User login
Can the test for human papillomavirus DNA be used as the stand-alone, first-line screening test for cervical cancer?
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
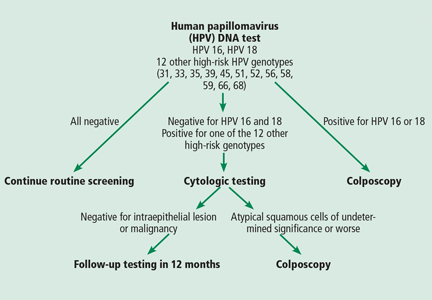
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
Yes. Growing evidence demonstrates that the human papillomavirus (HPV) DNA test is more sensitive than the Papanicolaou (Pap) test, with a better negative predictive value—ie, women who have negative test results can be more certain that they are truly free of cervical cancer.1–3
On April 24, 2014, the US Food and Drug Administration (FDA) approved the Cobas HPV test developed by Roche for use as the first-line screening test for cervical cancer in women age 25 and older.4 The approval follows the unanimous recommendation from an independent panel of experts, the Microbiology Devices Panel of the FDA’s Medical Devices Advisory Committee, on March 12, 2014.
PAP-HPV COTESTING IS EFFECTIVE BUT NOT PERFECT
Based on conclusive evidence of a direct link between HPV infection (specifically, infection with certain high-risk HPV genotypes) and almost all cases of invasive cervical cancer,5,6 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), American Society for Clinical Pathology (ASCP), US Preventive Services Task Force (USPSTF), and American Congress of Obstetricians and Gynecologists (ACOG) issued a consensus recommendation for Pap-HPV cotesting as the preferred screening strategy starting at age 30 and continuing through age 65.7–9
Compared with Pap testing alone, cotesting offers improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) and the ability to safely extend the screening interval to every 5 years in women who have negative results on both tests. It is an effective screening strategy and remains the standard of care today.
However, this strategy is not perfect and presents several problems for clinicians. The results of the two tests often conflict—the results of the Pap test might be positive while those of the HPV test are negative, or vice versa. Integrating the results of cotesting into triaging can be confusing and complicated. In addition, performing two tests on all women increases the cost of care. And furthermore, the cotesting strategy increases the number of women who require immediate or short-term follow-up,1,2,10–12 such as colposcopy, which is unnecessary for many.
THE HPV TEST DETECTS 14 HIGH-RISK GENOTYPES
The FDA-approved HPV test detects 14 high-risk genotypes. The results for 12 of these are pooled and reported collectively as either positive or negative, while the other two—HPV 16 and HPV 18—are reported separately. (HPV 16 and HPV 18 are the highest-risk genotypes, and together they account for more than two-thirds of cases of invasive cervical cancer.)
ADVANTAGES OF HPV-ONLY TESTING: FINDINGS FROM THE ATHENA TRIAL
The FDA’s decision to approve the Cobas HPV test for use by itself for screening was based on the landmark ATHENA (Addressing the Need for Advanced HPV Diagnostics) trial.13 ATHENA, the largest prospective study of cervical cancer screening performed in the United States to date, enrolled 47,208 women at 61 sites in 23 states. The study revealed the following findings:
- The HPV DNA test had higher sensitivity for detecting CIN3+ (37% higher than the Pap test) and equivalent specificity.
- The HPV test’s positive predictive value was nearly twice as high (12.25% vs 6.47%), and it had a higher negative predictive value (99.58% vs 99.41%) in detecting CIN3+ than with the Pap test.
- HPV testing by itself performed better than Pap-HPV cotesting, with positive predictive values of 12.25% vs 11.04% and negative predictive values of 99.58% vs 99.52% (data presented to the FDA Medical Devices Advisory Committee, Microbiology Panel. March 12, 2014. FDA Executive Summary).
For women whose results were negative for HPV 16 and 18 but positive for the 12-genotype pooled panel, the sample was automatically submitted for cytologic (Pap) testing. Reserving Pap testing for samples in this category improved the specificity of the test and resulted in fewer colposcopy referrals. The ATHENA researchers found that 11.4% of the participants who tested positive for either HPV 16 or 18 had CIN2+.13 Other large cohort studies14,15 also showed that the short-term risk of developing CIN3+ reached 10% over 1 to 5 years in women who tested positive for HPV 16 or 18.
The proposed algorithm for screening (Figure 1) takes advantage of the superior sensitivity of the HPV test, the built-in risk stratification of HPV 16 and 18 genotyping, and the excellent specificity of the Pap test in triaging women whose results are positive for high-risk HPV genotypes other than HPV 16 and 18. Thus, women who have a negative HPV test result can be assured of remaining disease-free for 3 years. The algorithm also identifies women who are at highest risk, ie, those who test positive for HPV 16 or 18. In contrast, the current cotesting approach uses the Qiagen Hybrid Capture HPV testing system, which is a panel of 13 high-risk genotypes, but, if the result is positive, it does not tell you which one the patient has. Furthermore, the new algorithm provides efficient triage, using the Pap test, for women who test positive for the 12 other high-risk HPV genotypes.
Data from large clinical trials other than ATHENA are limited.
FDA APPROVAL DOES NOT CHANGE THE GUIDELINES—YET
The cervical cancer screening guidelines are developed by several organizations other than the FDA. The current guidelines issued by the ACS, ASCCP, ASCP, USPSTF, and ACOG in 2012 call for Pap testing every 3 years in women younger than 30 and Pap-HPV cotesting every 5 years in women ages 30 to 65.7–9 However, FDA approval of the new indication of the HPV DNA test as a stand-alone first-line screening test is an important milestone. It heralds the shifting of the practice paradigm from morphologically based Pap testing to molecular testing in cervical cancer screening.
The ACS and ASCCP have announced that they are reviewing the evidence and may issue updated guidelines for clinicians in the near future.16,17 We anticipate that other organizations may take similar steps. As primary care physicians, we need to stay tuned and follow the most up-to-date evidence-based practice guidelines to provide the best care for our patients.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomized controlled trial. Lancet Oncol 2010; 11:249–257.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- US Food and Drug Administration. FDA approves first human papillomavirus test for primary cervical cancer screening. www.fda.gov/newsevents/newsroom/pressannouncements/ucm394773.htm. Accessed March 3, 2015.
- Muñoz N, Castellsagué X, de González AB, Gissmann L. Chapter 1: HPV in the etiology of human cancer. Vaccine 2006; 24(suppl 3):S3/1–S3/10.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society; American Society for Colposcopy and Cervical Pathology; American Society for Clinical Pathology. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VA; US Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Castle PE, Stoler MH, Wright TC Jr, Sharma A, Wright TL, Behrens CM. Performance of carcinogenic human papillomavirus (HPV) testing and HPV16 or HPV18 genotyping for cervical cancer screening of women aged 25 years and older: a subanalysis of the ATHENA study. Lancet Oncol 2011; 12:880–890.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomized controlled trial. Lancet Oncol 2009; 10:672–682.
- Naucler P, Ryd W, Tornberg S, et al. Efficacy of HPV DNA testing with cytology triage and/or repeat HPV DNA testing in primary cervical cancer screening. J Natl Cancer Inst 2009; 101:88–99.
- Wright TC Jr, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing The Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Kjaer SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- American Cancer Society. FDA approves HPV test as first line screening for cervical cancer. www.cancer.org/cancer/news/fda-approves-hpv-test-as-first-line-screening-for-cervical-cancer. Accessed March 3, 2015.
- American Society for Colposcopy and Cervical Pathology. Medical societies recommend consideration of primary HPV testing for cervical cancer screening. www.asccp.org/About-ASCCP/News-Announcements. Accessed March 3, 2015.
Cervical cancer screening: What’s new and what’s coming?
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
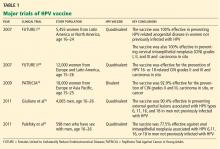
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
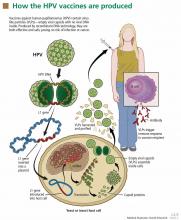
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
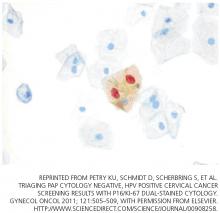
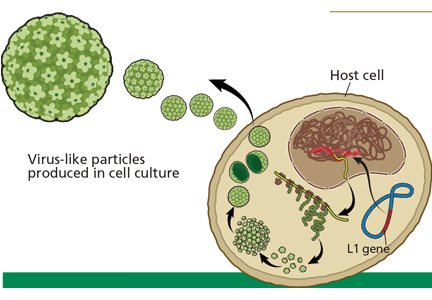
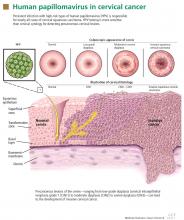
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
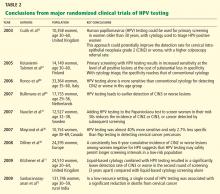
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
Advances in our understanding of the pathogenesis of cervical cancer, new tests for human papillomavirus (HPV), and the development of HPV vaccines in the last decade are transforming the way we screen for cervical cancer.
As a result, screening guidelines are evolving rapidly, requiring clinicians to keep up-to-date with the evidence and rationales supporting the latest guidelines to properly convey best practices to patients.1–3
For example, we must understand why it is safe to extend the screening interval in women at low risk (as recommended in the new guidelines), and we need to be familiar with the options for women who test positive for HPV. Patients and providers may often find such new recommendations frustrating, and patients may feel that they are being denied something necessary by insurers rather than being treated according to scientific evidence.
This article will review the newest screening guidelines and the evidence supporting these recommendations for primary care providers. We will also review the potential role of novel biomarkers, newer HPV tests, and possible future strategies for cervical cancer screening.
WHAT’S NEW IN THE LATEST SCREENING GUIDELINES
Over the years, various organizations have issued separate screening guidelines, sometimes agreeing with each other, sometimes disagreeing.4 Now, for the first time, several of these organizations have developed guidelines collaboratively, and we have consensus in the screening recommendations.
Shortly after the American Congress of Obstetricians and Gynecologists (ACOG) issued its screening guidelines in December 2009,1 the American Cancer Society (ACS), American Society for Colposcopy and Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP) convened an expert panel to review the available evidence and develop a new joint screening guideline. Concurrently, the US Preventive Services Task Force (USPSTF) commissioned a targeted systematic review of the latest evidence.
Both the ACS/ASCCP/ASCP group2 and the USPSTF3 released their new guidelines on March 14, 2012. In November 2012, ACOG issued its latest recommendation on cervical cancer screening.4 The following discussion highlights the consensus recommendations from these organizations (Table 1).
These guidelines apply to the general population only. They do not apply to women at high risk who may require more intensive screening, such as those who have a history of cervical cancer, are immunocompromised (eg, positive for human immunodeficiency virus [HIV]), or were exposed in utero to diethylstilbestrol.
Start screening at age 21
According to the new guidelines, women younger than 21 years should not be screened, regardless of the age at which they start having sex.1–3 This is a change from the 2002 and 2003 ACS recommendations, which said screening should begin 3 years after the onset of vaginal intercourse.5,6
Evidence. The rationale for the recommendation not to screen before age 21 stems from two pieces of evidence:
- Invasive cervical cancer is rare in this age group.7
- Screening can cause harm. For example, unnecessary treatment of preinvasive lesions can lead to long-term complications such as cervical stenosis, preterm delivery, and preterm premature rupture of membranes.8,9
Additionally, one study found that screening before age 21 has little or no impact on the incidence of invasive cervical cancer.10
Longer screening intervals
The 2012 ACS/ASCCP/ASCP guidelines2 and the latest ACOG guidelines4 lengthen the interval between cytology (Papanicolaou) testing to every 3 years in women age 21 to 29. Previous recommendations from these groups were to screen every 2 years, and the USPSTF first recommended the 3-year interval in 2003.11
For women age 30 to 65, the ACS/ASCCP/ASCP, ACOG, and the USPSTF now recommend screening every 5 years if the patient’s results on combined cytology and HPV testing are negative. However, cytologic testing alone every 3 years is also acceptable.2–4
Evidence. The evidence supporting a 3-year screening interval in women age 21 to 29 is primarily from modeling studies—no randomized clinical trial has been done. These studies found no significant difference in outcomes with a 2-year vs a 3-year screening interval.12,13 In particular, the predicted lifetime risk of cervical cancer in women screened every 3 years was 5 to 8 new cases of cancer per 1,000 women, compared with 4 to 6 cases per 1,000 women screened every 2 years.14
Similarly, screening women younger than age 30 at 2-year or 3-year intervals carried the same predicted lifetime risk of death from cervical cancer of 0.05 per 1,000 women, yet women screened every 2 years underwent 40% more colposcopies than those screened every 3 years.2 Therefore, screening every 3 years offers the best balance of benefits and risks in this age group.
Adding HPV testing to cytologic testing increases the sensitivity of screening—thus the recommendation to lengthen the screening interval to every 5 years in women age 30 to 65 who are at low risk and who have negative results on both tests. (Previously, the interval was 3 years.)
Specifically, adding HPV testing improves the sensitivity of screening for cervical intraepithelial neoplasia grade 3 (CIN3), so that, in subsequent rounds of screening, fewer cases of CIN3 or worse (CIN3+) or cancer are detected.15–17 The longer diagnostic lead time with combined testing is associated with a lower risk of CIN3+ or cancer following a double-negative test result than screening with cytology alone at shorter intervals. Combined testing at 5-year intervals is associated with a similar or lower cancer risk than cytology-alone screening at 3-year intervals.9
Moreover, modeling studies have shown that combined testing of women age 30 and older at 5-year intervals leads to fewer colposcopies and a similar or lower cancer risk than with cytology screening at 3-year intervals.18,19
A stronger endorsement for HPV testing
Combined cytologic and HPV testing has received its strongest endorsement to date from the ACS/ASCCP/ASCP, ACOG, and USPSTF in their latest guidelines.2–4
In 2003, ACOG gave HPV and cytology combined testing an “optional” recommendation for women over age 30; in 2009, it upgraded its recommendation to the highest level of recommendation.1 At that time, the USPSTF did not recommend for or against HPV testing, while the ACS did recommend HPV testing (with cytology testing alone every 2 to 3 years as an alternative screening strategy).5
Now, the ACS/ASCCP/ASCP and ACOG recommend HPV and cytology combined testing as the preferred strategy for screening women age 30 or over.2,4 Similarly, the USPSTF gives combined testing for women age 30 to 65 a grade A (its highest) recommendation.3 (In 2003, it had given it a grade I—insufficient evidence to assess the balance of benefit and harm.)
Evidence. Several recent studies provide compelling evidence that HPV testing has high sensitivity and excellent negative predictive value, supporting the stronger endorsement of HPV testing and longer screening intervals.
The Joint European Cohort study,20 in 24,295 women, conclusively showed that the 6-year risk of CIN3+ following a negative HPV test was significantly lower than that following a negative cytology result alone (0.27% vs 0.97%).
Katki et al,21 in another retrospective study, analyzed data from 330,000 women age 30 and older who underwent combined HPV and cytology testing. Looking at the tests separately, they found the risk of CIN3+ was comparable in the 3 years following a negative cytology test by itself and in the 5 years following negative combined HPV and cytology testing. In fact, combined testing at 5- or 6-year intervals offered better protection than cytology alone at 3-year intervals.
Furthermore, combined testing is also more sensitive for detecting cervical adenocarcinoma.22 (Most cancers of the cervix are squamous cell carcinomas, but approximately 10% are adenocarcinomas.)
Stop screening sooner
In 2002, the ACS recommended ending screening at age 70,11 and in 2009 ACOG said to stop at age 65 to 70.1 Now, the ACS/ASCCP/ASCP group2 and ACOG4 recommend stopping screening sooner—at age 65—provided that:
- The patient has had adequate negative screening until then. (Adequate negative prior screening is defined as three consecutive negative cytology results or two consecutive negative combined HPV and cytologic testing results within the 10 years before ceasing screening, with the most recent test performed within the last 5 years.)
- The patient has no history of CIN2+ within the last 20 years.
- The patient is not at high risk of cervical cancer, eg, no history of a high-grade precancerous cervical lesion or cervical cancer, in utero exposure to diethylstilbestrol, or immunosuppression (eg, HIV infection).
The USPSTF had already adopted this position.
Evidence. In women over age 65 who have had good screening, cervical cancer is rare and CIN2+ is uncommon.2,23,24 Kulasingam et al,9 in a modeling study performed for the USPSTF, calculated that continuing to screen until age 90 prevents only 1.6 cancer cases and 0.5 cancer deaths and extends life expectancy by only 1 year per 1,000 women.
Other studies also suggest that newly acquired high-risk HPV infection in women age 65 or older is associated with a very low absolute risk of HPV persistence and CIN3+ progression.25,26
In addition, cervical cancer takes a median of 20 to 25 years to develop after infection with high-risk HPV.2 Also, continuing to screen this older population will detect only a very small number of new cases of CIN2+ and may lead to harm from overtreatment.
Finally, postmenopausal women often have smaller and less accessible cervical transformation zones that may require more interventions to obtain adequate samples and to treat.
