User login
Psoriasis vulgaris is a chronic inflammatory skin condition associated with notable elevation in helper T cell (TH) production of TH1/TH17-mediated inflammatory cytokines, including IL-17A.1 Upon binding of IL-17A to IL-17 receptors in the skin, an inflammatory cascade is triggered, resulting in the classic clinical appearance of psoriasis. Moderate to severe psoriasis often is managed by suppressing TH1/TH17-mediated inflammation using targeted immune therapy such as secukinumab, an IL-17A inhibitor.2 Atopic dermatitis (AD), another chronic inflammatory dermatosis, is associated with substantial elevation in TH2-mediated inflammatory cytokines, such as IL-4.3 Dupilumab, which interacts with IL-4R, disrupts the IL-4 and IL-13 signaling pathways and demonstrates considerable efficacy in the treatment of moderate to severe AD.4
A case series has shown that suppression of the TH1/TH17-mediated inflammation of psoriasis may paradoxically result in the development of TH2-mediated AD.5 Similarly, a recent case report described a patient who developed psoriasis following treatment of AD with dupilumab.6 Herein, we describe a patient with a history of psoriasis that was well controlled with secukinumab who developed severe refractory erythrodermic AD that resolved with dupilumab treatment. Following clearance of AD with dupilumab, he exhibited psoriasis recurrence.
Case Report
