User login
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
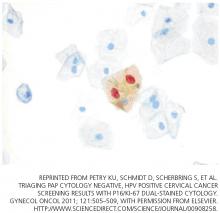
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
KEY POINTS
- The new guidelines still recommend starting screening with cytologic (Papanicolaou) testing at age 21, but now recommend repeating the test less often, ie, every 3 years rather than every 2 years for women age 21 to 29.
- Women age 30 and older who are screened by combined cytologic and HPV testing should be rescreened every 5 years if both tests are negative (instead of every 3 years, as previously recommended). Alternatively, they can be screened by cytology alone every 3 years.
- We can stop screening women at age 65 if they have had adequate screening until then and no history of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in the past 20 years. Once screening is discontinued, it should not resume, even if the patient has a new sexual partner.
- Screening should not change after HPV vaccination.
- When women have negative cytology but positive HPV results, tests for the HPV 16 and 18 genotypes can help to identify those at higher risk of developing CIN2+.