Stop screening after hysterectomy
The ACS/ASCCP/ASCP group, ACOG, and the USPSTF reaffirmed their recommendation against screening in women who have had a hysterectomy with removal of the cervix for a reason other than cancer and who have had no history of CIN2+ or cervical cancer.2–4
Evidence. Several lines of evidence suggest stopping screening after a woman has a hysterectomy. The incidence of vaginal cancer is extremely low,27 and the positive predictive value of cytologic testing of the vaginal cuff for vaginal cancer was zero in one study.28 Also, a large cross-sectional study of 5,330 screening cytology tests in women who had a hysterectomy found only one case of dysplasia and no cancer.29
Continue to screen after HPV vaccination
For the first time since HPV vaccines were introduced in 2006, the ACS/ASCCP/ASCP, ACOG, and the USPSTF have had to consider what to do for vaccinated women. All of their new guidelines say to keep screening them.
Evidence. The currently available HPV vaccines protect against cervical cancer,30 but only against cervical cancer caused by HPV types 16 and 18. Other oncogenic types of HPV exist, and the current vaccines do not protect against them.
Furthermore, many women are vaccinated who are already infected. In addition, as of 2010, only about 32% of eligible girls and women in the United States had received all three recommended doses of the vaccine.31 And modeling studies predict that the impact of the HPV vaccine will not be apparent for at least another decade.32
HPV 16/18 genotyping
The ACS/ASCCP/ASCP and ACOG now recommend HPV 16/18 genotyping as a triage option in women who have positive results on HPV testing but negative cytology results, and immediate referral for colposcopy if the genotyping test is positive.2 The alternative option in this situation is to repeat combined HPV and cytologic testing in 12 months.2,33
Evidence. The standard tests for HPV can detect DNA from about a dozen of the oncogenic types of HPV depending on the test, but they do not tell you which one the patient has. This information may be relevant, since not all “high-risk” HPV types are equally bad. HPV 16 and HPV 18 are the worst of all, together accounting for more than 70% of cases of cervical cancer.
Large cohort studies34,35 have shown that the risk of CIN3 reaches 10% over 1 to 4 years in women who test positive for HPV 16, and over 2 to 5 years if they test positive for HPV 18. This clinically relevant short-term risk supports immediate referral for colposcopy.
In March 2009, the US Food and Drug Administration (FDA) approved a test for HPV 16 and HPV 18—Cervista HPV 16/18 (Hologic, Bedford, MA).36
More recently, researchers from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial,37 in 47,208 women, reported that they found CIN2+ in 11.4% of women who tested positive for either HPV 16 or HPV 18, and CIN3+ in 9.8%. Of those who were positive for HPV 16, 13.6% had CIN2+ and 11.7% had CIN3+.
WHAT’S COMING?
As we gain knowledge of the molecular oncogenesis of cervical cancer, we appreciate more the complex relation between HPV oncoproteins and cervical dysplasia. Recent studies demonstrated the clinical utility of detecting novel markers in women who have positive HPV results.38,39
At present, however, there is insufficient evidence to integrate these strategies into our standard of care for cervical cancer screening.
Novel biomarkers: p16 and Ki-67
Although HPV testing is sensitive, it has poor specificity and positive predictive value.40,41 In a primary screening setting, women with normal cytology results who test positive for high-risk HPV may carry a risk of only 3% to 7% for high-grade CIN.42,43
HPV 16/18 genotyping can be useful in this situation (see above). However, not everyone who carries HPV 16 or 18 goes on to develop CIN or cancer.44
A novel biomarker, p16, has been shown to be overexpressed in cervical dysplasia and associated with high-risk HPV oncogenic transformation. Another novel marker, Ki-67, can be regarded as a surrogate marker of deregulated cell proliferation (Figure 1).38
A recent study reported that a combined test for both of these markers (dual-stained cytology) had a sensitivity of 91.9% for detecting CIN2+ and 96.4% for CIN3+. This test was also highly specific: 82.1% for CIN2+ and 76.9% for CIN3+.38
An Italian randomized trial reported that p16 immunostaining improved the specificity of HPV testing in detecting CIN2+.45
In addition, the European Equivocal or Mildly Abnormal Papanicolaou Cytology Study46 found that the dual-stained cytology test had excellent sensitivity for CIN2+ in women with atypical squamous cells of undetermined significance (ASCUS) or low-grade squamous intraepithelial lesion (LSIL) cytology results (92.2% for ASCUS, 94.2% for LSIL). The specificity for CIN2+ in ASCUS and LSIL was 80.6% and 68%, respectively.
A US study also showed that the sensitivity and specificity to detect CIN3+ by using p16/Ki-67 were 97.2% and 60%, respectively, in women age 30 and older.47
If confirmed in more studies, p16/Ki-67 dual staining could help us in deciding which women who have positive HPV but negative cytology results should be referred for colposcopy.
HPV oncogene E6/E7 mRNA testing
In October 2011, the FDA approved the clinical use of a new-generation HPV test, the Aptima HPV assay (Hologic Gen-Probe, San Diego, CA), which detects mRNA for the proteins E6 and E7 from high-risk HPV.39
HPV E6/E7 mRNA expression has been found in virtually all HPV-positive cancer cases and demonstrates a stronger correlation with cervical disease than detection of HPV DNA.48 High-risk HPV E6 and E7 proteins immortalize and malignantly transform infected cells by inhibiting two host cellular anticancer proteins, p53 and retinoblastoma protein (pRB).44,49
The recent FDA approval was based on data from the CLEAR (Clinical Evaluation of Aptima HPV RNA) trial.39 In this trial, in more than 11,000 women, the test was as sensitive for detecting CIN2+ as the HPV DNA-based test, and it was more specific. This advantage was statistically significant. The higher specificity may reduce the number of unnecessary colposcopies and allow for more effective management.50,51
A promising future screening strategy: HPV testing first, then cytology
HPV testing is more sensitive than cytology, while cytology is more specific. Thus, it would be logical to test for HPV first, and then to perform cytologic testing in patients who have positive results on HPV testing.
In the past 5 years, several large randomized clinical trials within national screening programs in Italy, England, Sweden, and the Netherlands examined the value of a primary HPV-based screening strategy.15–17,52 These studies confirmed the superior sensitivity of HPV testing for detection of CIN2+.
A large Canadian randomized trial53 compared HPV testing and cytologic testing as screening tests in women age 30 to 69. HPV DNA testing was 94.6% sensitive in detecting CIN2 or CIN3, compared with 55.4% for cytology. The specificity of HPV testing was nearly as high as that of cytology, 94.1% vs 96.8%. Furthermore, HPV testing followed (in those positive for HPV) by cytology resulted in a lower referral rate for colposcopy than did either test alone (1.1% vs 2.9% with cytology alone or 6.1% with HPV testing alone).
More randomized trial data are needed to evaluate the validity of this promising new approach in varied populations. The HPV FOCAL trial is comparing HPV-then-cytology testing vs cytology-then (in women with ASCUS)-HPV testing.54 In addition, the aforementioned novel biomarkers for HPV oncogenic activity may eventually play a greater role in primary screening.
With the latest evidence-based screening guidelines, we can implement a more sensitive and effective screening strategy for better prevention and early detection of cervical cancer. Newer cutting-edge molecular technologies appear promising; however, their cost-effectiveness needs to be further evaluated.
A MORAL AND ETHICAL RESPONSIBILITY
Our unscreened and underscreened populations carry a higher burden of cervical cancer and of death from cervical cancer. Identifying and reaching out to these women is our moral and ethical responsibility and yet poses the biggest challenge in screening. Arguably, this could have the most significant impact on rates of death from cervical cancer.
Innovative measures in overcoming healthcare barriers and in making testing cheaper will help to close the gap between well-screened and underscreened populations in the United States and globally. Examples would be a low-cost, point-of-care screening test for the general population, and a government-subsidized global vaccination program. It is entirely conceivable that women will no longer die from cervical cancer in the near future, thanks to global effective screening and preventive efforts through widespread HPV vaccination.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Saslow D, Solomon D, Lawson HW, et al; American Cancer Society. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol 2012; 137:516–542.
- Moyer VAUS Preventive Services Task Force. Screening for cervical cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med 2012; 156:880–891.
- Committee on Practice Bulletins—Gynecology. ACOG practice bulletin number 131: screening for cervical cancer. Obstet Gynecol 2012; 120:1222–1238.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Recommendations and rationale. AHRQ Publication No. 03-515A. Rockville, MD: Agency for Healthcare Research and Quality, 2003.
- Castle PE, Carreon JD. Practice improvement in cervical screening and management: symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:238–340.
- Moscicki AB, Cox JT. Practice improvement in cervical screening and management (PICSM): symposium on management of cervical abnormalities in adolescents and young women. J Low Genit Tract Dis 2010; 14:73–80.
- Kulasingam SL, Havrilesky L, Ghebre R, Myers ER. Screening for cervical cancer: a decision analysis for the US Preventive Services Task Force. AHRQ Publication No. 11-05157-EF-1. Rockville, MD: Agency for Healthcare Research and Quality, 2011.
- Sasieni P, Castanon A, Cuzick J. Effectiveness of cervical screening with age: population based case-control study of prospectively recorded data. BMJ 2009; 339:b2968.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sasieni PD, Cuzick J, Lynch-Farmery E. Estimating the efficacy of screening by auditing smear histories of women with and without cervical cancer. The National Co-ordinating Network for Cervical Screening Working Group. Br J Cancer 1996; 73:1001–1005.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Goldie SJ, Kim JJ, Wright TC. Cost-effectiveness of human papillomavirus DNA testing for cervical cancer screening in women aged 30 years or more. Obstet Gynecol 2004; 103:619–631.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol 2010; 11:249–257.
- Vijayaraghavan A, Efrusy MB, Mayrand MH, Santas CC, Goggin P. Cost-effectiveness of high-risk human papillomavirus testing for cervical cancer screening in Québec, Canada. Can J Public Health 2010; 101:220–225.
- Koliopoulos G, Arbyn M, Martin-Hirsch P, Kyrgiou M, Prendiville W, Paraskevaidis E. Diagnostic accuracy of human papillomavirus testing in primary cervical screening: a systematic review and metaanalysis of non-randomized studies. Gynecol Oncol 2007; 104:232–246.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Katki HA, Kinney WK, Fetterman B, et al. Cervical cancer risk for women undergoing concurrent testing for human papillomavirus and cervical cytology: a population-based study in routine clinical practice. Lancet Oncol 2011; 12:663–672.
- Anttila A, Kotaniemi-Talonen L, Leinonen M, et al. Rate of cervical cancer, severe intraepithelial neoplasia, and adenocarcinoma in situ in primary HPV DNA screening with cytology triage: randomised study within organised screening programme. BMJ 2010; 340:c1804.
- Castle PE, Schiffman M, Wheeler CM, Solomon D. Evidence for frequent regression of cervical intraepithelial neoplasia-grade 2. Obstet Gynecol 2009; 113:18–25.
- Copeland G, Datta SD, Spivak G, Garvin AD, Cote ML. Total burden and incidence of in situ and invasive cervical carcinoma in Michigan, 1985–2003. Cancer 2008; 113(suppl 10):2946–2954.
- Chen HC, Schiffman M, Lin CY, et al; CBCSP-HPV Study Group. Persistence of type-specific human papillomavirus infection and increased long-term risk of cervical cancer. J Natl Cancer Inst 2011; 103:1387–1396.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Wu X, Matanoski G, Chen VW, et al. Descriptive epidemiology of vaginal cancer incidence and survival by race, ethnicity, and age in the United States. Cancer 2008; 113(suppl 10):2873–2882.
- Pearce KF, Haefner HK, Sarwar SF, Nolan TE. Cytopathological findings on vaginal Papanicolaou smears after hysterectomy for benign gynecologic disease. N Engl J Med 1996; 335:1559–1562.
- Fox J, Remington P, Layde P, Klein G. The effect of hysterectomy on the risk of an abnormal screening Papanicolaou test result. Am J Obstet Gynecol 1999; 180:1104–1109.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years—United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Wright TC, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 ASCCP-Sponsored Consensus Conference. 2006 consensus guidelines for the management of women with abnormal cervical screening tests. J Low Genit Tract Dis 2007; 11:201–222.
- Kjær SK, Frederiksen K, Munk C, Iftner T. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst 2010; 102:1478–1488.
- Khan MJ, Castle PE, Lorincz AT, et al. The elevated 10-year risk of cervical precancer and cancer in women with human papillomavirus (HPV) type 16 or 18 and the possible utility of type-specific HPV testing in clinical practice. J Natl Cancer Inst 2005; 97:1072–1079.
- US Food and Drug Administration (FDA). FDA approved first DNA test for two types of human papillomavirus: agency also approved second DNA test for wider range of HPV types. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2009/ucm149544.htm. Accessed February 5, 2013.
- Wright TC, Stoler MH, Sharma A, Zhang G, Behrens C, Wright TL; ATHENA (Addressing THE Need for Advanced HPV Diagnostics) Study Group. Evaluation of HPV-16 and HPV-18 genotyping for the triage of women with high-risk HPV+ cytology-negative results. Am J Clin Pathol 2011; 136:578–586.
- Petry KU, Schmidt D, Scherbring S, et al. Triaging Pap cytology negative, HPV positive cervical cancer screening results with p16/Ki-67 dual-stained cytology. Gynecol Oncol 2011; 121:505–509.
- Clad A, Reuschenbach M, Weinschenk J, Grote R, Rahmsdorf J, Freudenberg N. Performance of the Aptima high-risk human papillomavirus mRNA assay in a referral population in comparison with Hybrid Capture 2 and cytology. J Clin Microbiol 2011; 49:1071–1076.
- Cárdenas-Turanzas M, Nogueras-Gonzalez GM, Scheurer ME, et al. The performance of human papillomavirus high-risk DNA testing in the screening and diagnostic settings. Cancer Epidemiol Biomarkers Prev 2008; 17:2865–2871.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Castle PE, Fetterman B, Poitras N, Lorey T, Shaber R, Kinney W. Fiveyear experience of human papillomavirus DNA and Papanicolaou test cotesting. Obstet Gynecol 2009; 113:595–600.
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006; 110:525–541.
- Carozzi F, Confortini M, Dalla Palma P, et al; New Technologies for Cervival Cancer Screening (NTCC) Working Group. Use of p16-INK4A overexpression to increase the specificity of human papillomavirus testing: a nested substudy of the NTCC randomised controlled trial. Lancet Oncol 2008; 9:937–945.
- Schmidt D, Bergeron C, Denton KJ, Ridder R; European CINtec Cytology Study Group. p16/ki-67 dual-stain cytology in the triage of ASCUS and LSIL papanicolaou cytology: results from the European equivocal or mildly abnormal Papanicolaou cytology study. Cancer Cytopathol 2011; 119:158–166.
- Wentzensen N, Schwartz L, Zuna RE, et al. Performance of p16/Ki-67 immunostaining to detect cervical cancer precursors in a colposcopy referral population. Clin Cancer Res 2012; 18:4154–4162.
- Nakagawa S, Yoshikawa H, Yasugi T, et al. Ubiquitous presence of E6 and E7 transcripts in human papillomavirus-positive cervical carcinomas regardless of its type. J Med Virol 2000; 62:251–258.
- Oren M. Decision making by p53: life, death and cancer. Cell Death Differ 2003; 10:431–442.
- Cuschieri K, Wentzensen N. Human papillomavirus mRNA and p16 detection as biomarkers for the improved diagnosis of cervical neoplasia. Cancer Epidemiol Biomarkers Prev 2008; 17:2536–2545.
- Dockter J, Schroder A, Hill C, Guzenski L, Monsonego J, Giachetti C. Clinical performance of the APTIMA HPV Assay for the detection of high-risk HPV and high-grade cervical lesions. J Clin Virol 2009; 45(suppl 1):S55–S61.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ogilvie GS, van Niekerk DJ, Krajden M, et al. A randomized controlled trial of human papillomavirus (HPV) testing for cervical cancer screening: trial design and preliminary results (HPV FOCAL Trial). BMC Cancer 2010; 10:111.
KEY POINTS
- The new guidelines still recommend starting screening with cytologic (Papanicolaou) testing at age 21, but now recommend repeating the test less often, ie, every 3 years rather than every 2 years for women age 21 to 29.
- Women age 30 and older who are screened by combined cytologic and HPV testing should be rescreened every 5 years if both tests are negative (instead of every 3 years, as previously recommended). Alternatively, they can be screened by cytology alone every 3 years.
- We can stop screening women at age 65 if they have had adequate screening until then and no history of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) in the past 20 years. Once screening is discontinued, it should not resume, even if the patient has a new sexual partner.
- Screening should not change after HPV vaccination.
- When women have negative cytology but positive HPV results, tests for the HPV 16 and 18 genotypes can help to identify those at higher risk of developing CIN2+.
Human papillomavirus vaccine: Safe, effective, underused
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
The vaccines against human papillomavirus (HPV) are the only ones designed to prevent cancer caused by a virus1,2—surely a good goal. But because HPV is sexually transmitted, HPV vaccination has met with public controversy.3 To counter the objections and better protect their patients’ health, primary care providers and other clinicians need a clear understanding of the benefits and the low risk of HPV vaccination—and the reasons so many people object to it.3
In this article, we will review:
- The impact of HPV-related diseases
- The basic biologic features of HPV vaccines
- The host immune response to natural HPV infection vs the response to HPV vaccines
- The clinical efficacy and safety of HPV vaccines
- The latest guidelines for HPV vaccination
- The challenges to vaccination implementation
- Frequently asked practical questions about HPV vaccination.