A 39-year-old man with a lifelong history of psoriasis was admitted to the hospital for management of severe erythroderma. Four years prior, secukinumab was initiated for treatment of psoriasis, resulting in excellent clinical response. He discontinued secukinumab after 2 years of treatment because of insurance coverage issues and managed his condition with only topical corticosteroids. He restarted secukinumab 10 months before admission because of a psoriasis flare. Shortly after resuming secukinumab, he developed a severe exfoliative erythroderma that was not responsive to corticosteroids, etanercept, methotrexate, or ustekinumab.
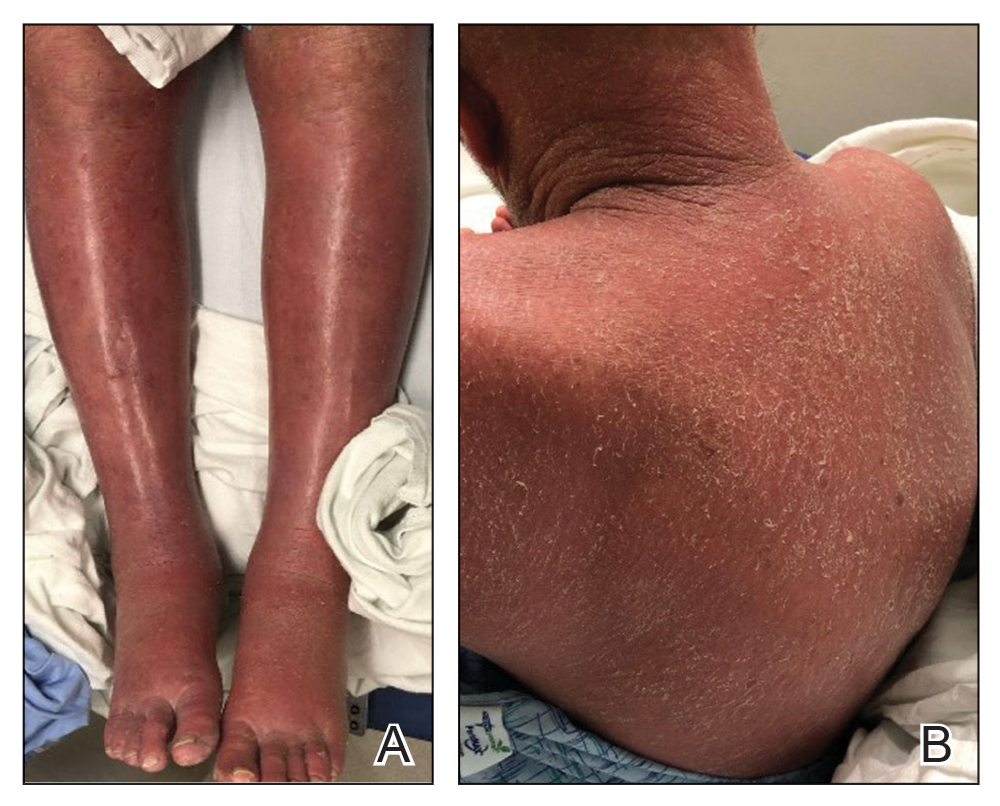
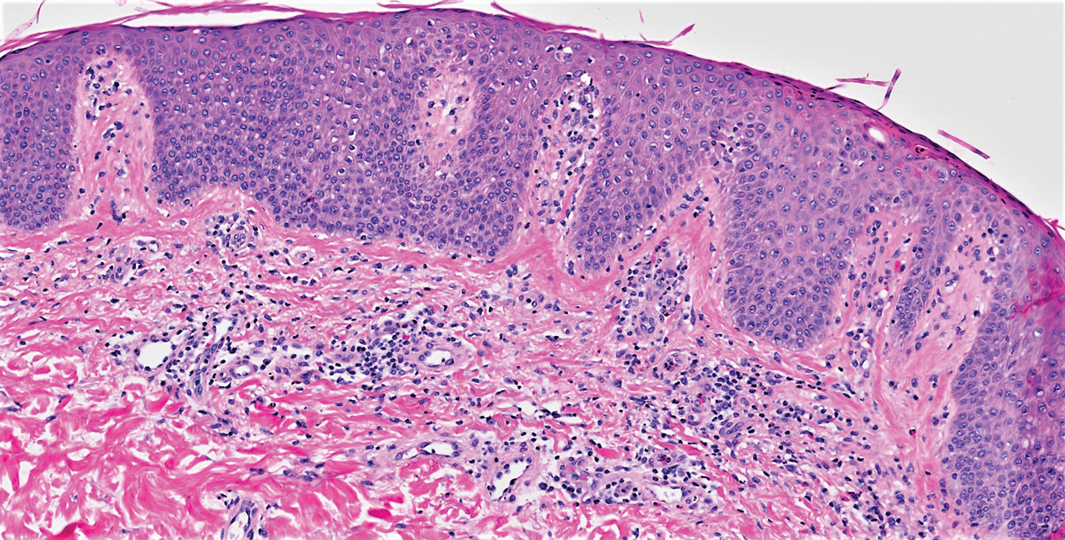
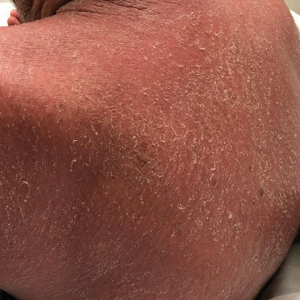
On initial presentation, physical examination revealed diffuse erythema and scaling with associated edema of the face, trunk, and extremities (Figure 1). A biopsy from the patient’s right arm demonstrated a superficial perivascular inflammatory infiltrate composed of lymphocytes, histiocytes, and scattered eosinophils consistent with spongiotic dermatitis (Figure 2). Cyclosporine 225 mg twice daily and topical corticosteroids were started.
Over the next several months, the patient had several admissions secondary to recurrent skin abscesses in the setting of refractory erythroderma. He underwent trials of infliximab, corticosteroids, intravenous immunoglobulin, guselkumab, and acitretin with minimal improvement. He underwent an extensive laboratory and radiologic workup, which was notable for cyclical peripheral eosinophilia and elevated IgE levels correlating with the erythroderma flares. A second biopsy was obtained and continued to demonstrate changes consistent with AD.
