User login
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
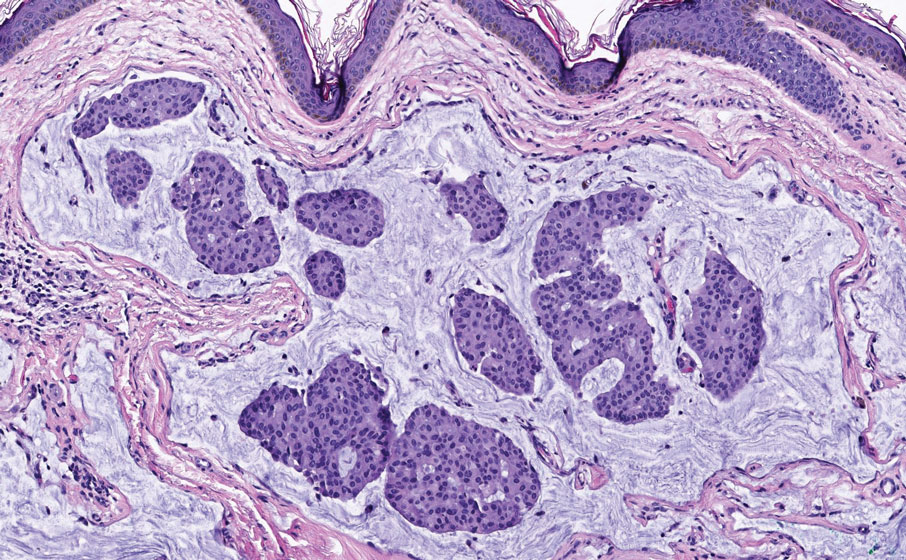
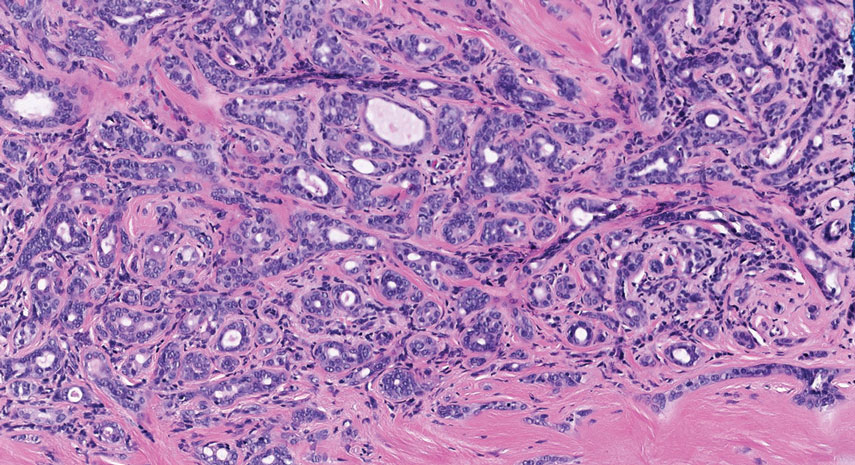
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
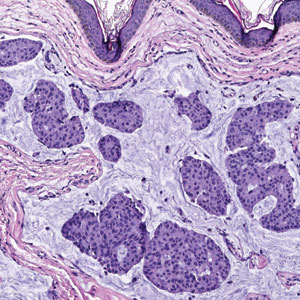
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7
Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2
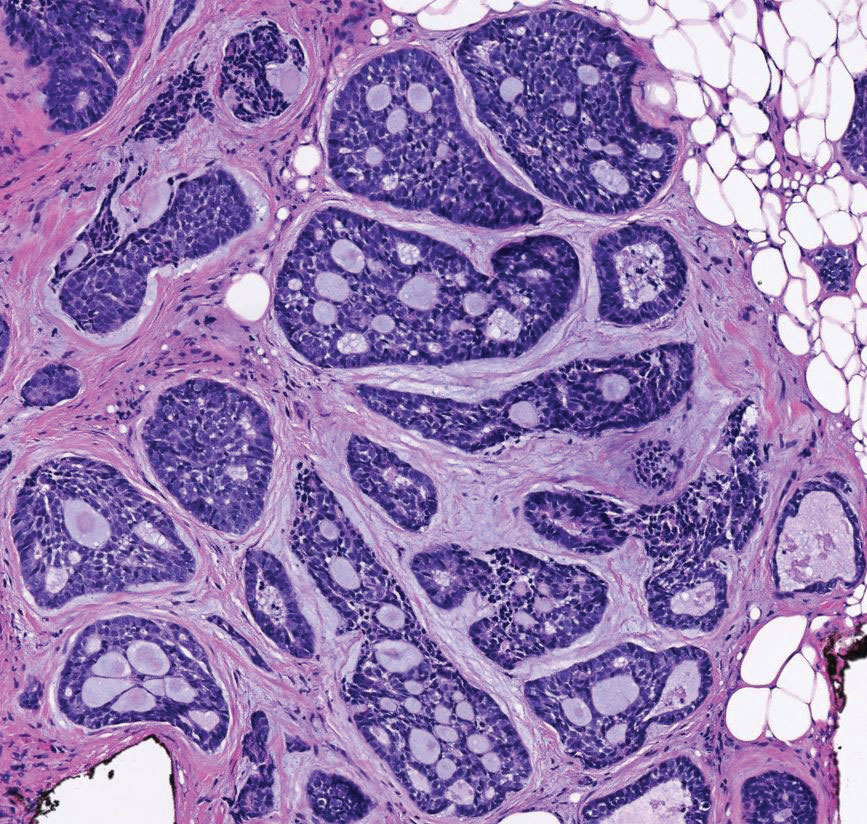
Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2
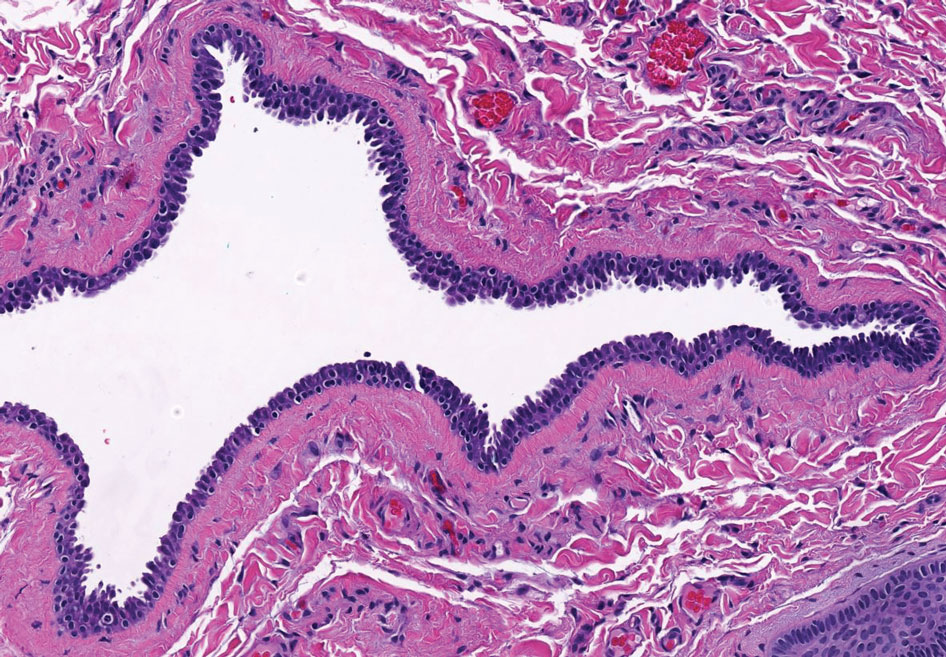
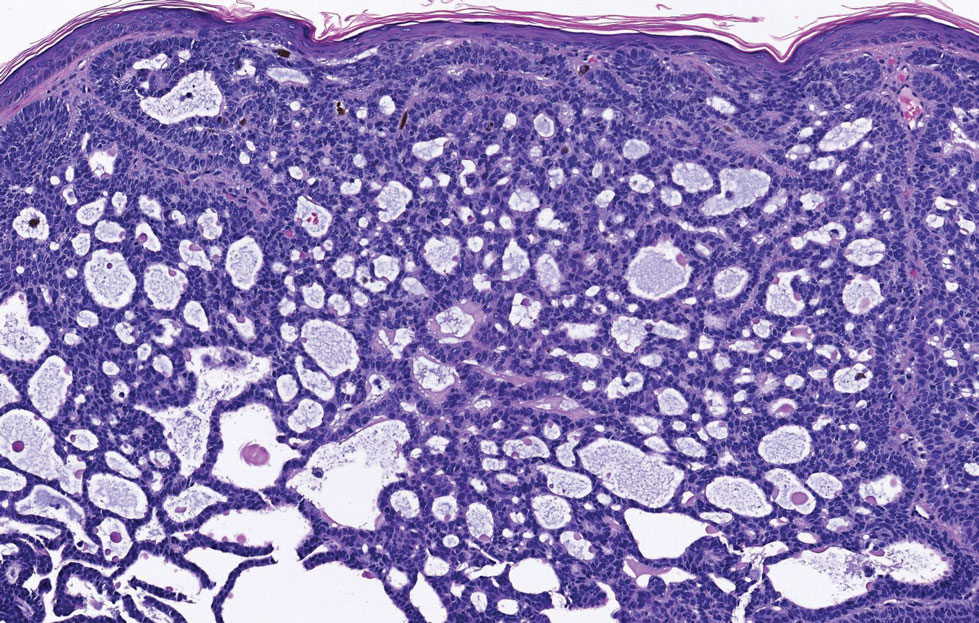
Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.
- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

The Diagnosis: Endocrine Mucin-Producing Sweat Gland Carcinoma
Endocrine mucin-producing sweat gland carcinoma (EMPSGC) is a rare cutaneous adnexal tumor that characteristically presents as slowgrowing, flesh-colored papules, nodules, or cystic lesions around the periorbital skin in elderly female patients.1 Histopathology of EMPSGCs reveals well-circumscribed multinodular dermal lesions that can be either cystic or solid and often are arranged in papillary and cribriform patterns (quiz image). Nests of uniform tumor cells are composed of small- to medium-sized epithelial cells with monomorphic nuclei showing fine to stippled chromatin.2 Histologically, EMPSGC resembles a solid papillary carcinoma of the breast, which is attributed to their common embryologic origin.3 Intracytoplasmic and extracellular mucin often are seen on hematoxylin and eosin staining.2 Variable immunohistochemical stain expression has been reported, including positive staining with synaptophysin and chromogranin. Other markers include cytokeratin CAM 5.2, epithelial membrane antigen, estrogen or progesterone receptors, and cytokeratin 7.4 Endocrine