HPV-RELATED DISEASES: FROM BOTHERSOME TO DEADLY
Clinical sequelae of HPV infection include genital warts; cancers of the cervix, vulva, vagina, anus, penis, and oropharynx; and recurrent respiratory papillomatosis.4–6
Genital warts
HPV types 6 and 11 are responsible for more than 90% of the 1 million new cases of genital warts diagnosed annually in the United States.7–10
Bothersome and embarrassing, HPV-related genital warts can cause itching, burning, erythema, and pain, as well as epithelial erosions, ulcerations, depigmentation, and urethral and vaginal bleeding and discharge.11,12 Although they are benign in the oncologic sense, they can cause a good deal of emotional and financial stress. Patients may feel anxiety, embarrassment,13 and vulnerability. Adolescents and adults who have or have had genital warts need to inform their current and future partners or else risk infecting them—and facing the consequences.
Direct health care costs of genital warts in the United States have been estimated to be at least $200 million per year.14
Cervical cancer
Cervical cancer cannot develop unless the cervical epithelium is infected with one of the oncogenic HPV types. Indeed, oncogenic HPV is present in as many as 99.8% of cervical cancer specimens.15 HPV 16 and 18 are the most oncogenic HPV genotypes and account for 75% of all cases of cervical cancer. Ten other HPV genotypes account for the remaining 25%.16
In 2012, there were an estimated 12,170 new cases of invasive cervical cancer in the United States and 4,220 related deaths.17 The cost associated with cervical cancer screening, managing abnormal findings, and treating invasive cervical cancer in the United States is estimated to be $3.3 billion per year.18
Although the incidence and the mortality rates of cervical cancer have decreased more than 50% in the United States over the past 3 decades thanks to screening,19 cervical cancer remains the second leading cause of death from cancer in women worldwide. Each year, an estimated 500,000 women contract the disease and 240,000 die of it.20
Anal cancer
A recent study indicated that oncogenic HPV can also cause anal cancer, and the proportion of such cancers associated with HPV 16 or HPV 18 infection is as high as or higher than for cervical cancers, and estimated at 80%.21
The incidence of anal cancer is increasing by approximately 2% per year in both men and women in the general population,22 and rates are even higher in men who have sex with men and people infected with the human immunodeficiency virus.23
Hu and Goldie24 estimated that the lifetime costs of caring for all the people in the United States who in just 1 year (2003) acquired anal cancer attributable to HPV would total $92 million.
Oropharyngeal cancer
HPV types 16, 18, 31, 33, and 35 also cause oropharyngeal cancer. HPV 16 accounts for more than 90% of cases of HPV-related oropharyngeal cancer.25
Chaturvedi et al6 tested tissue samples from three national cancer registries and found that the number of oropharyngeal cancers that were HPV-positive increased from 16.3% in 1984–1989 to 71.7% in 2000–2004, while the number of HPV-negative oropharyngeal cancers fell by 50%, paralleling the drop in cigarette smoking in the United States.
Hu and Goldie24 estimated that the total lifetime cost for all new HPV-related oropharyngeal cancers that arose in 2003 would come to $38.1 million.24
Vulvar and vaginal cancers
HPV 16 and 18 are also responsible for approximately 50% of vulvar cancers and 50% to 75% of vaginal cancers.4,5
Recurrent respiratory papillomatosis
HPV 6 and 11 cause almost all cases of juvenile- and adult-onset recurrent respiratory papillomatosis.26 The annual cost for surgical procedures for this condition in the United States has been estimated at $151 million.27
HPV VACCINES ARE NONINFECTIOUS AND NONCARCINOGENIC
Currently, two HPV vaccines are available: a quadrivalent vaccine against types 6, 11, 16, and 18 (Gardasil; Merck) and a bivalent vaccine against types 16 and 18 (Cervarix; Glaxo-SmithKline). The quadrivalent vaccine was approved by the US Food and Drug Administration (FDA) in 2006, and the bivalent vaccine was approved in 2009.28,29
Both vaccines contain virus-like particles, ie, viral capsids that contain no DNA. HPV has a circular DNA genome of 8,000 nucleotides divided into two regions: the early region, for viral replication, and the late region, for viral capsid production. The host produces neutralizing antibodies in response to the L1 capsid protein, which is different in different HPV types.
In manufacturing the vaccines, the viral L1 gene is incorporated into a yeast genome or an insect virus genome using recombinant DNA technology (Figure 1). Grown in culture, the yeast or the insect cells produce the HPV L1 major capsid protein, which has the intrinsic capacity to self-assemble into virus-like particles.30–33 These particles are subsequently purified for use in the vaccines.34
Recombinant virus-like particles are morphologically indistinguishable from authentic HPV virions and contain the same typespecific antigens present in authentic virions. Therefore, they are highly effective in inducing a host humoral immune response. And because they do not contain HPV DNA, the recombinant HPV vaccines are noninfectious and noncarcinogenic.35
VACCINATION INDUCES A STRONGER IMMUNE RESPONSE THAN INFECTION
HPV infections trigger both a humoral and a cellular response in the host immune system.
The humoral immune response to HPV infection involves producing neutralizing antibody against the specific HPV type, specifically the specific L1 major capsid protein. This process is typically somewhat slow and weak, and only about 60% of women with a new HPV infection develop antibodies to it.36,37
HPV has several ways to evade the host immune system. It does not infect or replicate within the antigen-presenting cells in the epithelium. In addition, HPV-infected keratinocytes are less susceptible to cytotoxic lymphocytic-mediated lysis. Moreover, HPV infection cause very little tissue destruction. And finally, natural cervical HPV infection does not result in viremia. As a result, antigen-presenting cells have no chance to engulf the virions and present virion-derived antigen to the host immune system. The immune system outside the epithelium has limited opportunity to detect the virus because HPV infection does not have a blood-borne phase.38,39
The cell-mediated immune response to early HPV oncoproteins may help eliminate established HPV infection.40 In contrast to antibodies, the T-cell response to HPV has not been shown to be specific to HPV type.41 Clinically, cervical HPV infection is common, but most lesions go into remission or resolve as a result of the cell-mediated immune response.40,41
In contrast to the weak, somewhat ineffective immune response to natural HPV infection, the antibody response to HPV vaccines is rather robust. In randomized controlled trials, almost all vaccinated people have seroconverted. The peak antibody concentrations are 50 to 10,000 times greater than in natural infection. Furthermore, the neutralizing antibodies induced by HPV vaccines persist for as long as 7 to 9 years after immunization.42 However, the protection provided by HPV vaccines against HPV-related cervical intraepithelial neoplasia does not necessarily correlate with the antibody concentration.43–47
Why does the vaccine work so well?
Why are vaccine-induced antibody responses so much stronger than those induced by natural HPV infection?
The first reason is that the vaccine, delivered intramuscularly, rapidly enters into blood vessels and the lymphatic system. In contrast, in natural intraepithelial infection, the virus is shed from mucosal surfaces and does not result in viremia.48
In addition, the strong immunogenic nature of the virus-like particles induces a robust host antibody response even in the absence of adjuvant because of concentrated neutralizing epitopes and excellent induction of the T-helper cell response.35,49,50
The neutralizing antibody to L1 prevents HPV infection by blocking HPV from binding to the basement membrane as well as to the epithelial cell receptor during epithelial microabrasion and viral entry. The subsequent micro-wound healing leads to serous exudation and rapid access of serum immunoglobulin G (IgG) to HPV virus particles and encounters with circulatory B memory cells.
Furthermore, emerging evidence suggests that even very low antibody concentrations are sufficient to prevent viral entry into cervical epithelial cells.46–48,51–53
THE HPV VACCINES ARE HIGHLY EFFECTIVE AND SAFE
The efficacy and safety of the quadrivalent and the bivalent HPV vaccines have been evaluated in large randomized clinical trials.23,28,29,54,55 Table 1 summarizes the key findings.
The Females United to Unilaterally Reduce Endo/ectocervical Disease (FUTURE I)54 and FUTURE II28 trials showed conclusively that the quadrivalent HPV vaccine is 98% to 100% efficacious in preventing HPV 16- and 18-related cervical intraepithelial neoplasia, carcinoma in situ, and invasive cervical cancer in women who had not been infected with HPV before. Similarly, the Papilloma Trial against Cancer in Young Adults (PATRICIA) concluded that the bivalent HPV vaccine is 93% efficacious.29
Giuliano et al55 and Palefsky et al23 conducted randomized clinical trials of the quadrivalent HPV vaccine for preventing genital disease and anal intraepithelial neoplasia in boys and men; the efficacy rates were 90.4%55 and 77.5%.23
A recent Finnish trial in boys age 10 to 18 found 100% seroconversion rates for HPV 16 and HPV 18 antibodies after they received bivalent HPV vaccine.56 Similar efficacy has been demonstrated for the quadrivalent HPV vaccine in boys.57
Adverse events after vaccination
After the FDA approved the quadrivalent HPV vaccine for girls in 2006, the US Centers for Disease Control and Prevention (CDC) conducted a thorough survey of adverse events after immunization from June 1, 2006 through December 31, 2008.58 There were about 54 reports of adverse events per 100,000 distributed vaccine doses, similar to rates for other vaccines. However, the incidence rates of syncope and venous thrombosis were disproportionately higher, according to data from the US Vaccine Adverse Event Reporting System. The rate of syncope was 8.2 per 100,000 vaccine doses, and the rate of venous thrombotic events was 0.2 per 100,000 doses.58
There were 32 reports of deaths after HPV vaccination, but these were without clear causation. Hence, this information must be interpreted with caution and should not be used to infer causal associations between HPV vaccines and adverse outcomes. The causes of death included diabetic ketoacidosis, pulmonary embolism, prescription drug abuse, amyotrophic lateral sclerosis, meningoencephalitis, influenza B viral sepsis, arrhythmia, myocarditis, and idiopathic seizure disorder.58
Furthermore, it is important to note that vasovagal syncope and venous thromboembolic events are more common in young females in general.59 For example, the background rates of venous thromboembolism in females age 14 to 29 using oral contraceptives is 21 to 31 per 100,000 woman-years.60
Overall, the quadrivalent HPV vaccine is well tolerated and clinically safe. Postlicensure evaluation found that the quadrivalent and bivalent HPV vaccines had similar safety profiles.61
Vaccination is contraindicated in people with known hypersensitivity or prior severe allergic reactions to vaccine or yeast or who have bleeding disorders.
HPV VACCINATION DOES MORE THAN PREVENT CERVICAL CANCER IN FEMALES
The quadrivalent HPV vaccine was licensed by the FDA in 2006 for use in females age 9 to 26 to prevent cervical cancer, cervical cancer precursors, vaginal and vulval cancer precursors, and anogenital warts caused by HPV types 6, 11, 16, and 18. The CDC’s Advisory Committee on Immunization Practices (ACIP) issued its recommendation for initiating HPV vaccination for females age 11 to 12 in March 2007. The ACIP stated that the vaccine could be given to girls as early as age 9 and recommended catch-up vaccinations for those age 13 to 26.62,63
The quadrivalent HPV vaccine was licensed by the FDA in 2009 for use in boys and men for the prevention of genital warts. In December 2010, the quadrivalent HPV vaccine received extended licensure from the FDA for use in males and females for the prevention of anal cancer. In October 2011, the ACIP voted to recommend routine use of the quadrivalent HPV vaccine for boys age 11 to 12; catch-up vaccination should occur for those age 13 to 22, with an option to vaccinate men age 23 to 26.
These recommendations replace the “permissive use” recommendations from the ACIP in October 2009 that said the quadrivalent HPV vaccine may be given to males age 9 to 26.64 This shift from a permissive to an active recommendation connotes a positive change reflecting recognition of rising oropharyngeal cancer rates attributable to oncogenic, preventable HPV, rising HPV-related anal cancer incidence, and the burden of the disease in female partners of infected men, with associated rising health care costs.
The bivalent HPV vaccine received FDA licensure in October 2009 for use in females age 10 to 25 to prevent cervical cancer and precursor lesions. The ACIP included the bivalent HPV vaccine in its updated recommendations in May 2010 for use in girls age 11 to 12. Numerous national and international organizations have endorsed HPV vaccination.65–71
Table 2 outlines the recommendations from these organizations.
HPV VACCINATION RATES ARE STILL LOW
HPV vaccine offers us the hope of eventually eradicating cervical cancer. However, the immunization program still faces many challenges, since HPV vaccination touches on issues related to adolescent sexuality, parental autonomy, and cost. As a result, HPV immunization rates remain relatively low in the United States according to several national surveys. Only 40% to 49% of girls eligible for the vaccine received even one dose, and of those who received even one dose, only 32% to 53.3% came back for all three doses.72–75 Furthermore, indigent and minority teens were less likely to finish the three-dose HPV vaccine series.
Why are the vaccination rates so low?
Parental barriers. In one survey,73 reasons that parents gave for not having their daughters vaccinated included:
- Lack of knowledge of the vaccine (19.4%)
- Lack of perceived need for the vaccine (18.8%)
- Belief that their daughter was not sexually active (18.3%)
- Clinician not recommending vaccination (13.1%).
In an effort to improve HPV vaccination rates,41 several states proposed legislation for mandatory HPV vaccination of schoolgirls shortly after licensure of the quadrivalent HPV vaccine.3 Since then, we have seen a wave of public opposition rooted in concerns and misinformation about safety, teenage sexuality, governmental coercion, and cost. Widespread media coverage has also highlighted unsubstantiated claims about side effects attributable to the vaccine that can raise parents’ mistrust of vaccines.76 Concerns have also been raised about a threat to parental autonomy in how and when to educate their children about sex.77
Moreover, the vaccine has raised ethical concerns in some parents and politicians that mandatory vaccination could undermine abstinence messages in sexual education and may alter sexual activity by condoning risky behavior.78 However, a recent study indicated that there is no significant change in sexual behavior related to HPV vaccination in young girls.79
In 2012, Mullins et al80 also found that an urban population of adolescent girls (76.4% black, 57.5% sexually experienced) did not feel they could forgo safer sexual practices after first HPV vaccination, although the girls did perceive less risk from HPV than from other sexually transmitted infections after HPV vaccination (P < .001).80 Inadequate knowledge about HPV-related disease and HPV vaccine correlated with less perceived risk from HPV after vaccination among the girls, and a lack of knowledge about HPV and less communication with their daughters about HPV correlated with less perceived risk from HPV in the mothers of the study population.81
Health-care-provider barriers. Physician endorsement of vaccines represents a key predictor of vaccine acceptance by patients, families, and other clinicians.82–84 In 2008, a cross-sectional, Internet-based survey of 1,122 Texas pediatricians, family practice physicians, obstetricians, gynecologists, and internal medicine physicians providing direct patient care found that only 48.5% always recommended HPV vaccination to girls.74 Of all respondents, 68.4% were likely to recommend the vaccine to boys, and 41.7% agreed with mandated vaccination. Thus, more than half of the physicians were not following the current recommendations for universal HPV vaccination for 11- to -12-year-olds.
In a survey of 1,013 physicians during the spring and summer of 2009, only 34.6% said they always recommend HPV vaccination to early adolescents, 52.7% to middle adolescents, and 50.2% to late adolescents and young adults.85 Pediatricians were more likely than family physicians and obstetrician-gynecologists to always recommend HPV vaccine across all age groups (P < .001). Educational interventions targeting various specialties may help overcome physician-related barriers to immunization.85
Financial barriers. HPV vaccine, which must be given in three doses, is more expensive than other vaccines, and this expense is yet another barrier, especially for the uninsured.86 Australia launched a government-funded program of HPV vaccination (with the quadrivalent vaccine) in schools in 2007, and it has been very successful. Garland et al87 reported that new cases of genital warts have decreased by 73% since the program began, and the rate of high-grade abnormalities on Papanicolaou testing has declined by a small but significant amount.
For HPV vaccination to have an impact on public health, vaccination rates in the general population need to be high. In order to achieve these rates, we need to educate our patients on vaccine safety and efficacy and counsel vaccine recipients about the prevention of sexually transmitted infections and the importance of regular cervical cancer screening after age 21. Clinicians can actively “myth-bust” with patients, who may not realize that the vaccine should be given despite a history of HPV infection or abnormal Pap smear.
FREQUENTLY ASKED QUESTIONS
What if the patient is late for a shot?
The current recommended vaccination schedule for the bivalent and quadrivalent HPV vaccines is a three-dose series administered at 0, 2, and 6 months, given as an intramuscular injection, preferably in the deltoid muscle. The minimal dosing interval is 4 weeks between the first and second doses and 12 weeks between the second and third doses.
The vaccines use different adjuncts with different specific mechanisms for immunogenicity; therefore, it is recommended that the same vaccine be used for the entire three-dose series. However, if circumstances preclude the completion of a series with the same vaccine, the other HPV vaccine may be used.63 Starting the series over is not recommended.
Long-term studies demonstrated clinical efficacy 8.5 years after vaccination.47 Amnestic response by virtue of activation of pools of memory B cells has been demonstrated, suggesting the vaccine may afford lifelong immunity.88
Is a pregnancy test needed before HPV vaccination?
The ACIP states that pregnancy testing is not required before receiving either of the available HPV vaccines.