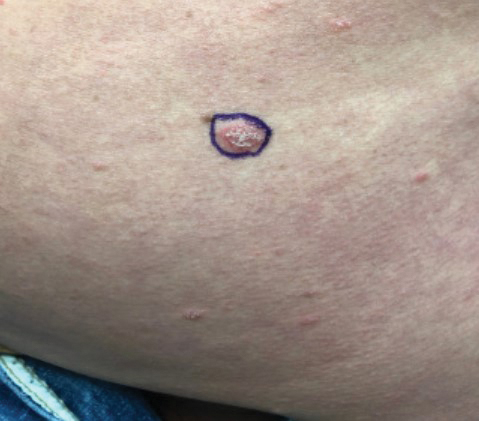
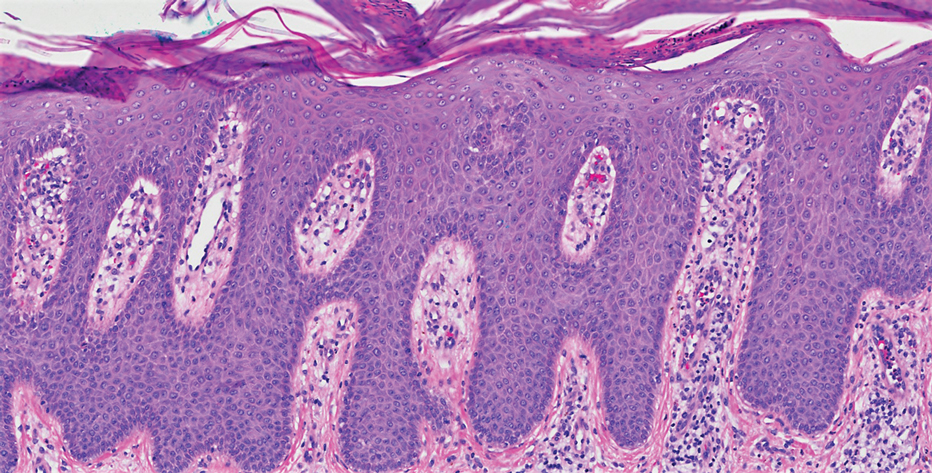
Four months after the initial hospitalization, all psoriasis medications were stopped, and the patient was started on dupilumab 300 mg/2 mL every 2 weeks and an 8-week oral prednisone taper. This combination led to notable clinical improvement and resolution of peripheral eosinophilia. Several months after disease remission, he began to develop worsening erythema and pruritus on the trunk and extremities, followed by the development of new psoriatic lesions (Figure 3) with a biopsy consistent with psoriasis (Figure 4). The patient was continued on dupilumab, but cyclosporine was added. The patient self-discontinued dupilumab owing to injection-site discomfort and has been slowly weaning off oral cyclosporine with 1 to 2 remaining eczematous plaques and 1 to 2 psoriatic plaques managed by topical corticosteroids.
Comment
We present a patient with psoriasis that was well controlled on secukinumab who developed severe AD following treatment with secukinumab. The AD resolved following treatment with dupilumab and a tapering dose of prednisone. However, after several months of treatment with dupilumab alone, he began to develop psoriatic lesions again. This case supports findings in a case series describing the development of AD in patients with psoriasis treated with IL-17 inhibitors5 and a recent case report describing a patient with AD who developed psoriasis following treatment with an IL-4/IL-13 inhibitor.6
Recognized adverse effects demonstrate biologic medications’ contributions to both normal as well as aberrant immunologic responses. For example, IL-17 plays an essential role in innate and adaptive immune responses against infections at mucosal and cutaneous interfaces, as demonstrated by chronic mucocutaneous candidiasis in patients with genetic defects in IL-17–related pathways.7 Similarly, in patients taking IL-17 antagonists, an increase in the incidence of Candida infections has been observed.8 In patients with concurrent psoriasis and inflammatory bowel disease (IBD), treatment with IL-17 inhibitors is contraindicated due to the risk of exacerbating the IBD. This observation is somewhat paradoxical, as increased IL-17 release by TH17 cells is implicated in the pathogenesis of IBD.9 Interestingly, it is now thought that IL-17 may play a protective role in T-cell–driven intestinal inflammation through induction of protective intestinal epithelial gene expression and increased mucosal defense against gut microbes, explaining the worsening of IBD in patients on IL-17 inhibitors.10 These adverse effects illustrate the complicated and varied roles biologic medications play in immunologic response.
Given that TH1 and TH2 exert opposing immune mechanisms, it is uncommon for psoriasis and AD to coexist in a single patient. However, patients who exhibit concurrent findings may represent a unique population in which psoriasis and AD coexist, perhaps because of an underlying genetic predisposition. Moreover, targeted treatment of pathways unique to these disease processes may result in paradoxical flaring of the nontargeted pathway. It also is possible that inhibition of a specific T-cell pathway in a subset of patients will result in an immunologic imbalance, favoring increased activity of the opposing pathway in the absence of coexisting disease. In the case presented here, the findings may be explained by secukinumab’s inhibition of TH1/TH17-mediated inflammation, which resulted in a shift to a TH2-mediated inflammatory response manifesting as AD, as well as dupilumab’s inhibition of TH2-mediated inflammation, which caused a shift back to TH1-mediated inflammatory pathways. Additionally, for patients with changing morphologies exacerbated by biologic medications, alternative diagnoses, such as cutaneous T-cell lymphoma, may be considered.