mucin-producing sweat gland carcinoma is thought to be a precursor to invasive neuroendocrine-type primary cutaneous mucinous carcinoma. Primary cutaneous mucinous carcinoma has been associated with EMPSGC in approximately 35.7% of cases. Histologically, primary cutaneous mucinous carcinoma that has transformed from EMPSGC would show an infiltration of tumor nests with desmoplastic stroma or mucin pools with clusters of tumor cells.2
Primary cutaneous adenoid cystic carcinoma is a rare malignant tumor that often presents on the head and neck. It usually appears as a single, slowly growing subcutaneous nodule or multinodular plaque.5,6 Histologic features include basaloid cells in alternating tubular and cribriform patterns. The cribriform areas are composed of pseudoglandular adenoid spaces that contain mucin, basement membrane zone material, and cellular debris from necrotic neoplastic cells (Figure 1).7 Primary cutaneous adenoid cystic carcinoma predominantly is dermal with extension to the subcutaneous tissue. True ductal structures that demonstrate decapitation secretion also may be present.7

Basal cell carcinoma (adenoid type) presents as a pigmented or nonpigmented nodule or ulcer on sunexposed areas of the head and neck. Histopathology reveals basaloid cells surrounding islands of connective tissue resulting in a lacelike pattern (Figure 2). The lumina may contain a colloidal substance or amorphous granular material.8 The characteristic features of basal cell carcinomas, such as nests of basaloid cells with peripheral palisading cells, retraction of adjacent stroma, increased apoptosis and mitotic figures, and connection to the epidermis, can be helpful to distinguish basal cell carcinoma histologically from EMPSGC.2