A recent retrospective review of phase III efficacy trials and pregnancy registry surveillance data for both vaccines revealed no increase in spontaneous abortions, fetal malformations, or adverse pregnancy outcomes.89 Data are limited on bivalent and quadrivalent HPV vaccine given within 30 days of pregnancy and subsequent pregnancy and fetal outcomes. Both vaccines have been assigned a pregnancy rating of category B; however, the ACIP recommends that neither vaccine be given if the recipient is known to be pregnant. If pregnancy occurs, it is recommended that the remainder of the series be deferred until after delivery.62
It is not known whether the vaccine is excreted in breast milk. The manufacturers of both the bivalent and quadrivalent HPV vaccines recommend caution when vaccinating lactating women.30,31
Can HPV vaccine be given with other vaccines?
In randomized trials, giving the bivalent HPV vaccine with the combined hepatitis A, hepatitis B, meningococcal conjugate and the combined tetanus, diphtheria, and acellular pertussis vaccines did not interfere with the immunogenic response, was safe, and was well tolerated.90,91 Coadministration of the quadrivalent HPV vaccine has been studied only with hepatitis B vaccine, with similar safety and efficacy noted.
The ACIP recommends giving HPV vaccine at the same visit with other age-appropriate immunizations to increase the likelihood of adherence to recommended vaccination schedules.62
Is HPV vaccination cost-effective?
Kim and Goldie86 performed a cost-effectiveness analysis of HPV vaccination of girls at age 12 and catch-up vaccination up to the ages of 18, 21, and 26. For their analysis, they considered prevention of cancers associated with HPV types 16 and 18, of genital warts associated with types 6 and 11, and of recurrent respiratory papillomatosis. They also assumed that immunity would be lifelong, and current screening practices would continue.
They calculated that routine vaccination of 12-year-old girls resulted in an incremental cost-effective ratio of $34,900 per quality-adjusted life-year (QALY) gained. A threshold of less than $50,000 per QALY gained is considered reasonably cost-effective, with an upper limit of $100,000 considered acceptable.92
In the same analysis by Kim and Goldie,86 catch-up vaccination of girls through age 18 resulted in a cost of $50,000 to $100,000 per QALY gained, and catch-up vaccination of females through age 26 was significantly less cost-effective at more then $130,000 per QALY gained. The vaccine was also significantly less cost-effective if 5% of the population was neither screened nor vaccinated, if a 10-year booster was required, and if frequent cervical cancer screening intervals were adopted.
This analysis did not include costs related to the evaluation and treatment of abnormal Pap smears and cross-protection against other HPV-related cancers.
The cost-effectiveness of HPV vaccination depends on reaching more girls at younger ages (ideally before sexual debut) and completing the three-dose schedule to optimize duration of immunity.92 Appropriate modification of the current recommendations for the intervals of cervical cancer screening for vaccinated individuals will further improve the cost-effectiveness of vaccination. The inclusion of male vaccination generally has more favorable cost per QALY in scenarios in which female coverage rates are less than 50%93 and among men who have sex with men.94
TO ERADICATE CERVICAL CANCER
Given the remarkable efficacy and expected long-term immunogenicity of HPV vaccines, we anticipate a decline in HPV-related cervical cancer and other related diseases in the years to come. However, modeling studies predicting the impact of HPV vaccination suggest that although substantial reductions in diseases can be expected, the benefit, assuming high vaccination rates, will not be apparent for at least another decade.95 Furthermore, the current HPV vaccines contain only HPV 16 and 18 L1 protein for cancer protection and, therefore, do not provide optimal protection against all oncogenic HPV-related cancers.
The real hope of eradicating cervical cancer and all HPV-related disease relies on a successful global implementation of multivalent HPV vaccination, effective screening strategies, and successful treatment.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
- Baden LR, Curfman GD, Morrissey S, Drazen JM. Human papillomavirus vaccine—opportunity and challenge. N Engl J Med 2007; 356:1990–1991.
- Schiffman M, Wacholder S. From India to the world—a better way to prevent cervical cancer. N Engl J Med 2009; 360:1453–1455.
- Colgrove J, Abiola S, Mello MM. HPV vaccination mandates—law-making amid political and scientific controversy. N Engl J Med 2010; 363:785–791.
- World Health Organization. WHO/ICO Information Centre on Human Papilloma Virus (HPV) and Cervical Cancer. 2010Summary Report. www.who.int/hpvcentre. Accessed November 12, 2012.
- Muñoz N, Bosch FX, de Sanjosé S, et al; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med 2003; 348:518–527.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol 2011; 29:4294–4301.
- Jansen KU, Shaw AR. Human papillomavirus vaccines and prevention of cervical cancer. Annu Rev Med 2004; 55:319–331.
- Fleischer AB, Parrish CA, Glenn R, Feldman SR. Condylomata acuminata (genital warts): patient demographics and treating physicians. Sex Transm Dis 2001; 28:643–647.
- Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev 2005; 14:1157–1164.
- Schiffman M, Solomon D. Findings to date from the ASCUS-LSIL Triage Study (ALTS). Arch Pathol Lab Med 2003; 127:946–949.
- Insinga RP, Dasbach EJ, Myers ER. The health and economic burden of genital warts in a set of private health plans in the United States. Clin Infect Dis 2003; 36:1397–1403.
- Kodner CM, Nasraty S. Management of genital warts. Am Fam Physician 2004; 70:2335–2342.
- Maw RD, Reitano M, Roy M. An international survey of patients with genital warts: perceptions regarding treatment and impact on lifestyle. Int J STD AIDS 1998; 9:571–578.
- Insinga RP, Dasbach EJ, Elbasha EH. Assessing the annual economic burden of preventing and treating anogenital human papillomavirus-related disease in the US: analytic framework and review of the literature. Pharmacoeconomics 2005; 23:1107–1122.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- de Sanjose S, Quint WG, Alemany L, et al; Retrospective International Survey and HPV Time Trends Study Group. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol 2010; 11:1048–1056.
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin 2012; 62:10–29.
- Insinga RP, Glass AG, Rush BB. The health care costs of cervical human papillomavirus—related disease. Am J Obstet Gynecol 2004; 191:114–120.
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review,1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER Web site, 2009. Accessed November 12, 2012.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24(suppl 3):S3/11–S3/25.
- Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–2383.
- Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973–2000. Cancer 2004; 101:281–288.
- Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011; 365:1576–1585.
- Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500.e1–500.e7.
- Herrero R, Castellsagué X, Pawlita M, et al; IARC Multicenter Oral Cancer Study Group. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst 2003; 95:1772–1783.
- Lacey CJ, Lowndes CM, Shah KV. Chapter 4: Burden and management of non-cancerous HPV-related conditions: HPV-6/11 disease. Vaccine 2006; 24(suppl 3):S3/35–S3/41.
- Derkay CS. Task force on recurrent respiratory papillomas. A preliminary report. Arch Otolaryngol Head Neck Surg 1995; 121:1386–1391.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Paavonen J, Naud P, Salmerón J, et al; HPV PATRICIA Study Group. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet 2009; 374:301–314.
- Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Whitehouse Station, NJ. Patient information about Gardasil (human papillomavirus quadrivalent type 6,11,16 and 18 vaccine, recombinant. http://www.gardasil.com/. Accessed November 12, 2012.
- GlaxoSmithKline Biologicals, Rixensart, Belgium. Highlights of prescribing information. Cervarix (human papillomavirus bivalent type 16 and 18 vaccine, recombinant. http://us.gsk.com/products/assets/us_cervarix.pdf. Accessed November 12, 2012.
- Zhou J, Sun XY, Stenzel DJ, Frazer IH. Expression of vaccinia recombinant HPV 16 L1 and L2 ORF proteins in epithelial cells is sufficient for assembly of HPV virion-like particles. Virology 1991; 185:251–257.
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA 1992; 89:12180–12184.
- Schiller JT, Lowy DR. Papillomavirus-like particles and HPV vaccine development. Semin Cancer Biol 1996; 7:373–382.
- Harro CD, Pang YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst 2001; 93:284–292.
- af Geijersstam V, Kibur M, Wang Z, et al. Stability over time of serum antibody levels to human papillomavirus type 16. J Infect Dis 1998; 177:1710–1714.
- Safaeian M, Porras C, Schiffman M, et al; Costa Rican Vaccine Trial Group. Epidemiological study of anti-HPV16/18 seropositivity and subsequent risk of HPV16 and -18 infections. J Natl Cancer Inst 2010; 102:1653–1662.
- Tindle RW. Immune evasion in human papillomavirus-associated cervical cancer. Nat Rev Cancer 2002; 2:59–65.
- Scott M, Nakagawa M, Moscicki AB. Cell-mediated immune response to human papillomavirus infection. Clin Diagn Lab Immunol 2001; 8:209–220.
- Roden R, Wu TC. Preventative and therapeutic vaccines for cervical cancer. Expert Rev Vaccines 2003; 2:495–516.
- Wang SS, Hildesheim A. Chapter 5: Viral and host factors in human papillomavirus persistence and progression. J Natl Cancer Inst Monogr 2003; 31:35–40.
- De Carvalho N, Teixeira J, Roteli-Martins CM, et al. Sustained efficacy and immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine up to 7.3 years in young adult women. Vaccine 2010; 28:6247–6255.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br J Cancer 2006; 95:1459–1466.
- Villa LL, Ault KA, Giuliano AR, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine 2006; 24:5571–5583.
- Smith JF, Brownlow M, Brown M, et al. Antibodies from women immunized with Gardasil cross-neutralize HPV 45 pseudovirions. Hum Vaccin 2007; 3:109–115.
- Rowhani-Rahbar A, Mao C, Hughes JP, et al. Longer term efficacy of a prophylactic monovalent human papillomavirus type 16 vaccine. Vaccine 2009; 27:5612–5619.
- Stanley M. HPV - immune response to infection and vaccination. Infect Agent Cancer 2010; 5:19.
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol 2010; 117(suppl 2):S5–S10.
- Yan M, Peng J, Jabbar IA, et al. Activation of dendritic cells by human papillomavirus-like particles through TLR4 and NF-kappaB-mediated signalling, moderated by TGF-beta. Immunol Cell Biol 2005; 83:83–91.
- Roberts JN, Buck CB, Thompson CD, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med 2007; 13:857–861.
- Kines RC, Thompson CD, Lowy DR, Schiller JT, Day PM. The initial steps leading to papillomavirus infection occur on the basement membrane prior to cell surface binding. Proc Natl Acad Sci USA 2009; 106:20458–20463.
- Day PM, Kines RC, Thompson CD, et al. In vivo mechanisms of vaccine-induced protection against HPV infection. Cell Host Microbe 2010; 8:260–270.
- Garland SM, Hernandez-Avila M, Wheeler CM, et al; Females United to Unilaterally Reduce Endo/Ectocervical Disease (FUTURE) I Investigators. Quadrivalent vaccine against human papillomavirus to prevent anogenital diseases. N Engl J Med 2007; 356:1928–1943.
- Giuliano AR, Palefsky JM, Goldstone S, et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med 2011; 364:401–411.
- Petäjä T, Keränen H, Karppa T, et al. Immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in healthy boys aged 10–18 years. J Adolesc Health 2009; 44:33–40.
- Reisinger KS, Block SL, Lazcano-Ponce E, et al. Safety and persistent immunogenicity of a quadrivalent human papillomavirus types 6, 11, 16, 18 L1 virus-like particle vaccine in preadolescents and adolescents: a randomized controlled trial. Pediatr Infect Dis J 2007; 26:201–209.
- Slade BA, Leidel L, Vellozzi C, et al. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA 2009; 302:750–757.
- Block SL, Brown DR, Chatterjee A, et al. Clinical trial and post-licensure safety profile of a prophylactic human papillomavirus (types 6, 11, 16, and 18) l1 virus-like particle vaccine. Pediatr Infect Dis J 2010; 29:95–101.
- Farmer RD, Lawrenson RA, Thompson CR, Kennedy JG, Hambleton IR. Population-based study of risk of venous thromboembolism associated with various oral contraceptives. Lancet 1997; 349:83–88.
- Labadie J. Postlicensure safety evaluation of human papilloma virus vaccines. Int J Risk Saf Med 2011; 23:103–112.
- Markowitz LE, Dunne EF, Saraiya M, Lawson HW, Chesson H, Unger ER; Centers for Disease Control and Prevention (CDC). Quadrivalent human papillomavirus vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2007; 56:1–24.
- Centers for Disease Control and Prevention (CDC). FDA licensure of bivalent human papillomavirus vaccine (HPV2, Cervarix) for use in females and updated HPV vaccination recommendations from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:626–629.
- Centers for Disease Control and Prevention (CDC). FDA licensure of quadrivalent human papillomavirus vaccine (HPV4, Gardasil) for use in males and guidance from the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2010; 59:630–632.
- World Health Organization (WHO). Weekly Epidemiological Record (WER). January 2009; 84:1–16. http://www.who.int/wer/2009/wer8401_02/en/index.html. Accessed November 12, 2012.
- Saslow D, Castle PE, Cox JT, et al. American Cancer Society guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin 2007; 57:7–28.
- Committee opinion no. 467: human papillomavirus vaccination. Obstet Gynecol 2010; 116:800–803.
- American College of Physicians. ACP Guide to Adult Immunization. 4th ed. 2011:58–60. http://immunization.acponline.org/. Accessed November 12, 2012.
- Vaughn JA, Miller RA. Update on immunizations in adults. Am Fam Physician 2011; 84:1015–1020.
- American Academy of Pediatrics Committee on Infectious Diseases. Prevention of human papillomavirus infection: provisional recommendations for immunization of girls and women with quadrivalent human papillomavirus vaccine. Pediatrics 2007; 120:666–668.
- Friedman L, Bell DL, Kahn JA, et al. Human papillomavirus vaccine: an updated position statement of the Society for Adolescent Health and Medicine. J Adolesc Health 2011; 48:215–216.
- Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13 through 17 years--United States, 2010. MMWR Morb Mortal Wkly Rep 2011; 60:1117–1123.
- Dorell CG, Yankey D, Santibanez TA, Markowitz LE. Human papillomavirus vaccination series initiation and completion, 2008–2009. Pediatrics 2011; 128:830–839.
- Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: a statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev 2009; 18:2325–2332.
- Schwartz JL, Caplan AL, Faden RR, Sugarman J. Lessons from the failure of human papillomavirus vaccine state requirements. Clin Pharmacol Ther 2007; 82:760–763.
- Cooper LZ, Larson HJ, Katz SL. Protecting public trust in immunization. Pediatrics 2008; 122:149–153.
- Olshen E, Woods ER, Austin SB, Luskin M, Bauchner H. Parental acceptance of the human papillomavirus vaccine. J Adolesc Health 2005; 37:248–251.
- Zimmerman RK. Ethical analysis of HPV vaccine policy options. Vaccine 2006; 24:4812–4820.
- Al Romaih WRR, Srinivas A, Shahtahmasebi S, Omar HA. No significant change in sexual behavior in association with human papillomavirus vaccination in young girls. Int J Child Adolesc Health 2011; 4:1–5.
- Mullins TL, Zimet GD, Rosenthal SL, et al. Adolescent perceptions of risk and need for safer sexual behaviors after first human papillomavirus vaccination. Arch Pediatr Adolesc Med 2012; 166:82–88.
- Middleman AB, Tung JS. School-located immunization programs: do parental p predict behavior? Vaccine 2011; 29:3513–3516.
- Samoff E, Dunn A, VanDevanter N, Blank S, Weisfuse IB. Predictors of acceptance of hepatitis B vaccination in an urban sexually transmitted diseases clinic. Sex Transm Dis 2004; 31:415–420.
- Gnanasekaran SK, Finkelstein JA, Hohman K, O’Brien M, Kruskal B, Lieu T. Parental perspectives on influenza vaccination among children with asthma. Public Health Rep 2006; 121:181–188.
- Daley MF, Crane LA, Chandramouli V, et al. Influenza among healthy young children: changes in parental attitudes and predictors of immunization during the 2003 to 2004 influenza season. Pediatrics 2006; 117:e268–e277.
- Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine 2011; 29:8634–8641.
- Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med 2008; 359:821–832.
- Garland SM, Skinner SR, Brotherton JM. Adolescent and young adult HPV vaccination in Australia: achievements and challenges. Prev Med 2011; 53(suppl 1):S29–S35.
- Rowhani-Rahbar A, Alvarez FB, Bryan JT, et al. Evidence of immune memory 8.5 years following administration of a prophylactic human papillomavirus type 16 vaccine. J Clin Virol 2012; 53:239–243.
- Forinash AB, Yancey AM, Pitlick JM, Myles TD. Safety of the HPV bivalent and quadrivalent vaccines during pregnancy (February) Ann Pharmacother 2011; [epub ahead of print]
- Wheeler CM, Harvey BM, Pichichero ME, et al. Immunogenicity and safety of human papillomavirus-16/18 AS04-adjuvanted vaccine coadministered with tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine and/or meningococcal conjugate vaccine to healthy girls 11 to 18 years of age: results from a randomized open trial. Pediatr Infect Dis J 2011; 30:e225–e234.
- Pedersen C, Breindahl M, Aggarwal N, et al. Randomized trial: immunogenicity and safety of coadministered human papillomavirus-16/18 AS04-adjuvanted vaccine and combined hepatitis A and B vaccine in girls. J Adolesc Health 2012; 50:38–46.