Conclusion
We report an unusual case of secukinumab-induced AD in a patient with psoriasis that resolved following several months of treatment with dupilumab and a tapering dose of prednisone. Subsequently, this same patient developed re-emergence of psoriatic lesions with continued use of dupilumab, which was eventually discontinued by the patient despite appropriate disease control. In addition to illustrating the underlying pathophysiologic mechanisms of 2 common inflammatory dermatologic conditions, this case highlights how pharmacologic interventions targeted at specific immunologic pathways may have unintended consequences. Further investigation into the effects of targeted biologics on the TH1/TH2 immune axis is warranted to better understand the mechanism and possible implications of the phenotypic switching presented in this case.
- Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res. 2016;2016:7692024.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- van der Heijden FL, Wierenga EA, Bos JD, et al. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991;97:389-394.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Lai FYX, Higgins E, Smith CH, et al. Morphologic switch from psoriasiform to eczematous dermatitis after anti-IL-17 therapy: a case series. JAMA Dermatol. 2019;155:1082-1084.
- Varma A, Levitt J. Dupilumab-induced phenotype switching from atopic dermatitis to psoriasis. JAAD Case Rep. 2020;6:217-218.
- Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(suppl 1):34-40.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Hölttä V, Klemetti P, Sipponen T, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175-1184.
- Smith MK, Pai J, Panaccione R, et al. Crohn’s-like disease in a patient exposed to anti-interleukin-17 blockade (ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019;19:162.
Psoriasis vulgaris is a chronic inflammatory skin condition associated with notable elevation in helper T cell (TH) production of TH1/TH17-mediated inflammatory cytokines, including IL-17A.1 Upon binding of IL-17A to IL-17 receptors in the skin, an inflammatory cascade is triggered, resulting in the classic clinical appearance of psoriasis. Moderate to severe psoriasis often is managed by suppressing TH1/TH17-mediated inflammation using targeted immune therapy such as secukinumab, an IL-17A inhibitor.2 Atopic dermatitis (AD), another chronic inflammatory dermatosis, is associated with substantial elevation in TH2-mediated inflammatory cytokines, such as IL-4.3 Dupilumab, which interacts with IL-4R, disrupts the IL-4 and IL-13 signaling pathways and demonstrates considerable efficacy in the treatment of moderate to severe AD.4
A case series has shown that suppression of the TH1/TH17-mediated inflammation of psoriasis may paradoxically result in the development of TH2-mediated AD.5 Similarly, a recent case report described a patient who developed psoriasis following treatment of AD with dupilumab.6 Herein, we describe a patient with a history of psoriasis that was well controlled with secukinumab who developed severe refractory erythrodermic AD that resolved with dupilumab treatment. Following clearance of AD with dupilumab, he exhibited psoriasis recurrence.
Case Report
A 39-year-old man with a lifelong history of psoriasis was admitted to the hospital for management of severe erythroderma. Four years prior, secukinumab was initiated for treatment of psoriasis, resulting in excellent clinical response. He discontinued secukinumab after 2 years of treatment because of insurance coverage issues and managed his condition with only topical corticosteroids. He restarted secukinumab 10 months before admission because of a psoriasis flare. Shortly after resuming secukinumab, he developed a severe exfoliative erythroderma that was not responsive to corticosteroids, etanercept, methotrexate, or ustekinumab.
On initial presentation, physical examination revealed diffuse erythema and scaling with associated edema of the face, trunk, and extremities (Figure 1). A biopsy from the patient’s right arm demonstrated a superficial perivascular inflammatory infiltrate composed of lymphocytes, histiocytes, and scattered eosinophils consistent with spongiotic dermatitis (Figure 2). Cyclosporine 225 mg twice daily and topical corticosteroids were started.
Over the next several months, the patient had several admissions secondary to recurrent skin abscesses in the setting of refractory erythroderma. He underwent trials of infliximab, corticosteroids, intravenous immunoglobulin, guselkumab, and acitretin with minimal improvement. He underwent an extensive laboratory and radiologic workup, which was notable for cyclical peripheral eosinophilia and elevated IgE levels correlating with the erythroderma flares. A second biopsy was obtained and continued to demonstrate changes consistent with AD.
