ºF. Capillary glucose was 120 mg/dL, and oxygen saturation was 100% on room air. An electrocardiogram (ECG) revealed sinus tachycardia with normal QRS and QTc intervals.
What is the toxicological differential diagnosis?
Toxicity from several different classes of drugs may cause an altered level of consciousness, tachycardia, and hyperthermia. Serotonin agonists, such as selective serotonin reuptake inhibitors, may result in serotonin toxicity—a syndrome that includes altered cognition, autonomic changes (eg, tachycardia, hyperthermia), and neuromuscular effects (eg, rigidity, clonus), along with mydriasis and diaphoresis. Neuroleptic malignant syndrome (NMS) occurs following exposure to dopamine antagonists, such as antipsychotic medications.
Neuroleptic malignant syndrome presents in a similar manner to serotonin toxicity but tends to have a more indolent course compared with the abrupt onset and resolution of serotonin toxicity. Sympathomimetic medications (eg, methylphenidate) or drugs of abuse (eg, cocaine, methamphetamines) result in catecholamine effects including tachycardia, hypertension, diaphoresis, and mydriasis. Acetylsalicylic-acid (aspirin) toxicity (salicylism) often causes tinnitus, hyperpnea, and gastrointestinal (GI) effects following exposure. Severe toxicity may cause altered level of consciousness and hyperthermia; however, these are ominous and late findings. Mydriasis is not common.
What is the anticholinergic toxidrome?
Acetylcholine is a neurotransmitter present both in the central and peripheral nervous systems. In the periphery, acetylcholine acts at both the sympathetic and parasympathetic components of the autonomic nervous system and at somatic motor fibers. Acetylcholine acts at two classes of receptors, namely, nicotinic and muscarinic types. Muscarinic receptors are found in the central nervous system (CNS) (specifically the brain) and peripherally on effector cells of the parasympathetic nervous system and on sympathetically innervated sweat glands.1 Anticholinergic toxicity results from antagonism of muscarinic receptors and is more appropriately referred to as antimuscarinic poisoning, though the terms are used interchangeably. Nicotinic receptor antagonists are used primarily for neuromuscular blockade and do not cause this syndrome.
- “Hot as a hare” (anhidrosis with temperature elevation);
- “Red as a beet” (vasodilation with skin hyperemia);
- “Blind as a bat” (pupillary dilation with loss of accommodation);
- “Dry as a bone” (drying of mucosal surfaces and skin);
- “Full as a flask” (urinary retention); “Stuffed as a pepper” (constipation); and
- “Mad as a hatter” (describing the central anticholinergic effects that are often present—eg, altered mental status manifested as agitation, delirium, hallucinations, abnormal picking movements, rarely seizures).
Elderly patients and those with underlying medical illness or psychiatric disorders may be more prone to the CNS manifestations of anticholinergic medications. Anticholinergic effects can occur through ingestion, smoking, inhalation, and topical absorption (including transdermal or ophthalmic routes). Delayed or prolonged effects may occur due to slow gastric emptying and prolonged GI absorption. The duration of effects is variable and central anticholinergic manifestations of confusion or agitation may be present for several days, even after peripheral manifestations have resolved (termed the central anticholinergic syndrome).
What are common causes of anticholinergic toxicity?
Although anticholinergic effects are often described in terms of “toxicity,” these effects are often used for therapeutic benefit. Such roles of anticholinergic agents include the following:
- Atropine to treat bradycardia;
- Ipratropium bromide to manage asthma;
- Antinauseants (eg, scopolamine, meclizine) for symptom relief;
- Tolterodine to treat urge incontinence and overactive bladder; and
- Ophthalmologic medications (eg, scopolamine, homatropine) to inhibit ciliary spasm in patients with iritis.
Although the above medications are being used for a specific anticholinergic property, other unintended and troublesome anticholinergic effects are often seen. Similarly, many other medications often have unintended anticholinergic effects (see Table). Anticholinergic “toxicity” is simply an extension of the effects that occur with therapeutic use.
What is the treatment for patients with anticholinergic toxicity?
Most patients with anticholinergic toxicity do well with supportive management. Benzodiazepines are the treatment of choice for agitation. Haloperidol and other antipsychotics are relatively contraindicated for treatment of agitation as they may impair temperature regulation and lead to hyperthermia. Although likely of limited overall benefit, oral activated charcoal may reduce the amount of drug absorbed.
Antidotal therapy with physostigmine should be considered for select patients presenting with altered mental status due to an anticholinergic. Physostigmine is an acetylcholinesterase inhibitor that prevents the breakdown of acetylcholine in the synaptic cleft, thus antagonizing the effects of anticholinergic drugs. A retrospective study noted a lower incidence of complications and shorter time to recovery with the use of physostigmine compared with benzodiazepines in patients with anticholinergic toxicity.2 The use of physostigmine in select patients may obviate the need for a further delirium workup, which often includes computed tomography or lumbar puncture.