Apocrine hidrocystomas clinically present as round, flesh-colored, shiny or translucent, dome-shaped papules or nodules near the eyelid margin or lateral canthus.9 Histologically, they are composed of proliferating apocrine secretory coils with an epithelial side of cuboidal or columnar cells and a luminal side exhibiting decapitation secretion (Figure 3).2 An epidermal connection is absent.9 Apocrine hidrocystomas may exhibit complex architecture and papillary ductal hyperplasia that are difficult to distinguish from EMPSGC, especially if EMPSGC presents with cystic morphology. Apocrine cytomorphology and the lack of neuroendocrine marker expression and mucin production distinguish apocrine hidrocystomas. Furthermore, hidrocystomas infrequently demonstrate the nodular, solid, cribriform areas appreciated in EMPSGC.2

Microcystic adnexal carcinoma is a rare, slowly growing, locally aggressive sweat gland tumor that commonly presents as a flesh-colored to yellow papule, nodule, or plaque on the central face.10 Histopathologic examination reveals both eccrine and follicular differentiation. Keratin cysts, bland keratinocyte cords, and epithelium with ductal differentiation is observed in the superficial layers (Figure 4). Deep invasion into the subcutis and perineural invasion frequently is observed.

- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
- Mulay K, Menon V, Lahane S, et al. Endocrine mucinproducing sweat gland carcinoma (EMPSGC) of the eyelid: clinicopathologic features, immunohistochemical findings and review of literature. Indian J Ophthalmol. 2019;67:1374-1377. doi:10.4103/ijo.IJO_1745_18
- Au RTM, Bundele MM. Endocrine mucin-producing sweat gland carcinoma and associated primary cutaneous mucinous carcinoma: review of the literature. J Cutan Pathol. 2021;48:1156-1165. doi:10.1111/cup.13983