Four months after the initial hospitalization, all psoriasis medications were stopped, and the patient was started on dupilumab 300 mg/2 mL every 2 weeks and an 8-week oral prednisone taper. This combination led to notable clinical improvement and resolution of peripheral eosinophilia. Several months after disease remission, he began to develop worsening erythema and pruritus on the trunk and extremities, followed by the development of new psoriatic lesions (Figure 3) with a biopsy consistent with psoriasis (Figure 4). The patient was continued on dupilumab, but cyclosporine was added. The patient self-discontinued dupilumab owing to injection-site discomfort and has been slowly weaning off oral cyclosporine with 1 to 2 remaining eczematous plaques and 1 to 2 psoriatic plaques managed by topical corticosteroids.
Comment
We present a patient with psoriasis that was well controlled on secukinumab who developed severe AD following treatment with secukinumab. The AD resolved following treatment with dupilumab and a tapering dose of prednisone. However, after several months of treatment with dupilumab alone, he began to develop psoriatic lesions again. This case supports findings in a case series describing the development of AD in patients with psoriasis treated with IL-17 inhibitors5 and a recent case report describing a patient with AD who developed psoriasis following treatment with an IL-4/IL-13 inhibitor.6
Recognized adverse effects demonstrate biologic medications’ contributions to both normal as well as aberrant immunologic responses. For example, IL-17 plays an essential role in innate and adaptive immune responses against infections at mucosal and cutaneous interfaces, as demonstrated by chronic mucocutaneous candidiasis in patients with genetic defects in IL-17–related pathways.7 Similarly, in patients taking IL-17 antagonists, an increase in the incidence of Candida infections has been observed.8 In patients with concurrent psoriasis and inflammatory bowel disease (IBD), treatment with IL-17 inhibitors is contraindicated due to the risk of exacerbating the IBD. This observation is somewhat paradoxical, as increased IL-17 release by TH17 cells is implicated in the pathogenesis of IBD.9 Interestingly, it is now thought that IL-17 may play a protective role in T-cell–driven intestinal inflammation through induction of protective intestinal epithelial gene expression and increased mucosal defense against gut microbes, explaining the worsening of IBD in patients on IL-17 inhibitors.10 These adverse effects illustrate the complicated and varied roles biologic medications play in immunologic response.
Given that TH1 and TH2 exert opposing immune mechanisms, it is uncommon for psoriasis and AD to coexist in a single patient. However, patients who exhibit concurrent findings may represent a unique population in which psoriasis and AD coexist, perhaps because of an underlying genetic predisposition. Moreover, targeted treatment of pathways unique to these disease processes may result in paradoxical flaring of the nontargeted pathway. It also is possible that inhibition of a specific T-cell pathway in a subset of patients will result in an immunologic imbalance, favoring increased activity of the opposing pathway in the absence of coexisting disease. In the case presented here, the findings may be explained by secukinumab’s inhibition of TH1/TH17-mediated inflammation, which resulted in a shift to a TH2-mediated inflammatory response manifesting as AD, as well as dupilumab’s inhibition of TH2-mediated inflammation, which caused a shift back to TH1-mediated inflammatory pathways. Additionally, for patients with changing morphologies exacerbated by biologic medications, alternative diagnoses, such as cutaneous T-cell lymphoma, may be considered.
Conclusion
We report an unusual case of secukinumab-induced AD in a patient with psoriasis that resolved following several months of treatment with dupilumab and a tapering dose of prednisone. Subsequently, this same patient developed re-emergence of psoriatic lesions with continued use of dupilumab, which was eventually discontinued by the patient despite appropriate disease control. In addition to illustrating the underlying pathophysiologic mechanisms of 2 common inflammatory dermatologic conditions, this case highlights how pharmacologic interventions targeted at specific immunologic pathways may have unintended consequences. Further investigation into the effects of targeted biologics on the TH1/TH2 immune axis is warranted to better understand the mechanism and possible implications of the phenotypic switching presented in this case.
Psoriasis vulgaris is a chronic inflammatory skin condition associated with notable elevation in helper T cell (TH) production of TH1/TH17-mediated inflammatory cytokines, including IL-17A.1 Upon binding of IL-17A to IL-17 receptors in the skin, an inflammatory cascade is triggered, resulting in the classic clinical appearance of psoriasis. Moderate to severe psoriasis often is managed by suppressing TH1/TH17-mediated inflammation using targeted immune therapy such as secukinumab, an IL-17A inhibitor.2 Atopic dermatitis (AD), another chronic inflammatory dermatosis, is associated with substantial elevation in TH2-mediated inflammatory cytokines, such as IL-4.3 Dupilumab, which interacts with IL-4R, disrupts the IL-4 and IL-13 signaling pathways and demonstrates considerable efficacy in the treatment of moderate to severe AD.4
A case series has shown that suppression of the TH1/TH17-mediated inflammation of psoriasis may paradoxically result in the development of TH2-mediated AD.5 Similarly, a recent case report described a patient who developed psoriasis following treatment of AD with dupilumab.6 Herein, we describe a patient with a history of psoriasis that was well controlled with secukinumab who developed severe refractory erythrodermic AD that resolved with dupilumab treatment. Following clearance of AD with dupilumab, he exhibited psoriasis recurrence.