When administering physostigmine, atropine should be present at the bedside with airway equipment readily available as cholinergic effects may develop (specifically bronchospasm, bronchorrhea, or bradycardia). Dosing of physostigmine in adult patients is 1 to 2 mg via slow intravenous (IV) push, in aliquots of 0.2 to 0.3 mg each, over 5 minutes; pediatric dosing is 20 mcg/kg to maximum 0.5 mg. Onset of effects can be expected within minutes of administration.3 Since the duration of physostigmine is less than that of many anticholinergic drugs, recurrence of anticholinergic effects should be anticipated.
Historically, physostigmine was included in the “coma cocktail,” along with thiamine, dextrose, and naloxone for treating undifferentiated patients with altered level of consciousness. Concern for its ubiquitous use arose following reports of asystole in two patients who presented with tricyclic antidepressant (TCA) overdose, although these patients actually had more complicated multidrug overdoses.4 Nevertheless, an ECG should be performed in all patients for whom physostigmine is being considered, and it should not be administered (or perhaps only extremely cautiously) if the ECG demonstrates a QRS complex duration >100 ms.3 Relative contraindications include reactive airways disease, peripheral vascular disease, or intestinal or bladder-outlet obstruction.
Prolongation of the QRS interval is not always indicative of TCA ingestion as certain other antimuscarinic drugs, such as diphenhydramine, may cause sodium-channel blockade. Based on extrapolation from TCA literature,5 if the QRS >100 ms, a bolus of 1 to 2 mEq/kg sodium bicarbonate should be given with monitoring of the QRS interval for narrowing.
Case conclusion
The clinicians at the bedside felt that the infant’s presentation was consistent with anticholinergic toxicity. Physostigmine was administered by slow IV push for a total dose of 1.5 mg. The patient had immediate improvement of symptoms, including decreased skin redness, decreased agitation, and improved vital signs (BP, 118/80 mm Hg and HR, 160 beats/minute). He was admitted to the pediatric intensive care unit for monitoring and was subsequently discharged home with complete symptom resolution 2 days later.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
- Gerretsen P, Pollock BG. Drugs with anticholinergic properties: a current perspective on use and safety. Expert Opin Drug Saf. 2011;10(5):751-765.
- Burns MJ, Linden CH, Graudins A, Brown RM, Fletcher KE. A comparison of physostigmine and benzodiazepines for the treatment of anticholinergic poisoning. Ann Emerg Med. 2000;35(4):374-381.
- Howland MA. Physostigmine salicylate. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:759-762.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med. 1980;9(11):588-590.
- Boehnert MT, Lovejoy FH, Jr. Value of the QRS duration versus the serum drug level in predicting seizures and ventricular arrhythmias after an acute overdose of tricyclic antidepressants. N Engl J Med. 1985;313(8):474-479.
Tiny Bubbles: Or, the Dangers of Cleaning Fruit
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
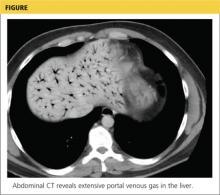
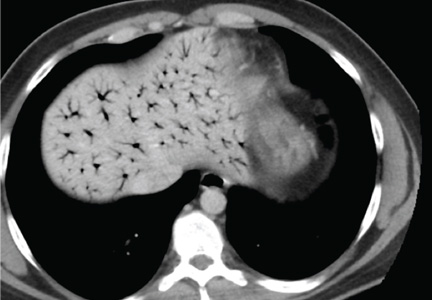
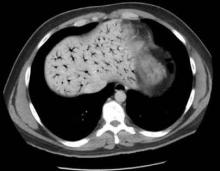
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
A previously healthy 32-year-old man presented to the emergency department (ED) after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs included a blood pressure of 140/92 mm Hg; heart rate, 93 beats/min; respiratory rate, 18 breaths/min; and temperature, 96.4°F. His O2 saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
WHAT ARE THE POTENTIAL EXPOSURES TO HYDROGEN PEROXIDE?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the FDA for any such purpose.3 When diluted sufficiently, this concoction is not harmful but is unlikely to provide any health benefits.
Continue reading for the toxic effects of concentrated hydrogen peroxide...
WHAT ARE THE TOXIC EFFECTS OF CONCENTRATED HYDROGEN PEROXIDE?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 mL of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.
In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Continue reading for the case continuation...
CASE CONTINUATION
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal CT scan, which revealed the presence of extensive air throughout the portal venous system (see the figure).
DO ALL PATIENTS PRESENTING WITH H2O2 INGESTION REQUIRE IMAGING TO ASSESS FOR THE PRESENCE OF PORTAL VENOUS AIR?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a work-up is unnecessary.
Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
Continue reading to find out what to do if portal venous air is detected...
IF PORTAL VENOUS AIR IS DETECTED, DO PATIENTS REQUIRE HYPERBARIC OXYGEN THERAPY?
The management of patients with portal venous gas following H2O2 ingestion is controversial and has not been established. Hyperbaric oxygen therapy involves increasing the ambient pressure by several atmospheres inside a specially designed chamber—the same therapy used for diving-related bubble injury.
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs, where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10
Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Continue reading for the case conclusion...
CASE CONCLUSION
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
REFERENCES
1. Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
2. 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. www.theoneminutemiracleinc.com/pages/h2o2-benefits/. Accessed January 20, 2013.
3. FDA. FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. July 27, 2006. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2006/ucm108701.htm. Accessed January 20, 2013.
4. Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
5. Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
6. Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
7. French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
8. Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
9. Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
10. Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23
Case Studies in Toxicology: Tiny Bubbles (Or, the Dangers of Cleaning Your Fruit)
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
Case
A previously healthy 32-year-old man presented to the ED after unintentionally ingesting a mouthful of concentrated (35%) hydrogen peroxide (H2O2) from an unmarked bottle he kept in his refrigerator. Upon realizing his error, he immediately drank a liter of water, which promptly induced vomiting. In the ED, the patient complained of mild throat and chest discomfort as well as “abdominal fullness.”
His initial vital signs were: blood pressure, 140/92 mm Hg; heart rate, 93 beats/minute; respiratory rate, 18 breaths/minute; temperature, 96.4° F. Oxygen saturation was 98% on room air. Physical examination revealed tenderness in the epigastric region with no peritoneal findings. Oropharynx and chest examination were normal, and standard laboratory investigations were all within normal limits.
What are the potential exposures to hydrogen peroxide?
Hydrogen peroxide is a colorless and odorless liquid. Solutions with concentrations ranging from 3% to 5% have many household applications, including use as a wound disinfectant and dentifrice; dilute solutions are also utilized for similar purposes in the hospital setting. Industrial-strength H2O2 (concentrations of 10% to 35%) is employed to bleach textiles and paper, and higher concentrations (70% to 90%) are used as an oxygen source for rocket engines.
Consumer application of concentrated H2O2 solutions has become increasingly common. Some, like this patient, clean the surfaces of fruits and vegetables with H2O2 to decrease transmission of bacteria during cutting.1 More concerning, however, is the purported medicinal benefits of ingesting “food-grade” (35%) H2O2 mixed with water—touted on many Internet sites as a treatment for illnesses such as emphysema, cancer, anemia, and HIV.2 Sometimes referred to as “hyperoxygenation therapy,” this so-called treatment has not been approved by the US Food and Drug Administration for any such purpose.3 When diluted sufficiently, this concoction is not harmful but unlikely to provide any health benefits.
Dr Lucyk is a fellow of medical toxicology in the department of emergency medicine at the New York University School of Medicine and the New York City Poison Control Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
What are the toxic effects of concentrated hydrogen peroxide?
Injury from concentrated H2O2 consumption is primarily from either direct caustic injury or the embolic obstruction of blood flow. Following ingestion, the enzyme catalase metabolizes the breakdown of H2O2 in accordance with the following equation: 2H2O2(aq) → 2H2O(l) + O2(g) + heat. A single milliliter of 35% H2O2 results in the liberation of 100 mL of O2. (The more common 3% household solution generates 10 mL of oxygen per 1 ml of H2O2.) The creation of a large intragastric pressure gradient from the liberation of gas, coupled with the caustic and exothermic injury of the bowel mucosa, may contribute to the movement of oxygen through epithelial interstices into the circulation.In addition, and perhaps more importantly, absorption of intact H2O2 with subsequent metabolism by catalase in the blood liberates oxygen directly within the vasculature. Oxygen bubbles may coalesce in blood circulation and occlude vascular flow. In canine studies, elevated oxygen tension in the portal venous system led to cessation of mesenteric flow in arteries and veins, though the mechanism of action is unclear.4 Furthermore, coalescence of bubbles can lead to disruption of bowel-cell architecture, fibrin plugging of capillaries, venous thrombosis, and infarction of tissues.4
Cases of cardiac and cerebral gas embolism have been reported, and present similarly to patients with diving-related decompression injuries (eg, stroke-like syndromes).5,6 The proposed mechanism for these latter effects involves the metabolism of H2O2 in the systemic circulation with production of oxygen bubbles. In the presence of an atrial septal defect, bubbles may move from the right atrium to the arterial circulation.7
Toxicity and death from H2O2 exposure associated with the historical treatment of inspissated meconium,4 as well as the irrigation of wounds,8 has been reported in the medical literature. Ingestion of a 3% solution is generally benign, resulting at worst in gastrointestinal symptoms or throat irritation.9 Rarely does significant toxicity occur at this low concentration,5 with the vast majority of such cases involving concentrated solutions of 35%.