- Eichler HG, Kong SX, Gerth WC, Mavros P, Jönsson B. Use of costeffectiveness analysis in health-care resource allocation decisionmaking: how are cost-effectiveness thresholds expected to emerge? Value Health 2004; 7:518–528.
- Chesson HW. HPV vaccine cost-effectiveness: updates and review. Presentation before the Advisory Committee on Immunization Practices (ACIP), June 22, 2011. Atlanta, GA: US Department of Health and Human Services, CDC; 2011. http://www.cdc.gov/vaccines/recs/acip/downloads/mtg-slides-jun11/07-5-hpv-cost-effect.pdf. Accessed August 31, 2012.
- Kim JJ. Targeted human papillomavirus vaccination of men who have sex with men in the USA: a cost-effectiveness modelling analysis. Lancet Infect Dis 2010; 10:845–852.
- Cuzick J, Castañón A, Sasieni P. Predicted impact of vaccination against human papillomavirus 16/18 on cancer incidence and cervical abnormalities in women aged 20–29 in the UK. Br J Cancer 2010; 102:933–939.
KEY POINTS
- Two HPV vaccines are available: a quadrivalent vaccine against HVP types 6, 11, 16, and 18, and a bivalent vaccine against types 16 and 18.
- HPV causes cervical cancer, genital warts, oropharyngeal cancer, anal cancer, and recurrent respiratory papillomatosis, creating a considerable economic and health burden.
- The host immune response to natural HPV infection is slow and weak. In contrast, HPV vaccine induces a strong and long-lasting immune response.
- The HPV vaccines have greater than 90% efficacy in preventing cervical dysplasia and genital warts that are caused by the HPV types the vaccine contains. They are as safe as other common prophylactic vaccines.
- HPV vaccination has been challenged by public controversy over the vaccine’s safety, teenage sexuality, mandatory legislation, and the cost of the vaccine.
2012–2013 Influenza update: Hitting a rapidly moving target
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
Despite our success in reducing the number of deaths from influenza in the last half-century, we must remain vigilant, since influenza still can kill.1,2 Gene mutations and reassortment among different strains of influenza viruses pose a significant public health threat, especially in an increasingly mobile world.3,4
In this article, we will present an update on influenza to better prepare primary care providers to prevent and treat this ongoing threat.
H3N2v: SWINE FLU DÉJÀ VU?
Outbreaks of swine flu at state and county fairs in 2012 are unprecedented and have raised concerns.
From 1990 to 2010, human infections with swine-origin influenza viruses were sporadic, and the US Centers for Disease Control and Prevention (CDC) confirmed a total of only 27 cases during this period.5 However, the number has been increasing since 2011: as of August 31, 2012, a total of 309 cases had been reported.6
Analysis of viral RNA in clinical respiratory specimens from 12 cases in 2011 revealed a variant strain, called H3N2v, which is a hybrid containing genetic material from swine H3N2 and the 2009 human pandemic virus H1N1pdm09. The M gene in this new variant came from the human virus, while the other seven came from the swine virus when a host was infected with both viruses simultaneously (Figure 1). As a result of this genetic reassortment, this variant virus is genetically and antigenically different from seasonal H3N2.
Epidemiologic data showed that children under 10 years of age are especially susceptible to this new variant because they lack immunity, whereas adolescents and adults may have some immunity from cross-reacting antibodies.7 Most infected people had been exposed to swine in agriculture, including county and state fairs. So far, evidence suggests only limited human-to-human transmission.8 The clinical diagnosis of H3N2v infection relies on the epidemiologic link to exposure to pigs in the week before the onset of illness, since the symptoms are indistinguishable from those of seasonal influenza A or B infections.
In suspected cases, the clinician should notify the local or state public health department and arrange for a special test to be performed on respiratory specimens: the CDC Flu Real-Time Reverse Transcriptase Polymerase Chain Reaction Dx Panel. The reason is that a negative rapid influenza diagnostic test does not rule out influenza infection, and a positive immunofluorescence assay (direct fluorescent antibody staining) cannot specifically detect H3N2v.7
The current seasonal influenza vaccine will not protect against H3N2v. The isolates tested to date were susceptible to the neuraminidase inhibitor drugs oseltamivir (Tamiflu) and zanamivir (Relenza) but resistant to amantadine (Symmetrel) and rimantadine (Flumadine).9
Whether H3N2v will become a significant problem during the upcoming flu season largely depends on the extent of human-to-human transmission. We need to closely follow updates on this virus.
H5N1: THE LOOMING THREAT OF A BIRD FLU PANDEMIC
Since 2003, influenza A H5N1, a highly pathogenic avian virus, has broken out in Asia, Africa, and the Middle East, killing more than 100 million birds. It also has crossed the species barrier to infect humans, with an unusually high death rate.10
As of August 10, 2012, the World Health Organization had reported 608 confirmed cases of this virus infecting humans and 359 associated deaths.11 Most infected patients had a history of close contact with diseased poultry, but limited, nonsustained human-to-human transmission can occur during very close, unprotected contact with a severely ill patient.12
Molecular studies of this virus revealed further insights into its pathogenesis. Some of the viruses isolated from humans have had mutations that allow them to bind to human-type receptors.13 Amino acid substitutions in the polymerase basic protein 2 (PB2) gene are associated with mammalian adaptation, virulence in mice, and viral replication at temperatures present in the upper respiratory tract.14 Furthermore, higher plasma levels of macrophage- and neutrophil-attractant chemokines and both inflammatory and anti-inflammatory cytokines (interleukin 6, interleukin 10, and interferon gamma) have been observed in patients with H5N1 infection, especially in fatal cases.15 A recent study found that H5N1 causes significant perturbations in the host’s protein synthesis machinery as early as 1 hour after infection, suggesting that this virus gains an early advantage in replication by using the host’s proteome.16 The effects of unrestrained viral infection and inflammatory responses induced by H5N1 infection certainly contributed to the primary pathologic process and to death in human fulminant viral pneumonia. The up-regulation of inflammatory cytokines in these infections contributes to the development of sepsis syndrome, acute respiratory distress syndrome, and an increased risk of death, particularly in pregnant women.
Most experts predict that pandemic influenza is probably inevitable.17 If avian H5N1 and a human influenza virus swap genes in a host such as swine, the new hybrid virus will contain genetic material from both strains and will have surface antigens that the human immune system does not recognize. This could lead to a devastating avian flu pandemic with a very high death rate.18
An inactivated whole-virus H5N1 vaccine has been developed by the US government to prevent H5N1 infection.19 For treatment, the neuraminidase inhibitor oseltamivir is the drug of choice.10 Oseltamivir resistance remains uncommon. 20 Fortunately, zanamivir is still active against oseltamivir-resistant variants that have N1 neuraminidase mutations.21
THE 2009 H1N1 PANDEMIC KILLED MORE PEOPLE THAN WE THOUGHT
The fourth flu pandemic of the last 100 years occurred in 2009. (The other three were in 1918, 1957, and 1968.) It was caused by a novel strain, H1N1 of swine origin.22 This 2009 pandemic strain had six genes from the North American swine flu virus and two genes from the Eurasian swine flu virus. The pandemic affected more children and young people (who completely lacked prior immunity to this virus), while older people, who had cross-reacting antibodies, were less affected.
Worldwide, 18,500 people were reported initially to have died in this pandemic from April 2009 to August 2010.23 However, a recent modeling study estimated the number of respiratory and cardiovascular deaths associated with this pandemic at 283,500—about 15 times higher.24
AN AUSTRALIAN OUTBREAK OF OSELTAMIVIR-RESISTANT H1N1
Many strains of influenza A virus are resistant to amantadine and rimantadine, owing to amino acid substitutions in the M2 protein.25 In contrast, resistance to the neuraminidase inhibitors oseltamivir and zanamivir has been reported only occasionally.26
Until recently, most oseltamivir-resistant viruses were isolated from immunocompromised hosts treated with oseltamivir.27–29 All the resistant viral isolates contained an amino acid substitution of histidine (H) to tyrosine (Y) at position 275 of the viral neuraminidase.30 In general, transmission of these oseltamivir-resistant strains has been limited and unsustained, but it can occur in settings of close contact, such as hospitals, school camps, or long train rides.31–35 Oseltamivir-resistant strains were detected in fewer than 1% of isolates from the community during the 2010–2011 influenza season in the Northern Hemisphere and most countries in the Southern Hemisphere during the 2011 flu season.36,37
However, an outbreak of oseltamivir-resistant H1N1 occurred in Australia between June and August 2011.38 In that outbreak, the isolates from only 15% of the 191 people infected with this virus, designated H1N1pdm09, carried the H257Y neuraminidase substitution.39 Further, only 1 of the 191 patients had received oseltamivir before. More importantly, genetic analysis suggested that the infection spread from a single source.
This was the first reported sustained community transmission of oseltamivir-resistant H1N1 in a community previously unexposed to this drug. As such, it is a warning sign of the potential for a widespread outbreak of this virus. In the event of such an outbreak, inhaled zanamivir would be the only effective treatment available.
THIS SEASON’S TRIVALENT INACTIVATED VACCINE
The trivalent inactivated influenza vaccine for the 2012–2013 season contains three inactivated viruses40:
- Influenza A/California/7/2009(H1N1)-like
- Influenza A/Victoria/361/2011(H3N2)-like
- Influenza B/Wisconsin/1/2010-like (Yamagata lineage).
The influenza A H3N2 and influenza B antigens are different from those in the 2011–2012 vaccine.41 The H1N1 strain is derived from H1N1pdm09, which had been contained in the 2011–2012 seasonal vaccine. This vaccine will not protect against H3N2v or H5N1.
LATEST RECOMMENDATIONS ON VACCINATION
Since 2010, the Advisory Committee on Immunization Practices (ACIP) has recommended annual flu shots for all people older than 6 months in the United States.42
Vaccination should be done before the onset of influenza activity in the community as soon as vaccine is available for the season. However, one should continue offering vaccination throughout the influenza season as long as influenza viruses are circulating in the community.
Children ages 6 months through 8 years not previously vaccinated against influenza should receive two doses of influenza vaccine at least 4 weeks apart for an optimal immune response. The US-licensed Afluria vaccine (CSL Biotherapies, King of Prussia, PA), a trivalent inactivated vaccine, is not recommended for children under 9 years of age because of concern about febrile seizures.43,44
There is no contraindication to giving inactivated trivalent influenza vaccine to immunosuppressed people.
The live-attenuated influenza vaccine is indicated only for healthy, nonpregnant people age 2 through 49 years and not for people who care for severely immunosuppressed patients who require a protective environment.
For indications for and details about the different available influenza vaccines, see the ACIP’s current recommendations (www.cdc.gov/mmwr/pdf/wk/mm6132.pdf).40
Updated recommendations for people allergic to eggs
All currently available influenza vaccines are made by growing the virus in chicken eggs. Therefore, severe allergic and anaphylactic reactions can occur in people with egg allergy. The ACIP recommends that if people experienced only hives after egg exposure, they should still receive the trivalent inactivated vaccine. Recently, the ACIP reviewed data from the Vaccine Adverse Event Reporting System45 and issued the following recommendations for the 2012–2013 influenza season40:
- In people who are allergic to eggs, only trivalent inactivated vaccine should be used, not the live-attenuated vaccine, because of lack of data for use of the latter in this group.
- Vaccine should be given by providers who are familiar with the signs of egg allergy.
- Patients with a history of egg allergy who have experienced only hives after exposure to eggs should be observed for a minimum of 30 minutes after vaccination.
- Patients who experience lightheadedness, respiratory distress, angioedema, or recurrent emesis or who require epinephrine or emergency medical attention after egg exposure should be referred before vaccination to a physician who has expertise in managing allergic conditions.
- Tolerance to egg-containing foods does not exclude the possibility of egg allergy. Egg allergy can be confirmed by a consistent medical history of adverse reactions to eggs or egg-containing foods, plus skin or blood testing for immunoglobulin E antibodies to egg proteins.
A high-dose vaccine is available for people 65 years and older
The rates of hospitalization and death due to seasonal flu in elderly people have increased significantly in the last 20 years despite rising rates of vaccination.46–48 This is largely due to lower serologic response rates and vaccine efficacy in older adults with weaker immune systems.
Several studies have shown that the development of protective antibody titers depends on the dose of antigen.49–53 A randomized, controlled clinical trial compared the immunogenicity of a high-dose vaccine and a standard-dose vaccine in older adults and found that the level of antibody response was significantly higher with the high-dose vaccine, and that the rate of adverse reactions was the same.54
In December 2009, the US Food and Drug Administration (FDA) licensed a new trivalent inactivated influenza vaccine with high doses of hemagglutinin antigens for adults over the age of 65.55 Postlicensure safety surveillance in 2010 revealed no serious safety concerns.56
At present, the ACIP expresses no preference for standard-dose or high-dose vaccine for adults 65 years of age and older.40 Importantly, if only the standard-dose vaccine is at hand, the opportunity for influenza vaccination should not be missed with the intention of giving high-dose vaccine at a later date.
A NEW QUADRIVALENT LIVE-ATTENUATED INFLUENZA VACCINE FOR THE 2013–2014 SEASON
In February 2012, the FDA approved the first quadrivalent live-attenuated influenza vaccine, which is expected to replace the currently available trivalent live-attenuated influenza vaccine in the 2013–2014 flu season. The quadrivalent vaccine will include both lineages of the circulating influenza B viruses (the Victoria and Yamagata lineages). The reasons for this inclusion is the difficulty in predicting which of these viruses will predominate in any given season, and the limited cross-resistance by immunization against one of the lineages.
A recent analysis57 estimated that such a vaccine is likely to further reduce influenza cases, related hospitalizations, and deaths compared with the current trivalent vaccine. Like the current trivalent live-attenuated vaccine, the quadrivalent vaccine is inhaled.
EVOLVING VACCINATION POLICY IN HEALTH CARE WORKERS
Starting in January 2013, the Centers for Medicare and Medicaid Services will require hospitals to report how many of their health care workers are vaccinated. These rates will be publicly reported as a measure of hospital quality. This has fueled the ongoing debate about mandating influenza vaccination for health care workers. Studies have shown that the most important factors in increasing influenza vaccination rates among health care workers are requiring vaccination as a condition for employment and making vaccination available on-site, for more than 1 day, at no cost to the worker.58
As an alternative, some institutions have implemented a “shot-or-mask” policy whereby a health care worker who elects not to be vaccinated because of medical or religious reasons would be asked to wear a mask during all faceto-face encounters with patients.
NEW ANTIVIRAL DRUGS ON THE HORIZON
The emergence of oseltamivir-resistant strains in recent years caused a great deal of concern in public health regarding the potential for outbreaks of drug-resistant influenza.34,35,59–61
A recent Asian randomized clinical trial reported the efficacy of a long-acting neuraminidase inhibitor, laninamivir octanoate, in the treatment of seasonal influenza.62 This study showed that a single inhalation of this drug is effective in treating seasonal influenza, including that caused by oseltamivir-resistant strains in adults. Laninamivir is currently approved in Japan.
CHALLENGES IN PREVENTING AND TREATING INFLUENZA
Despite the great advances that we have made in preventing and treating influenza in the last half-century, we still face many challenges. Each year in the United States, influenza infection results in an estimated 31 million outpatient visits, 226,000 hospital admissions, and 36,000 deaths.42
Antigenic drift and shift. Influenza viruses circulating among animals and humans vary genetically from season to season and within seasons. As a result of this changing viral antigenicity, the virus can evade the human immune system, causing widespread outbreaks.
One of the unique and most remarkable features of influenza virus is the antigenic variation: antigenic drift and antigenic shift. Antigenic drift is the relatively minor antigenic changes that occur frequently within an influenza subtype, typically resulting from genetic mutation of viral RNA coding for hemagglutinin or neuraminidase. This causes annual regional epidemics. In contrast, antigenic shift is the result of genetic material reassortment: the emerging of new viral RNA from different strains of different species. This often leads to global pandemics.
Therefore, it is challenging to accurately predict the antigenic makeup of influenza viruses for each season and to include new emerging antigens in the vaccine production, as we are facing a moving target. We prepare influenza vaccines each season based on past experience.63
Vaccination rates have hit a plateau of 60% to 70% in adults since the 1990s, in spite of greater vaccine supply and recommendations that all adults, regardless of underlying disease, be vaccinated annually.64 Similarly, only 51% of children age 6 months to 17 years were vaccinated in the 2010–2011 season.65 And vaccination rates are even lower in low-income populations.66,67
The emergence of oseltamivir-resistant strains in recent years, not only in people exposed to oseltamivir but also in those who haven’t been exposed to this drug, with sustained transmission, certainly raises the possibility of a more difficult epidemic to control.38
Global travel, global infection. Our last H1N1 pandemic in 2009 was an example of how easily the influenza virus can spread rapidly in today’s highly mobile global society.22
What we must do
As primary health care providers, we must closely monitor the community outbreak and the emergence of drug-resistant strains and strongly recommend vaccination for all patients older than 6 months, since timely vaccination is the cornerstone of influenza prevention. Although many have questioned the efficacy of influenza vaccination, a recent meta-analysis showed a 59% pooled efficacy of the trivalent inactivated vaccine in adults age 18 to 65 years in preventing virologically confirmed influenza, and 83% pooled efficacy of the live-attenuated influenza vaccine in children age 6 months to 7 years.68 Novel approaches for vaccination reminders, such as text messaging69 in addition to traditional mail or telephone reminders, can improve vaccination compliance in today’s highly mobile world that is more than ever connected.