Case Report
A 39-year-old man with a lifelong history of psoriasis was admitted to the hospital for management of severe erythroderma. Four years prior, secukinumab was initiated for treatment of psoriasis, resulting in excellent clinical response. He discontinued secukinumab after 2 years of treatment because of insurance coverage issues and managed his condition with only topical corticosteroids. He restarted secukinumab 10 months before admission because of a psoriasis flare. Shortly after resuming secukinumab, he developed a severe exfoliative erythroderma that was not responsive to corticosteroids, etanercept, methotrexate, or ustekinumab.
On initial presentation, physical examination revealed diffuse erythema and scaling with associated edema of the face, trunk, and extremities (Figure 1). A biopsy from the patient’s right arm demonstrated a superficial perivascular inflammatory infiltrate composed of lymphocytes, histiocytes, and scattered eosinophils consistent with spongiotic dermatitis (Figure 2). Cyclosporine 225 mg twice daily and topical corticosteroids were started.
Over the next several months, the patient had several admissions secondary to recurrent skin abscesses in the setting of refractory erythroderma. He underwent trials of infliximab, corticosteroids, intravenous immunoglobulin, guselkumab, and acitretin with minimal improvement. He underwent an extensive laboratory and radiologic workup, which was notable for cyclical peripheral eosinophilia and elevated IgE levels correlating with the erythroderma flares. A second biopsy was obtained and continued to demonstrate changes consistent with AD.
Four months after the initial hospitalization, all psoriasis medications were stopped, and the patient was started on dupilumab 300 mg/2 mL every 2 weeks and an 8-week oral prednisone taper. This combination led to notable clinical improvement and resolution of peripheral eosinophilia. Several months after disease remission, he began to develop worsening erythema and pruritus on the trunk and extremities, followed by the development of new psoriatic lesions (Figure 3) with a biopsy consistent with psoriasis (Figure 4). The patient was continued on dupilumab, but cyclosporine was added. The patient self-discontinued dupilumab owing to injection-site discomfort and has been slowly weaning off oral cyclosporine with 1 to 2 remaining eczematous plaques and 1 to 2 psoriatic plaques managed by topical corticosteroids.
Comment
We present a patient with psoriasis that was well controlled on secukinumab who developed severe AD following treatment with secukinumab. The AD resolved following treatment with dupilumab and a tapering dose of prednisone. However, after several months of treatment with dupilumab alone, he began to develop psoriatic lesions again. This case supports findings in a case series describing the development of AD in patients with psoriasis treated with IL-17 inhibitors5 and a recent case report describing a patient with AD who developed psoriasis following treatment with an IL-4/IL-13 inhibitor.6
Recognized adverse effects demonstrate biologic medications’ contributions to both normal as well as aberrant immunologic responses. For example, IL-17 plays an essential role in innate and adaptive immune responses against infections at mucosal and cutaneous interfaces, as demonstrated by chronic mucocutaneous candidiasis in patients with genetic defects in IL-17–related pathways.7 Similarly, in patients taking IL-17 antagonists, an increase in the incidence of Candida infections has been observed.8 In patients with concurrent psoriasis and inflammatory bowel disease (IBD), treatment with IL-17 inhibitors is contraindicated due to the risk of exacerbating the IBD. This observation is somewhat paradoxical, as increased IL-17 release by TH17 cells is implicated in the pathogenesis of IBD.9 Interestingly, it is now thought that IL-17 may play a protective role in T-cell–driven intestinal inflammation through induction of protective intestinal epithelial gene expression and increased mucosal defense against gut microbes, explaining the worsening of IBD in patients on IL-17 inhibitors.10 These adverse effects illustrate the complicated and varied roles biologic medications play in immunologic response.