Case continuation
Case 2
Based on this patient’s continued symptoms, an abdominal radiograph was obtained to assess the presence of portal venous air. Although radiographic findings were normal, continued abdominal examination findings warranted a subsequent abdominal computed tomography (CT) scan, which revealed the presence of extensive air throughout the portal venous system (Figure.).
Do all patients presenting with H2O2 ingestion require imaging to assess for the presence of portal venous air?
Reportedly, ingestion of as little as a “sip” or “mouthful” of 35% H2O2 has resulted in venous and arterial gas embolism,6 occasionally with severe consequences, but no current consensus guidelines exist regarding imaging requirements. Some toxicologists and hyperbaric physicians believe that the presence of portal venous air does not adversely impact a patient’s prognosis or necessitate treatment, and therefore a workup is unnecessary. Others, however, suggest that the presence of portal venous air indicates oversaturation of oxygen in the blood, placing the patient at increased risk for cardiac and cerebral air embolism. Neither one of these theories is well supported in the literature. Although practice patterns vary by institution, it is reasonable that all patients presenting with abdominal complaints after ingestion of H2O2 undergo CT imaging to assess for portal venous air.
If portal venous air is detected, do patients require hyperbaric oxygen therapy?
The management of patients with portal venous gas following H2O2
Hyperbaric therapy increases the amount of oxygen that can be dissolved in the blood, thereby decreasing bubble formation and allowing transport of dissolved oxygen to the lungs where it can be exhaled. Some patients with portal venous air experience significant pain and portal venous hypertension, which may respond rapidly to this therapy.10 Based on available literature, hyperbaric therapy is reasonable for patients with significant abdominal pain and portal venous air following H2O2 ingestion; less controversial is the role of hyperbaric therapy in those with cerebral air embolism. Multiple case reports of patients with significant neurologic findings demonstrate resolution of symptoms following hyperbaric therapy.6
Case conclusion
Hyperbaric oxygen therapy was recommended for the patient in this case, but transfer to a hyperbaric facility was not possible. He was instead admitted to the hospital for continuous monitoring. Over the next 12 hours, his symptoms gradually resolved, and a repeat CT scan the following day showed complete resolution of the portal venous gas. The patient was subsequently discharged without any sequelae.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.
- Ukuku DO, Bari ML, Kawamoto S, Isshiki K. Use of hydrogen peroxide in combination with nisin, sodium lactate and citric acid for reducing transfer of bacterial pathogens from whole melon surfaces to fresh-cut pieces. Int J Food Microbiol. 2005;104(2):225-233.
- 35% H2O2 hydrogen peroxide food grade certified benefits. The One Minute Miracle Web site. http:// www.theoneminutemiracleinc.com/pages/h2o2- benefits/. Accessed November 20, 2013.
- FDA warns consumers against drinking high-strength hydrogen peroxide for medicinal use: ingestion can lead to serious health risk and death [news release]. Silver Spring, MD: US Food and Drug Administration; July 27, 2006. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ 2006/ucm108701.htm. Accessed November 20, 2013.
- Shaw A, Cooperman A, Fusco J. Gas embolism produced by hydrogen peroxide. N Engl J Med. 1967;277(5):238-241.
- Cina SJ, Downs JC, Conradi SE. Hydrogen peroxide: a source of lethal oxygen embolism. Case report and review of the literature. Am J Forensic Med Pathol. 1994;15(1):44-50.
- Rider SP, Jackson SB, Rusyniak DE. Cerebral air gas embolism from concentrated hydrogen peroxide ingestion. Clin Toxicol (Phila). 2008;46(9):815-818.
- French LK, Horowitz BZ, McKeown NJ. Hydrogen peroxide ingestion associated with portal venous gas and treatment with hyperbaric oxygen: a case series and review of the literature. Clin Toxicol (Phila). 2010;48(6):533-538.
- Bassan MM, Dudai M, Shalev O. Near-fatal systemic oxygen embolism due to wound irrigation with hydrogen peroxide. Postgrad Med J. 1982;58(681):448-450.
- Henry MC, Wheeler J, Mofenson HC, et al. Hydrogen peroxide 3% exposures. J Toxicol Clin Toxicol. 1996;34(3):323-327.
- Papafragkou S, Gasparyan A, Batista R, Scott P. Treatment of portal venous gas embolism with hyperbaric oxygen after accidental ingestion of hydrogen peroxide: a case report and review of the literature. J Emerg Med. 2012;43(1):e21-e23.