With the lessons learned from four pandemics in the last century, updated recommendations for prevention, and adequate vaccine supply, we should be ready to face the challenge of another flu season.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
- Doshi P. Trends in recorded influenza mortality: United States, 1900–2004. Am J Public Health 2008; 98:939–945.
- Centers for Disease Control and Prevention (CDC). Estimates of deaths associated with seasonal influenza — United States, 1976–2007. MMWR Morb Mortal Wkly Rep 2010; 59:1057–1062.
- Reid AH, Taubenberger JK, Fanning TG. Evidence of an absence: the genetic origins of the 1918 pandemic influenza virus. Nat Rev Microbiol 2004; 2:909–914.
- Lindstrom S, Garten R, Balish A, et al. Human infections with novel reassortant influenza A(H3N2)v viruses, United States, 2011. Emerg Infect Dis 2012; 18:834–837.
- Shu B, Garten R, Emery S, et al. Genetic analysis and antigenic characterization of swine origin influenza viruses isolated from humans in the United States, 1990–2010. Virology 2012; 422:151–160.
- Centers for Disease Control and Prevention (CDC). http://www.cdc.gov/flu/swineflu/h3n2v-outbreak.htm. Accessed September 27, 2012.
- Centers for Disease Control and Prevention (CDC). Evaluation of rapid influenza diagnostic tests for influenza A (H3N2)v virus and updated case count — United States, 2012. MMWR Morb Mortal Wkly Rep 2012; 61:619–621.
- Centers for Disease Control and Prevention (CDC). Update: Influenza A (H3N2)v transmission and guidelines — five states, 2011. MMWR Morb Mortal Wkly Rep 2012; 60:1741–1744.
- Centers for Disease Control and Prevention (CDC). Interim information for clinicians about human infections with H3N2v virus. http://www.cdc.gov/flu/swineflu/h3n2v-clinician.htm. Accessed September 27, 2012.
- Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus; Abdel-Ghafar AN, Chotpitayasunondh T, Gao Z, et al. Update on avian influenza A (H5N1) virus infection in humans. N Engl J Med 2008; 358:261–273.
- World Health Organization (WHO). http://www.who.int/influenza/human_animal_interface/H5N1_cumulative_table_archives/en/index.html. Accessed September 27, 2012.
- Ungchusak K, Auewarakul P, Dowell SF, et al. Probable person-to-person transmission of avian influenza A (H5N1). N Engl J Med 2005; 352:333–340.
- Yamada S, Suzuki Y, Suzuki T, et al. Haemagglutinin mutations responsible for the binding of H5N1 influenza A viruses to human-type receptors. Nature 2006; 444:378–382.
- Hatta M, Hatta Y, Kim JH, et al. Growth of H5N1 influenza A viruses in the upper respiratory tracts of mice. PLoS Pathog 2007; 3:1374–1379.
- de Jong MD, Simmons CP, Thanh TT, et al. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med 2006; 12:1203–1207.
- Cheung CY, Chan EY, Krasnoselsky A, et al. H5N1 virus causes significant perturbations in host proteome very early in influenza virus-infected primary human monocyte-derived macrophages. J Infect Dis 2012; 206:640–645.
- Gordon S. Avian influenza: a wake-up call from birds to humans. Cleve Clin J Med 2004; 71:273–274.
- Jin XW, Mossad SB. Avian influenza: an emerging pandemic threat. Cleve Clin J Med 2005; 72:1129–1234.
- Ehrlich HJ, Müller M, Oh HM, et al; Baxter H5N1 Pandemic Influenza Vaccine Clinical Study Team. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 2008; 358:2573–2584.
- de Jong MD, Tran TT, Truong HK, et al. Oseltamivir resistance during treatment of influenza A (H5N1) infection. N Engl J Med 2005; 353:2667–2672.
- Le QM, Kiso M, Someya K, et al. Avian flu: isolation of drug-resistant H5N1 virus. Nature 2005; 437:1108.
- Ison MG, Lee N. Influenza 2010–2011: lessons from the 2009 pandemic. Cleve Clin J Med 2010; 77:812–820.
- World Health Organization (WHO). Pandemic (H1N1) 2009 — update 112. http://www.who.int/csr/don/2010_08_06/en/index.html. Accessed September 27, 2012.
- Dawood FS, Iuliano AD, Reed C, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. Lancet Infect Dis 2012; 12:687–695.
- Bright RA, Shay DK, Shu B, Cox NJ, Klimov AI. Adamantane resistance among influenza A viruses isolated early during the 2005–2006 influenza season in the United States. JAMA 2006; 295:891–894.
- Nguyen HT, Fry AM, Gubareva LV. Neuraminidase inhibitor resistance in influenza viruses and laboratory testing methods. Antivir Ther 2012; 17:159–173.
- Graitcer SB, Gubareva L, Kamimoto L, et al. Characteristics of patients with oseltamivir-resistant pandemic (H1N1) 2009, United States. Emerg Infect Dis 2011; 17:255–257.
- Hurt AC, Deng YM, Ernest J, et al. Oseltamivir-resistant influenza viruses circulating during the first year of the influenza A(H1N1) 2009 pandemic in the Asia-Pacific region, March 2009 to March 2010. Euro Surveill 2011; 16:19770.
- Meijer A, Jonges M, Abbink F, et al. Oseltamivir-resistant pandemic A(H1N1) 2009 influenza viruses detected through enhanced surveillance in the Netherlands, 2009–2010. Antiviral Res 2011; 92:81–89.
- Gubareva LV, Kaiser L, Hayden FG. IInfluenza virus neuraminidase inhibitors. Lancet 2000; 355:827–835.
- Wolfe C, Greenwald I, Chen L. Pandemic (H1N1) 2009 and oseltamivir resistance in hematology/oncology patients. Emerg Infect Dis 2010; 16:1809–1811.
- Moore C, Galiano M, Lackenby A, et al. Evidence of person-to-person transmission of oseltamivir-resistant pandemic influenza A(H1N1) 2009 virus in a hematology unit. J Infect Dis 2011; 203:18–24.
- Chen LF, Dailey NJ, Rao AK, et al. Cluster of oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infections on a hospital ward among immunocompromised patients — North Carolina, 2009. J Infect Dis 2011; 203:838–846.
- Centers for Disease Control and Prevention (CDC). Oseltamivir-resistant 2009 pandemic influenza A (H1N1) virus infection in two summer campers receiving prophylaxis — North Carolina, 2009. MMWR Morb Mortal Wkly Rep 2009; 58:969–972.
- Le QM, Wertheim HF, Tran ND, van Doorn HR, Nguyen TH, Horby P; Vietnam H1N1 Investigation Team. A community cluster of oseltamivir-resistant cases of 2009 H1N1 influenza. N Engl J Med 2010; 362:86–87.
- Lackenby A, Moran Gilad J, Pebody R, et al. Continued emergence and changing epidemiology of oseltamivir-resistant influenza A(H1N1)2009 virus, United Kingdom, winter 2010/11. Euro Surveill 2011; 16:19784.
- World Health Organization (WHO). Summary of influenza antiviral susceptibility surveillance findings, September 2010 – March 2011. http://www.who.int/influenza/gisrs_laboratory/updates/antiviral_susceptibility/en/index.html. Accessed September 27, 2012.
- Hurt AC, Hardie K, Wilson NJ, et al. Community transmission of oseltamivir-resistant A(H1N1)pdm09 influenza. N Engl J Med 2011; 365:2541–2542.
- Hurt AC, Hardie K, Wilson NJ, et al. Characteristics of a widespread community cluster of H275Y oseltamivir-resistant A(H1N1)pdm09 influenza in Australia. J Infect Dis 2012; 206:148–157.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) — United States, 2012–13 Influenza Season. MMWR Morb Mortal Wkly Rep 2012; 61:613–618.
- Food and Drug Administration (FDA). Summary minutes: vaccines and related biological products advisory committee. February 28–29, 2012. Silver Spring, MD. http://www.fda.gov/downloads/Advisory-Committees/CommitteesMeetingMaterials/BloodVaccinesandOther-Biologics/VaccinesandRelatedBiologicalProductsAdvisoryCommittee/UCM296193.pdf. Accessed September 28, 2012.
- Fiore AE, Uyeki TM, Broder K, et al; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59:1–62.
- Centers for Disease Control and Prevention (CDC). Update: recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding use of CSL seasonal influenza vaccine (Afluria) in the United States during 2010–11. MMWR Morb Mortal Wkly Rep 2010; 59:989–992.
- Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep 2011; 60:1128–1132.
- Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices: Update on influenza vaccine safety monitoring. June 20–21, 2012. Atlanta, GA. http://www.cdc.gov/vaccines/acip/meetings/downloads/slides-jun-2012/03-influenza-Shimabukuro.pdf. Accessed September 28, 2012.
- Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med 2005; 165:265–272.
- Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA 2004; 292:1333–1340.
- Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003; 289:179–186.
- Mostow SR, Schoenbaum SC, Dowdle WR, Coleman MT, Kaye HS. Inactivated vaccines. 1. Volunteer studies with very high doses of influenza vaccine purified by zonal ultracentrifugation. Postgrad Med J 1973; 49:152–158.
- Keitel WA, Atmar RL, Cate TR, et al. Safety of high doses of influenza vaccine and effect on antibody responses in elderly persons. Arch Intern Med 2006; 166:1121–1127.
- Ruben FL, Jackson GG. A new subunit influenza vaccine: acceptability compared with standard vaccines and effect of dose on antigenicity. J Infect Dis 1972; 125:656–664.
- Palache AM, Beyer WE, Sprenger MJ, et al. Antibody response after influenza immunization with various vaccine doses: a double-blind, placebo-controlled, multi-centre, dose-response study in elderly nursing-home residents and young volunteers. Vaccine 1993; 11:3–9.
- Couch RB, Winokur P, Brady R, et al. Safety and immunogenicity of a high dosage trivalent influenza vaccine among elderly subjects. Vaccine 2007; 25:7656–7663.
- Falsey AR, Treanor JJ, Tornieporth N, Capellan J, Gorse GJ. Randomized, double-blind controlled phase 3 trial comparing the immunogenicity of high-dose and standard-dose influenza vaccine in adults 65 years of age and older. J Infect Dis 2009; 200:172–180.
- US Food and Drug Administration. Vaccines, Blood & Biologics. December 23,2009 approval letter—Fluzone high-dose. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm195481.htm. Accessed October 1, 2012.
- Moro PL, Arana J, Cano M, et al. Postlicensure safety surveillance for high-dose trivalent inactivated influenza vaccine in the Vaccine Adverse Event Reporting System, 1 July 2010–31 December 2010. Clin Infect Dis 2012; 54:1608–1614.
- Reed C, Meltzer MI, Finelli L, Fiore A. Public health impact of including two lineages of influenza B in a quadrivalent seasonal influenza vaccine. Vaccine 2012; 30:1993–1998.
- Centers for Disease Control and Prevention (CDC). Influenza vaccination coverage among health-care personnel — United States, 2010–11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1073–1077.
- Meijer A, Lackenby A, Hungnes O, et al; European Influenza Surveillance Scheme. Oseltamivir-resistant influenza virus A (H1N1), Europe, 2007–08 season. Emerg Infect Dis 2009; 15:552–560.
- Moscona A. Global transmission of oseltamivir-resistant influenza. N Engl J Med 2009; 360:953–956.
- World Health Organization (WHO). Influenza A virus resistance to oseltamivir. http://www.who.int/influenza/patient_care/antivirals/oseltamivir_summary/en/. Accessed September 28, 2012.
- Watanabe A, Chang SC, Kim MJ, Chu DW, Ohashi Y; MARVEL Study Group. Long-acting neuraminidase inhibitor laninamivir octanoate versus oseltamivir for treatment of influenza: a double-blind, randomized, noninferiority clinical trial. Clin Infect Dis 2010; 51:1167–1175.
- Deyde VM, Gubareva LV. Influenza genome analysis using pyro-sequencing method: current applications for a moving target. Expert Rev Mol Diagn 2009; 9:493–509.
- Schuchat A, Katz JM. Protecting adults from influenza: tis the season to learn from the pandemic. J Infect Dis 2012; 206:803–805.
- Centers for Disease Control and Prevention (CDC). Final state-level influenza vaccination coverage estimates for the 2010–11 season — United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/professionals/vaccination/coverage_1011estimates.htm. Accessed September 28, 2012.
- Bhatt P, Block SL, Toback SL, Ambrose CS. Timing of the availability and administration of influenza vaccine through the vaccines for children program. Pediatr Infect Dis J 2011; 30:100–106.
- Lee BY, Brown ST, Bailey RR, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health Aff (Millwood) 2011; 30:1141–1150.
- Osterholm MT, Kelley NS, Sommer A, Belongia EA. Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect Dis 2012; 12:36–44.
- Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA 2012; 307:1702–1708.
KEY POINTS
- A recent outbreak of swine flu in children exposed to pigs at agricultural fairs is unprecedented. Seasonal influenza vaccine does not protect against this strain, designated H3N2v. The neuraminidase inhibitors oseltamivir (Tamiflu) and zanamivir (Relenza) are the drugs of choice for treatment.
- A highly lethal bird flu, designated H5N1, is still a pandemic threat. In the event of an outbreak, an inactivated whole-virus vaccine is available.
- A community outbreak of oseltamivir-resistant H1N1 in Australia sounded an alarm for a potential drug-resistant flu epidemic. Inhaled zanamivir would be the only effective therapy available in the event of such an epidemic.
- An emerging new antiviral drug is effective against oseltamivir-resistant influenza.
In reply: Cervical cancer screening
In Reply: We thank Dr. Keller for his excellent comment. The rationale for discontinuing screening in a woman over 70 who has multiple sexual partners without a history of an abnormal Pap test is that she is at lower risk of new-onset cervical intraepithelial neoplasia (CIN) than a younger woman because of her decreased rate of metaplasia and less accessible transformation zone. In addition, postmenopausal mucosal atrophy may predispose to false-positive cytology. False-positive results are likely to be followed by additional invasive procedures, anxiety, and cost to the patient. However, she is still at risk for acquiring human papillomavirus (HPV) and CIN. Given that cervical cancer develops slowly and risk factors decrease with age, it is reasonable to stop screening at this point. Also, the recommendation of the 3-year screening interval in women over 30 with multiple sexual partners who had negative Pap and HPV tests is based on the fact that they can acquire HPV the day after screening and subsequently develop CIN, but we can detect HPV and CIN in the next round of screening (3 years later) and so will not miss the opportunity to treat cervical dysplasia.
However, practice guidelines are never meant to replace a physician’s sound clinical decision made on an individual basis.
In Reply: We thank Dr. Keller for his excellent comment. The rationale for discontinuing screening in a woman over 70 who has multiple sexual partners without a history of an abnormal Pap test is that she is at lower risk of new-onset cervical intraepithelial neoplasia (CIN) than a younger woman because of her decreased rate of metaplasia and less accessible transformation zone. In addition, postmenopausal mucosal atrophy may predispose to false-positive cytology. False-positive results are likely to be followed by additional invasive procedures, anxiety, and cost to the patient. However, she is still at risk for acquiring human papillomavirus (HPV) and CIN. Given that cervical cancer develops slowly and risk factors decrease with age, it is reasonable to stop screening at this point. Also, the recommendation of the 3-year screening interval in women over 30 with multiple sexual partners who had negative Pap and HPV tests is based on the fact that they can acquire HPV the day after screening and subsequently develop CIN, but we can detect HPV and CIN in the next round of screening (3 years later) and so will not miss the opportunity to treat cervical dysplasia.
However, practice guidelines are never meant to replace a physician’s sound clinical decision made on an individual basis.
In Reply: We thank Dr. Keller for his excellent comment. The rationale for discontinuing screening in a woman over 70 who has multiple sexual partners without a history of an abnormal Pap test is that she is at lower risk of new-onset cervical intraepithelial neoplasia (CIN) than a younger woman because of her decreased rate of metaplasia and less accessible transformation zone. In addition, postmenopausal mucosal atrophy may predispose to false-positive cytology. False-positive results are likely to be followed by additional invasive procedures, anxiety, and cost to the patient. However, she is still at risk for acquiring human papillomavirus (HPV) and CIN. Given that cervical cancer develops slowly and risk factors decrease with age, it is reasonable to stop screening at this point. Also, the recommendation of the 3-year screening interval in women over 30 with multiple sexual partners who had negative Pap and HPV tests is based on the fact that they can acquire HPV the day after screening and subsequently develop CIN, but we can detect HPV and CIN in the next round of screening (3 years later) and so will not miss the opportunity to treat cervical dysplasia.
However, practice guidelines are never meant to replace a physician’s sound clinical decision made on an individual basis.
Cervical cancer screening: Less testing, smarter testing
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
Cervical cancer screening and prevention have evolved rapidly in the last decade, especially in the 5 years since the introduction of the first cancer prevention vaccine, human papillomavirus (HPV) recombinant vaccine.1
Providers need to understand the rationale for the recommendations so that they can explain them to patients. In particular, patients may wonder why we now begin screening for cervical cancer later than we used to, and why some women do not need to be screened as often. Both of these changes result from our enhanced understanding of the role of HPV in cervical cancer genesis.