Given that TH1 and TH2 exert opposing immune mechanisms, it is uncommon for psoriasis and AD to coexist in a single patient. However, patients who exhibit concurrent findings may represent a unique population in which psoriasis and AD coexist, perhaps because of an underlying genetic predisposition. Moreover, targeted treatment of pathways unique to these disease processes may result in paradoxical flaring of the nontargeted pathway. It also is possible that inhibition of a specific T-cell pathway in a subset of patients will result in an immunologic imbalance, favoring increased activity of the opposing pathway in the absence of coexisting disease. In the case presented here, the findings may be explained by secukinumab’s inhibition of TH1/TH17-mediated inflammation, which resulted in a shift to a TH2-mediated inflammatory response manifesting as AD, as well as dupilumab’s inhibition of TH2-mediated inflammation, which caused a shift back to TH1-mediated inflammatory pathways. Additionally, for patients with changing morphologies exacerbated by biologic medications, alternative diagnoses, such as cutaneous T-cell lymphoma, may be considered.
Conclusion
We report an unusual case of secukinumab-induced AD in a patient with psoriasis that resolved following several months of treatment with dupilumab and a tapering dose of prednisone. Subsequently, this same patient developed re-emergence of psoriatic lesions with continued use of dupilumab, which was eventually discontinued by the patient despite appropriate disease control. In addition to illustrating the underlying pathophysiologic mechanisms of 2 common inflammatory dermatologic conditions, this case highlights how pharmacologic interventions targeted at specific immunologic pathways may have unintended consequences. Further investigation into the effects of targeted biologics on the TH1/TH2 immune axis is warranted to better understand the mechanism and possible implications of the phenotypic switching presented in this case.
- Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res. 2016;2016:7692024.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- van der Heijden FL, Wierenga EA, Bos JD, et al. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991;97:389-394.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Lai FYX, Higgins E, Smith CH, et al. Morphologic switch from psoriasiform to eczematous dermatitis after anti-IL-17 therapy: a case series. JAMA Dermatol. 2019;155:1082-1084.
- Varma A, Levitt J. Dupilumab-induced phenotype switching from atopic dermatitis to psoriasis. JAAD Case Rep. 2020;6:217-218.
- Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(suppl 1):34-40.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Hölttä V, Klemetti P, Sipponen T, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175-1184.
- Smith MK, Pai J, Panaccione R, et al. Crohn’s-like disease in a patient exposed to anti-interleukin-17 blockade (ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019;19:162.
- Diani M, Altomare G, Reali E. T helper cell subsets in clinical manifestations of psoriasis. J Immunol Res. 2016;2016:7692024.
- Langley RG, Elewski BE, Lebwohl M, et al. Secukinumab in plaque psoriasis—results of two phase 3 trials. N Engl J Med. 2014;371:326-338.
- van der Heijden FL, Wierenga EA, Bos JD, et al. High frequency of IL-4-producing CD4+ allergen-specific T lymphocytes in atopic dermatitis lesional skin. J Invest Dermatol. 1991;97:389-394.
- Beck LA, Thaçi D, Hamilton JD, et al. Dupilumab treatment in adults with moderate-to-severe atopic dermatitis. N Engl J Med. 2014;371:130-139.
- Lai FYX, Higgins E, Smith CH, et al. Morphologic switch from psoriasiform to eczematous dermatitis after anti-IL-17 therapy: a case series. JAMA Dermatol. 2019;155:1082-1084.
- Varma A, Levitt J. Dupilumab-induced phenotype switching from atopic dermatitis to psoriasis. JAAD Case Rep. 2020;6:217-218.
- Ling Y, Puel A. IL-17 and infections. Actas Dermosifiliogr. 2014;105(suppl 1):34-40.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Hölttä V, Klemetti P, Sipponen T, et al. IL-23/IL-17 immunity as a hallmark of Crohn’s disease. Inflamm Bowel Dis. 2008;14:1175-1184.
- Smith MK, Pai J, Panaccione R, et al. Crohn’s-like disease in a patient exposed to anti-interleukin-17 blockade (ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019;19:162.
Practice Points
- Treatment of psoriasis vulgaris, a helper T cell TH1/TH17-mediated skin condition, with secukinumab may result in phenotypic switching to TH2-mediated atopic dermatitis.
- Atopic dermatitis responds well to dupilumab but may result in phenotypic switching to psoriasis.
- Biologic therapies targeted at specific immunologic pathways may have unintended consequences on the TH1/TH2 immune axis.