User login
Cervical cancer screening: What’s new and what’s coming?
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
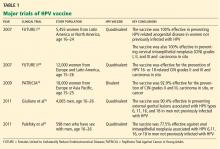
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
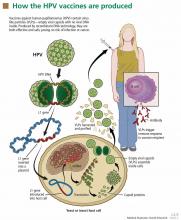
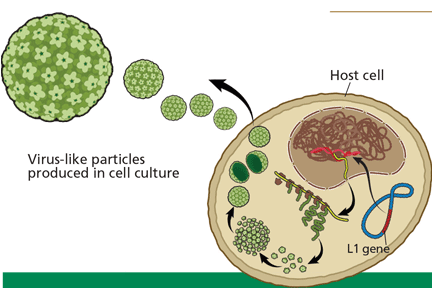
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
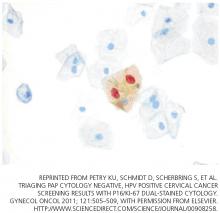
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
KEY POINTS
- The new guidelines still recommend starting screening with cytologic (Papanicolaou) testing at age 21, but now recommend repeating the test less often, ie, every 3 years rather than every 2 years for women age 21 to 29.
- Women age 30 and older who are screened by combined cytologic and HPV testing should be rescreened every 5 years if both tests are negative (instead of every 3 years, as previously recommended). Alternatively, they can be screened by cytology alone every 3 years.
- We can stop screening women at age 65 if they have had adequate screening until then and no history of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in the past 20 years. Once screening is discontinued, it should not resume, even if the patient has a new sexual partner.
- Screening should not change after HPV vaccination.
- When women have negative cytology but positive HPV results, tests for the HPV 16 and 18 genotypes can help to identify those at higher risk of developing CIN2+.
Human papillomavirus vaccine: Safe, effective, underused
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
KEY POINTS
- Two HPV vaccines are available: a quadrivalent vaccine against HVP types 6, 11, 16, and 18, and a bivalent vaccine against types 16 and 18.
- HPV causes cervical cancer, genital warts, oropharyngeal cancer, anal cancer, and recurrent respiratory papillomatosis, creating a considerable economic and health burden.
- The host immune response to natural HPV infection is slow and weak. In contrast, HPV vaccine induces a strong and long-lasting immune response.
- The HPV vaccines have greater than 90% efficacy in preventing cervical dysplasia and genital warts that are caused by the HPV types the vaccine contains. They are as safe as other common prophylactic vaccines.
- HPV vaccination has been challenged by public controversy over the vaccine’s safety, teenage sexuality, mandatory legislation, and the cost of the vaccine.