In this article we will briefly review:
- The current understanding of the natural history of cervical cancer
- Advantages and disadvantages of cervical cytology, ie, the Papanicolaou (Pap) test
- The role of HPV testing in cervical cancer screening
- The latest screening guidelines (the new standard of care)
- A possible future screening strategy
- The impact of HPV vaccination on screening.
500,000 NEW CASES EVERY YEAR
The incidence of cervical cancer and its mortality rate have decreased more than 50% in the United States over the past 3 decades, largely as a result of screening with the Pap test.2 In 2010, there were an estimated 12,200 new cases of invasive cervical cancer in the United States and 4,210 deaths from it,3 which are lower than the historical rates.
However, because most developing countries lack effective screening programs, cervical cancer remains the second-leading cause of death from cancer in women worldwide. According to a recent estimate, there are almost 500,000 new cases and 240,000 deaths from this disease worldwide every year.4 If effective global screening programs could be set up, they would markedly reduce the incidence of cervical cancer and deaths from it.5
HPV IS NECESSARY BUT NOT SUFFICIENT FOR CERVICAL CANCER TO DEVELOP
For cervical cancer to develop, the essential first step is infection of the cervical epithelium with one of the oncogenic (high-risk) types of HPV (see below).6–10 Walboomers et al9 tested cervical tissue samples taken from 932 women with cervical cancer and detected HPV DNA in 930 (99.8%) of them.
Fortunately, most HPV-infected women do not develop cervical cancer, as most young women clear the infection in an average of 8 to 24 months.11,12 However, if the infection persists, and if it is one of the high-risk types of HPV, precursor lesions can develop that can progress to cervical cancer.13 The evidence conclusively supports the association between oncogenic HPV infection and the subsequent development of virtually all cases of cervical cancer.6–10
Known risk factors for HPV persistence and the subsequent development of high-grade lesions are cigarette smoking and a compromised immune system.14,15
Terminology: Results of Pap smears
- Normal
- Atypical squamous cells of undetermined significance (ASC-US)
- Low-grade squamous intraepithelial lesions (LSIL)
- High-grade squamous intraepithelial lesions (HSIL)
- Cancer.
Terminology: Results of cervical biopsy
- Normal
- Cervical intraepithelial neoplasia grade 1 (CIN1)
- CIN2 (previously called moderate dysplasia)
- CIN3 (previously called severe dysplasia)
- Carcinoma in situ
- Invasive cervical cancer.
Lesions that are CIN2 or higher are considered high-grade.13
The 18 high-risk HPV types
More than 40 types of HPV infect the genital tract16; 18 of these (types 16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) are classified as high-risk because of their causative association with cervical cancer (ie, their oncogenic potential).17
How HPV causes cervical cancer
In laboratory cultures, normal human cells die out after a few generations. However, if human epithelial cells are infected by one of the high-risk types of HPV, they can go on dividing indefinitely.18,19
Two HPV proteins, E6 and E7, induce this cell “immortalization.”20,21 E6 from high-risk HPV binds to the human tumor-suppressor protein p53 and rapidly degrades it in a proteolytic process. The p53 protein normally suppresses cell proliferation by arresting growth in the G1 phase of the cell cycle. Therefore, with less p53, the cell cannot suppress uncontrolled cell growth.22–24
Similarly, E7 from high-risk HPV forms a complex with another human tumor suppressor, the retinoblastoma protein (pRB), and disrupts its binding to a transcriptional factor, E2F-1. The freed E2F-1 then stimulates DNA synthesis and uncontrolled cell growth.25
Furthermore, HPV-16 E6 and E7 proteins can collectively cause cellular genetic instability.26
The carcinogenic mechanism of high-risk HPV is complex. The host immune system and natural tumor suppression play important roles. However, the natural history of cervical intraepithelial neoplasia is not well understood. For example, it remains unclear if low-grade lesions such as CIN1 are necessary precursors to high-grade lesions and invasive cancer.6,7,10
THE PAP TEST: SPECIFIC BUT NOT VERY SENSITIVE, AND PRONE TO ERROR
The principal advantage of cervical cytologic testing (ie, the Pap test) in detecting cervical dysplasia is its overall high specificity. Many studies have found that the specificity of conventional Pap testing can reach approximately 98%.27
However, the conventional Pap test has drawbacks. Contaminants such as blood, discharge, and lubricant can make it difficult to interpret, and artifact can occur with air-drying of the Pap smear as it is transferred to the cell slide (“air-drying artifact”).
Liquid-based cytologic study has replaced the older method
To overcome these disadvantages, a liquid-based method of cervical cytologic study, ThinPrep (Hologic, Bedford, MA), was introduced in the mid-1990s. In this method, cell samples are first transferred to a liquid solution for mechanical separation from contaminants, and a representative sample of cells is then placed on a slide for review.
The liquid-based method filters out most contaminating blood, inflammatory cells, and debris. It also eliminates the air-drying artifact in the conventional Pap collection technique and improves specimen adequacy. Cytotechnologists find liquid-based specimens easier to read because the cells are more evenly distributed on a clearer background. Another advantage is that we can routinely test for HPV in the available residual specimen if the cytologic interpretation is abnormal.
The main disadvantages of the liquid-based method are that its specificity is lower than that of conventional Pap smears (around 78%) and that it costs more.28 Nevertheless, the liquid-based technique has become the main method of cervical cytology, used by nearly 90% of gynecologists in the United States since 2003.1
Cytology is still prone to false-negative results
Despite the success of both conventional Pap testing and liquid-based Pap testing, cervical cytology is inherently prone to sample-quality variation, subjective interpretation error, and false-negative results. False-negative results can be due to failure to transfer dysplastic cells to the slide or failure of the cytologist to recognize abnormal cells. In 30% of new cases of cervical cancer, the patient had recently had a Pap test that was interpreted as negative.1,29
Errors in interpretation are exacerbated by inconsistency among cytopathologists. In one study,6,30 when a group of quality-control pathologists reviewed nearly 5,000 cytology specimens, they came to the same conclusion that the original reviewers did more than 50% of the time only for negative and LSIL readings. Of the specimens initially reported as ASC-US, almost 40% were reclassified as negative on further review. Of those originally interpreted as HSIL, more than 50% were reclassified as LSIL, as ASC-US, or as negative.
Furthermore, many studies found that the sensitivity of conventional Pap testing was only around 50%.27 The new liquid-based Pap test uses computer imaging, which has improved the rate of detection of cervical dysplasia but may still miss 15% to 35% of cases of HSIL (severe dysplasia) or cancer.31 Failure to detect cervical dysplasia or cancer on Pap smear has led to a number of lawsuits.32
Clearly, with its relatively low sensitivity, cervical cytology is no longer good enough to use as a sole screening test in all situations. However, its high specificity is an advantage when it is combined with HPV testing in screening.
HPV TESTING AND PAP TESTING COMPLEMENT EACH OTHER
From 17% to 36% of HPV-infected women develop a cytologic abnormality within 5 years, compared with 4% to 15% of women who are HPV-negative.33,34
The usefulness of testing for HPV in women who have had an abnormal Pap test has been well demonstrated in multiple studies.35–38
The landmark Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS)39 found that 82.9% of women with LSIL were HPV-positive. The investigators concluded that HPV testing has little utility in women with LSIL, as the test would likely be positive and thus would not change the decision to perform colposcopy.
However, in women with ASC-US, the sensitivity of HPV testing for predicting CIN3 or cancer was 96.3% and the negative predictive value was 99.5%. In contrast, the sensitivity of a single repeat Pap test was only 44.1%. This large randomized trial conclusively validates the important role of HPV testing in triaging women with ASC-US.
More recently, a meta-analysis of 20 studies of HPV testing in women with ASC-US found that it had a sensitivity of 92.5% and a specificity of 62.5% for detecting CIN2 or worse lesions, and a sensitivity of 95.6% and a specificity of 59.2% for detecting CIN3 or worse lesions.40
Furthermore, HPV testing in primary cervical cancer screening is strongly supported by large cross-sectional studies41–45 and randomized clinical trials.46,47 These studies have conclusively shown that HPV testing is significantly more sensitive than Pap testing for detecting cervical intraepithelial neoplasia, and that, when combined with Pap testing, it can achieve nearly 100% clinical sensitivity and nearly 93% specificity in women age 30 or older. Women who have negative results on both the HPV test and the Pap test can be reassured that their risk of undetected CIN2, CIN3, or cervical cancer is extremely low, since HPV testing has a negative predictive value close to 100%.46
In large multinational European studies involving more than 24,000 women, the risk of CIN3 or cancer after 6 years of follow-up was only 0.28% in women who had negative results on both HPV and Pap testing at baseline. This rate was basically the same as in women who tested negative for HPV alone (0.27%). However, it was significantly lower than that of all women who had negative Pap test results (0.97%). The combination of HPV testing and Pap testing at 6-year intervals offered better protection than Pap testing alone at 3-year intervals.48
NEW STANDARD OF CARE: THE LATEST SCREENING GUIDELINES
Until the mid-1990s, the strategy for cervical cancer screening had remained largely unchanged for many years. Since then, several advances have prompted changes in the standard of care.
1996—The US Food and Drug Administration (FDA) approved liquid-based Thin-Prep for cervical cancer screening, which improved specimen adequacy and reduced ambiguous interpretations compared with the original slide-based method of collection.49
2001—The Bethesda terminology for reporting cervical cytology results was updated. First proposed in 1988 to replace the original Papanicolaou system and revised in 1991, this standardized terminology enabled better clinical decision-making.50
2001—The FDA approved HPV testing for women with ASC-US. This provided a better triage strategy for deciding which women need colposcopy to exclude true intraepithelial lesions. Following the FDA approval, the clinical effectiveness of HPV testing in women with ASC-US was validated by a large randomized clinical trial—the ALTS.51
2003—The FDA approved HPV testing in conjunction with Pap testing for women age 30 or older in routine primary screening.52
Guidelines available
Based on these new developments in technology and reporting terminology, and the incorporation of HPV testing, several organizations issued guidelines.
The American Society for Colposcopy and Cervical Pathology published a consensus guideline on management of abnormal cervical cytology in 2001 and revised it in 2006.53
The American Cancer Society issued its guideline for cervical cancer screening in 2002.54
The US Preventive Services Task Force published its screening guidelines in 2003.55
The American College of Obstetricians and Gynecologists (ACOG) also made new recommendations in 2003 and updated them in December 2009.1
Start screening at age 21
Cervical cancer screening should begin at age 21 regardless of the age of onset of vaginal intercourse, according to the 2009 ACOG guidelines.1 This represents a change from previous recommendations from ACOG, the American Cancer Society, and the US Preventive Services Task Force, which were to start screening within 3 years of the onset of vaginal intercourse.
Rationale. This latest recommendation is based on the high rates of clearance of HPV infection and of spontaneous dysplasia regression and the low incidence of cervical cancer in younger women.57,58 HPV infections are common in young women who have had vaginal intercourse. However, most such HPV infections are cleared by the immune system within 1 to 2 years without causing cervical dysplasia.11,12 Invasive cervical cancer in women younger than 21 years is very rare. The annual incidence is only one to two cases per 1 million women ages 15 to 19.2,55
Another reason for not screening before age 21 is that a positive test result may lead to unnecessary anxiety and potentially harmful evaluations and procedures.
Screening intervals extended
The 2009 ACOG guidelines lengthen the cervical cancer screening interval to every 2 years in women under age 30.1 (The 2003 ACOG guidelines said to screen every year.)
For women age 30 and older, the 2009 ACOG guidelines recommend extending the interval to every 3 years when combined Pap and HPV testing are negative (changed from every 2 to 3 years).1
Rationale. Studies have shown little advantage in screening every year in women under the age of 30, with no higher risk of cervical cancer in women screened at a 2- to 3-year interval.59–62 The absolute risk of cervical cancer in a well-screened population is very low.63 Moreover, the absolute number of cervical cancer cases in women age 30 to 64 years screened at 3-year intervals is only four per 100,000 women.64
HPV-plus-Pap testing for women over 30
Based on convincing evidence of the high sensitivity and the high negative predictive value of HPV testing, since 2003 ACOG had recommended HPV-plus-Pap testing in women over age 30. Its 2009 guidelines upgraded this recommendation to level A, ie, the highest grade, based on good and consistent scientific evidence.1 (Previously the recommendation was level B.)
The American Cancer Society also recommends combined HPV and Pap testing as the optimal screening approach in women age 30 or older, with the subsequent screening interval 3 years if both tests are negative. It also endorses Pap testing alone every 2 to 3 years as an alternative screening strategy in this age group.
The US Preventive Services Task Force recommends Pap testing every 3 years in women age 30 or older, and it does not recommend for or against HPV testing. However, neither the US Preventive Services Task Force nor the American Cancer Society has updated its guidelines in 8 years.
Rationale. Women who undergo HPV-plus-Pap testing and who test negative on both are at very low risk of developing CIN2 or CIN3 during the next 4 to 6 years. The risk is much lower than that for women who have a sole negative Pap test result.39,40 Because of this extremely high negative predictive value, women age 30 and older who had negative results on both Pap and HPV testing should be screened no more often than every 3 years.
We believe that the HPV-plus-Pap testing strategy recommended by the 2009 ACOG guidelines for women age 30 and older is the most effective screening approach. This strategy takes advantage of the high sensitivity and high negative predictive value of HPV testing, as well as the high specificity of Pap testing. It achieves almost 100% clinical sensitivity in detecting cervical dysplasia.46
When to stop screening
The 2009 ACOG guidelines for the first time call for stopping cervical cancer screening in women 65 to 70 years of age who have had three negative Pap tests in a row and no abnormal tests in the previous 10 years.1 The American Cancer Society recommends stopping screening at age 70,65 while the US Preventive Services Task Force recommends stopping at age 65.55
Rationale. Cervical cancer develops slowly, and risk factors tend to decline with age, Also, postmenopausal mucosal atrophy may predispose to false-positive Pap results, which can lead to additional procedures and unnecessary patient anxiety.66
However, it is probably reasonable to continue screening in women age 70 and older who are sexually active with multiple partners and who have a history of abnormal Pap test results.1
Women who have had a hysterectomy
According to the latest American Cancer Society, ACOG, and US Preventive Services Task Force guidelines, cervical cancer screening should be discontinued after total hysterectomy for benign indications in women who have no history of high-grade cervical intraepithelial neoplasia, ie, CIN2 or worse.1
Rationale. If the patient has no cervix, continued vaginal cytology screening is not indicated, since the incidence of primary vaginal cancer is one to two cases per 100,000 women per year, much lower than that of cervical cancer.65
However, before discontinuing screening, clinicians should verify that any Pap tests the patient had before the hysterectomy were all read as normal, that the hysterectomy specimen was normal, and that the cervix was completely removed during hysterectomy.
Be ready to explain the recommendations
It is very important for providers to understand the evidence supporting the latest guidelines, as many patients may not realize the significant technological improvements and improved understanding of the role of HPV in cervical cancer genesis that have resulted in the deferred onset of screening and the longer intervals between screenings. This knowledge gap for patients can result in anxiety when told they no longer need an annual Pap test or can start later, if the issue is not properly and thoroughly explained by a confident provider.
A FUTURE STRATEGY: HPV AS THE SOLE PRIMARY SCREENING TEST?
Since HPV testing is much more sensitive than Pap testing for detecting cervical lesions of grade CIN2 or higher, why not use HPV testing as the primary test and then do Pap testing (which is more specific) only if the HPV test is positive?
Mayrand et al46 conducted the first large randomized trial in which HPV testing was compared directly as a stand-alone test with the Pap test in a North American population with access to quality care. Results were published in 2007. In Canada, a total of 10,154 women ages 30 to 69 years in Montreal and St. John’s were randomly assigned to undergo either conventional Pap testing or HPV testing. The sensitivity of HPV testing for CIN2 or CIN3 was 94.6%, whereas the sensitivity of Pap testing was only 55.4%. The specificity was 94.1% for HPV testing and 96.8% for Pap testing. In addition, HPV screening followed by Pap triage resulted in fewer referrals for colposcopy than did either test alone (1.1% vs 2.9% with Pap testing alone or 6.1% with HPV testing alone). In other words, HPV testing was almost 40% more sensitive and only 2.7% less specific than Pap testing in detecting cervical cancer precursors.
However, more controlled trials are needed to validate such a strategy. Furthermore, it remains unclear if a change from Pap testing to a primary HPV testing screening strategy will further reduce the mortality rate of cervical cancer, since the burden of cervical cancer worldwide lies in less-screened populations in low-resource settings.
Dillner et al,48 in a 2008 European study, further demonstrated that HPV testing offers better long-term (6-year) predictive value for CIN3 or worse lesions than cytology does. These findings suggest that HPV testing, with its higher sensitivity and negative predictive value and its molecular focus on cervical carcinogenesis, may safely permit longer screening intervals in a low-risk population.