- Flieder A, Koerner FC, Pilch BZ, et al. Endocrine mucin-producing sweat gland carcinoma: a cutaneous neoplasm analogous to solid papillary carcinoma of breast. Am J Surg Pathol. 1997;21:1501-1506. doi:10.1097/00000478-199712000-00014
- Shimizu I, Dufresne R, Robinson-Bostom L. Endocrine mucinproducing sweat gland carcinoma. Cutis. 2014;93:47-49.
- Ahn CS, Sangüeza OP. Malignant sweat gland tumors. Hematol Oncol Clin North Am. 2019;33:53-71. doi:10.1016/j.hoc.2018.09.002
- Tonev ID, Pirgova YS, Conev NV. Primary adenoid cystic carcinoma of the skin with multiple local recurrences. Case Rep Oncol. 2015;8:251-255. doi:10.1159/000431082
- Coca-Pelaz A, Rodrigo JP, Bradley PJ, et al. Adenoid cystic carcinoma of the head and neck—an update. Oral Oncol. 2015;51:652-661. doi:10.1016/j.oraloncology.2015.04.005
- Tambe SA, Ghate SS, Jerajani HR. Adenoid type of basal cell carcinoma: rare histopathological variant at an unusual location. Indian J Dermatol. 2013;58:159. doi:10.4103/0019-5154.108080
- Kikuchi K, Fukunaga S, Inoue H, et al. Apocrine hidrocystoma of the lower lip: a case report and literature review. Head Neck Pathol. 2014;8:117-121. doi:10.1007/s12105-013-0451-2
- Zito PM, Mazzoni T. Microcystic adnexal carcinoma. StatPearls. StatPearls Publishing; 2021.
A 76-year-old woman presented with a slowly growing, asymptomatic, 5-mm, pink-brown, dome-shaped papule adjacent to the left lateral canthus of several years’ duration. Dermoscopic examination revealed fine linear peripheral blood vessels. The lesional cells were positive with cytokeratin 7, estrogen receptor, progesterone receptor, chromogranin, synaptophysin, and neuron-specific enolase. Cytokeratin 20 and p63 were negative, and the Ki-67 proliferative index was less than 5%.