Sankaranarayanan et al72 performed a randomized trial in rural India in which 131,746 women age 30 to 59 years were randomly assigned to four groups: screening by HPV testing, screening by Pap testing, screening by visual inspection with acetic acid, and counseling only (the control group). At 8 years of follow-up, the numbers of cases of cervical cancer and of cervical cancer deaths were as follows:
- With HPV testing: 127 cases, 34 deaths
- With Pap testing: 152 cases, 54 deaths
- With visual inspection: 157 cases, 56 deaths
- With counseling only: 118 cases, 64 deaths.
The authors concluded that in a low-resource setting, a single round of HPV testing was associated with a significant reduction in the number of deaths from cervical cancer. Not only did the HPV testing group have a lower incidence of cancer-related deaths, there were no cancer deaths among the women in this group who tested negative for HPV. This is the first randomized trial to suggest that using HPV testing as the sole primary cervical cancer screening test may have a benefit in terms of the mortality rate.
At present, to the best of our knowledge, there are no US data validating the role of HPV testing as a stand-alone screening test for cervical cancer.
HPV VACCINATION DOES NOT MEAN THE END OF SCREENING
The development of an effective HPV vaccine and FDA approval of the first quadrivalent (active against HPV 6, 11, 16, and 18) recombinant vaccine (Gardasil) in 2006 has opened a new era of cervical cancer prevention.73,74 At present, the Advisory Committee on Immunization Practices75 recommends vaccination for females 9 to 26 years old.
However, HPV vaccination will not make screening obsolete, since not all women will be vaccinated, and those who have already contracted one of these high-risk HPV types will not benefit.76,77 In addition, the current HPV vaccine does not protect against infection with other oncogenic HPV types. The experts estimate that the initial impact of the HPV vaccine on cervical cancer will not likely be apparent until at least 20 to 30 years after a nationwide vaccination program is implemented.78,79 Therefore, the HPV vaccine certainly does not portend the end of screening. Vaccination combined with continued screening will provide added benefit for cervical cancer prevention.80
The last decade has been an exciting period in the field of cervical cancer screening and prevention, with advances in technology, newly acquired knowledge, and the development of the HPV vaccine. As a result, our clinical practice has become a work in progress, continuing to evolve as we continue to discover more information. The possibility of eradicating cervical cancer has never been greater. The implementation of the most sensitive and effective screening strategy and of a worldwide HPV vaccination program will help us to eventually eradicate cervical cancer and make it a disease of the past.81
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin no. 109: Cervical cytology screening. Obstet Gynecol 2009; 114:1409–1420.
- Horner MJ, Ries LAG, Krapcho M, et al, editors. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- American Cancer Society. Cancer Facts & Figures 2010. Atlanta, GA: American Cancer Society; 2010.
- Parkin DM, Bray F. Chapter 2: The burden of HPV-related cancers. Vaccine 2006; 24( suppl 3):S3/11–S3/25.
- Herrero R. Epidemiology of cervical cancer. J Natl Cancer Inst Monogr 1996; 21:1–6.
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer 2007; 111:145–153.
- Moscicki AB, Schiffman M, Kjaer S, Villa LL. Chapter 5: updating the natural history of HPV and anogenital cancer. Vaccine 2006; 24(suppl 3):S3/42–S3/51.
- Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol 2002; 55:244–265.
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999; 189:12–19.
- Kiviat N. Natural history of cervical neoplasia: overview and update. Am J Obstet Gynecol 1996; 175:1099–1104.
- Ho GY, Bierman R, Beardsley L, Chang CJ, Burk RD. Natural history of cervicovaginal papillomavirus infection in young women. N Engl J Med 1998; 338:423–428.
- Insinga RP, Dasbach EJ, Elbasha EH, Liaw KL, Barr E. Incidence and duration of cervical human papillomavirus 6, 11, 16, and 18 infections in young women: an evaluation from multiple analytic perspectives. Cancer Epidemiol Biomarkers Prev 2007; 16:709–715.
- Kjaer SK, van den Brule AJ, Paull G, et al. Type specific persistence of high risk human papillomavirus (HPV) as indicator of high grade cervical squamous intraepithelial lesions in young women: population based prospective follow up study. BMJ 2002; 325:572.
- Sycuro LK, Xi LF, Hughes JP, et al. Persistence of genital human papillomavirus infection in a long-term follow-up study of female university students. J Infect Dis 2008; 198:971–978.
- Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst 2010; 102:315–324.
- Schiffman M, Castle PE, Jeronimo J, Rodriguez AC, Wacholder S. Human papillomavirus and cervical cancer. Lancet 2007; 370:890–907.
- Koutsky LA, Holmes KK, Critchlow CW, et al. A cohort study of the risk of cervical intraepithelial neoplasia grade 2 or 3 in relation to papillomavirus infection. N Engl J Med 1992; 327:1272–1278.
- Stöppler H, Stöppler MC, Schlegel R. Transforming proteins of the papillomaviruses. Intervirology 1994; 37:168–179.
- zur Hausen H, de Villiers EM. Human papillomaviruses. Annu Rev Microbiol 1994; 48:427–447.
- Scheffner M, Romanczuk H, Münger K, Huibregtse JM, Mietz JA, Howley PM. Functions of human papillomavirus proteins. Curr Top Microbiol Immunol 1994; 186:83–99.
- Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994; 68:4358–4368.
- Werness BA, Levine AJ, Howley PM. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990; 248:76–79.
- Scheffner M, Huibregtse JM, Vierstra RD, Howley PM. The HPV-16 E6 and E6-AP complex functions as a ubiquitin-protein ligase in the ubiquitination of p53. Cell 1993; 75:495–505.
- Havre PA, Yuan J, Hedrick L, Cho KR, Glazer PM. p53 inactivation by HPV16 E6 results in increased mutagenesis in human cells. Cancer Res 1995; 55:4420–4424.
- Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J 1989; 8:4099–4105.
- Duensing S, Lee LY, Duensing A, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci U S A 2000; 97:10002–10007.
- Agency for Healthcare Research and Quality. Evaluation of Cervical Cytology. Summary: Evidence Report/Technology Assessment: Number 5. http://archive.ahrq.gov/clinic/epcsums/cervsumm.htm. Accessed August 18, 2011.
- Belinson J, Qiao YL, Pretorius R, et al. Shanxi Province Cervical Cancer Screening Study: a cross-sectional comparative trial of multiple techniques to detect cervical neoplasia. Gynecol Oncol 2001; 83:439–444.
- Shingleton HM, Patrick RL, Johnston WW, Smith RA. The current status of the Papanicolaou smear. CA Cancer J Clin 1995; 45:305–320.
- Stoler MH, Schiffman M; Atypical Squamous Cells of Undetermined Significance-Low-grade Squamous Intraepithelial Lesion Triage Study (ALTS) Group. Interobserver reproducibility of cervical cytologic and histologic interpretations: realistic estimates from the ASCUS-LSIL Triage Study. JAMA 2001; 285:1500–1505.
- Kulasingam SL, Hughes JP, Kiviat NB, et al. Evaluation of human papillomavirus testing in primary screening for cervical abnormalities: comparison of sensitivity, specificity, and frequency of referral. JAMA 2002; 288:1749–1757.
- Allen KA, Zaleski S, Cohen MB. Review of negative Papanicolaou tests. Is the retrospective 5-year review necessary? Am J Clin Pathol 1994; 101:19–21.
- Schlecht NF, Kulaga S, Robitaille J, et al. Persistent human papillomavirus infection as a predictor of cervical intraepithelial neoplasia. JAMA 2001; 286:3106–3114.
- Castle PE, Wacholder S, Sherman ME, et al. Absolute risk of a subsequent abnormal Pap among oncogenic human papillomavirus DNA-positive, cytologically negative women. Cancer 2002; 95:2145–2151.
- Manos MM, Kinney WK, Hurley LB, et al. Identifying women with cervical neoplasia: using human papillomavirus DNA testing for equivocal Papanicolaou results. JAMA 1999; 281:1605–1610.
- Wright TC, Lorincz A, Ferris DG, et al. Reflex human papillomavirus deoxyribonucleic acid testing in women with abnormal Papanicolaou smears. Am J Obstet Gynecol 1998; 178:962–966.
- Shlay JC, Dunn T, Byers T, Barón AE, Douglas JM. Prediction of cervical intraepithelial neoplasia grade 2–3 using risk assessment and human papillomavirus testing in women with atypia on Papanicolaou smears. Obstet Gynecol 2000; 96:410–416.
- Bergeron C, Jeannel D, Poveda J, Cassonnet P, Orth G. Human papillomavirus testing in women with mild cytologic atypia. Obstet Gynecol 2000; 95:821–827.
- ASCUS-LSIL Triage Study (ALTS) Group. Results of a randomized trial on the management of cytology interpretations of atypical squamous cells of undetermined significance. Am J Obstet Gynecol 2003; 188:1383–1392.
- Arbyn M, Sasieni P, Meijer CJ, Clavel C, Koliopoulos G, Dillner J. Chapter 9: clinical applications of HPV testing: a summary of meta-analyses. Vaccine 2006; 24(suppl 3):S3/78–S3/89.
- Petry KU, Menton S, Menton M, et al. Inclusion of HPV testing in routine cervical cancer screening for women above 29 years in Germany: results for 8466 patients. Br J Cancer 2003; 88:1570–1577.
- Cuzick J, Szarewski A, Cubie H, et al. Management of women who test positive for high-risk types of human papillomavirus: the HART study. Lancet 2003; 362:1871–1876.
- Salmerón J, Lazcano-Ponce E, Lorincz A, et al. Comparison of HPV-based assays with Papanicolaou smears for cervical cancer screening in Morelos State, Mexico. Cancer Causes Control 2003; 14:505–512.
- Herrero R, Hildesheim A, Bratti C, et al. Population-based study of human papillomavirus infection and cervical neoplasia in rural Costa Rica. J Natl Cancer Inst 2000; 92:464–474.
- Cuzick J, Clavel C, Petry KU, et al. Overview of the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer 2006; 119:1095–1101.
- Mayrand MH, Duarte-Franco E, Rodrigues I, et al; Canadian Cervical Cancer Screening Trial Study Group. Human papillomavirus DNA versus Papanicolaou screening tests for cervical cancer. N Engl J Med 2007; 357:1579–1588.
- Ronco G, Giorgi-Rossi P, Carozzi F, et al; New Technologies for Cervical Cancer Screening Working Group New Technologies for Cervical Cancer Screening Working Group. Results at recruitment from a randomized controlled trial comparing human papillomavirus testing alone with conventional cytology as the primary cervical cancer screening test. J Natl Cancer Inst 2008; 100:492–501.
- Dillner J, Rebolj M, Birembaut P, et al; Joint European Cohort Study. Long term predictive values of cytology and human papillomavirus testing in cervical cancer screening: joint European cohort study. BMJ 2008; 337:a1754.
- Noller KL, Bettes B, Zinberg S, Schulkin J. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol 2003; 102:259–265.
- Solomon D, Davey D, Kurman R, et al; Forum Group Members; Bethesda 2001 Workshop. The 2001 Bethesda System: terminology for reporting results of cervical cytology. JAMA 2002; 287:2114–2119.
- The Atypical Squamous Cells of Undetermined Significance/Low-Grade Squamous Intraepithelial Lesions Triage Study (ALTS) Group. Human papillomavirus testing for triage of women with cytologic evidence of low-grade squamous intraepithelial lesions: baseline data from a randomized trial. J Natl Cancer Inst 2000; 92:397–402.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No. 99: management of abnormal cervical cytology and histology. Obstet Gynecol 2008; 112:1419–1444.
- Wright TC Jr, Massad LS, Dunton CJ, Spitzer M, Wilkinson EJ, Solomon D; 2006 American Society for Colposcopy and Cervical Pathology-sponsored Consensus Conference. 2006 consensus guidelines for the management of women with cervical intraepithelial neoplasia or adenocarcinoma in situ. Am J Obstet Gynecol 2007; 197:340–345.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- US Preventive Services Task Force. Screening for cervical cancer. Systematic Evidence Review No. 25. http://www.ahrq.gov/downloads/pub/prevent/pdfser/cervcanser.pdf. Accessed October 9, 2011.
- Smith RA, Cokkinides V, Brooks D, Saslow D, Brawley OW. Cancer screening in the United States, 2010: a review of current American Cancer Society guidelines and issues in cancer screening. CA Cancer J Clin 2010; 60:99–119.
- Moscicki AB, Shiboski S, Broering J, et al. The natural history of human papillomavirus infection as measured by repeated DNA testing in adolescent and young women. J Pediatr 1998; 132:277–284.
- Watson M, Saraiya M, Benard V, et al. Burden of cervical cancer in the United States, 1998–2003. Cancer 2008; 113(suppl 10):2855–2864.
- IARC Working Group on evaluation of cervical cancer screening programmes. Screening for squamous cervical cancer: duration of low risk after negative results of cervical cytology and its implication for screening policies. Br Med J (Clin Res Ed) 1986; 293:659–664.
- Sawaya GF, Kerlikowske K, Lee NC, Gildengorin G, Washington AE. Frequency of cervical smear abnormalities within 3 years of normal cytology. Obstet Gynecol 2000; 96:219–223.
- Eddy DM. The frequency of cervical cancer screening. Comparison of a mathematical model with empirical data. Cancer 1987; 60:1117–1122.
- Sasieni P, Adams J, Cuzick J. Benefit of cervical screening at different ages: evidence from the UK audit of screening histories. Br J Cancer 2003; 89:88–93.
- Miller MG, Sung HY, Sawaya GF, Kearney KA, Kinney W, Hiatt RA. Screening interval and risk of invasive squamous cell cervical cancer. Obstet Gynecol 2003; 101:29–37.
- Sawaya GF, McConnell KJ, Kulasingam SL, et al. Risk of cervical cancer associated with extending the interval between cervical-cancer screenings. N Engl J Med 2003; 349:1501–1509.
- Saslow D, Runowicz CD, Solomon D, et al; American Cancer Society. American Cancer Society guideline for the early detection of cervical neoplasia and cancer. CA Cancer J Clin 2002; 52:342–362.
- Sawaya GF, Grady D, Kerlikowske K, et al. The positive predictive value of cervical smears in previously screened postmenopausal women: the Heart and Estrogen/progestin Replacement Study (HERS). Ann Intern Med 2000; 133:942–950.
- Kotaniemi-Talonen L, Nieminen P, Anttila A, Hakama M. Routine cervical screening with primary HPV testing and cytology triage protocol in a randomised setting. Br J Cancer 2005; 93:862–867.
- Ronco G, Segnan N, Giorgi-Rossi P, et al; New Technologies for Cervical Cancer Working Group. Human papillomavirus testing and liquid-based cytology: results at recruitment from the new technologies for cervical cancer randomized controlled trial. J Natl Cancer Inst 2006; 98:765–774.
- Bulkmans NW, Berkhof J, Rozendaal L, et al. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet 2007; 370:1764–1772.
- Naucler P, Ryd W, Törnberg S, et al. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med 2007; 357:1589–1597.
- Kitchener HC, Almonte M, Thomson C, et al. HPV testing in combination with liquid-based cytology in primary cervical screening (ARTISTIC): a randomised controlled trial. Lancet Oncol 2009; 10:672–682.
- Sankaranarayanan R, Nene BM, Shastri SS, et al. HPV screening for cervical cancer in rural India. N Engl J Med 2009; 360:1385–1394.
- Harper DM, Franco EL, Wheeler CM, et al; HPV Vaccine Study group. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 2006; 367:1247–1255.
- Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol 2005; 6:271–278.
- Centers for Disease Control and Prevention. Quadrivalent human papillomavirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR 2007; 56(RR02):1–24. http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5602a1.htm?s_cid=rr5602a1_e. Accessed 8/30/2011.
- Koutsky LA, Harper DM. Chapter 13: Current findings from prophylactic HPV vaccine trials. Vaccine 2006; 24( suppl 3):S3/114–S3/121.
- FUTURE II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N Engl J Med 2007; 356:1915–1927.
- Garnett GP, Kim JJ, French K, Goldie SJ. Chapter 21: Modelling the impact of HPV vaccines on cervical cancer and screening programmes. Vaccine 2006; 24( suppl 3):S3/178–S3/186.
- Plummer M, Franceschi S. Strategies for HPV prevention. Virus Res 2002; 89:285–293.
- Franco EL, Cuzick J, Hildesheim A, de Sanjosé S. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine 2006; 24(suppl 3):S3/171–S3/177.
- Cuzick J, Mayrand MH, Ronco G, Snijders P, Wardle J. Chapter 10: New dimensions in cervical cancer screening. Vaccine 2006; 24(suppl 3:S3/90–S3/97.
KEY POINTS
- Persistent infection with one of the 18 high-risk types of HPV is associated with the development of nearly all cases of cervical cancer.
- The 2009 ACOG guidelines recommend starting to screen with the Pap test at an older age (21 years) than in the past, and they recommend a longer screening interval for women in their 20s, ie, every 2 years instead of yearly.
- Women age 30 and older should undergo both Pap and HPV testing. If both tests are negative, screening should be done again no sooner than 3 years. Alternatively, women age 30 or older who have had three consecutive negative Pap tests can be screened by Pap testing every 3 years.
- Although vaccination can prevent most primary infections with high-risk HPV, it does not eliminate the need for continuing cervical cancer screening, as it does not protect against all high-risk HPV subtypes.
- Screening can stop at age 65 to 70 in women who have had three negative Pap tests in a row and no abnormal tests within the past 10 years.