User login
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
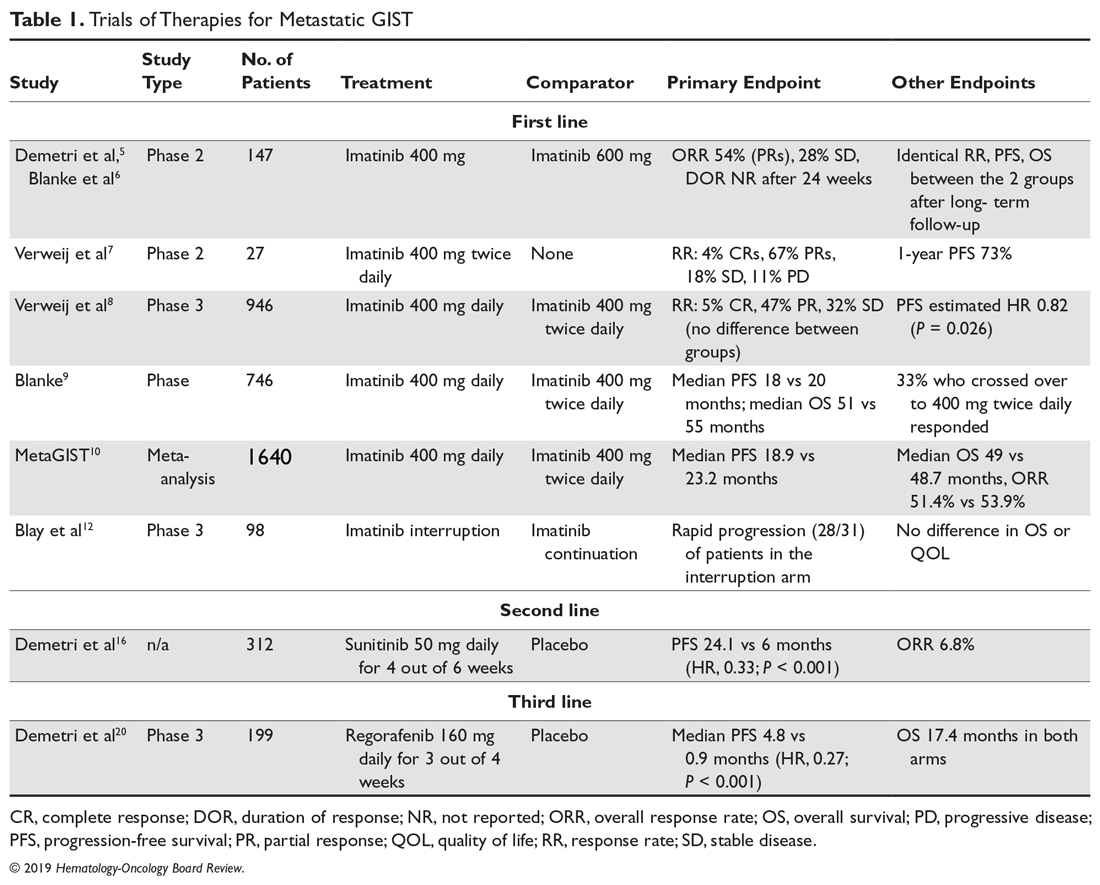
A list of landmark studies for advanced GISTs is provided in Table 1.
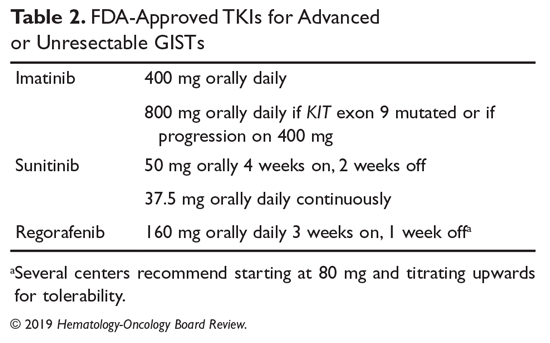
A summary of FDA-approved drugs for treating GISTs is provided in Table 2.
Clinical Trials
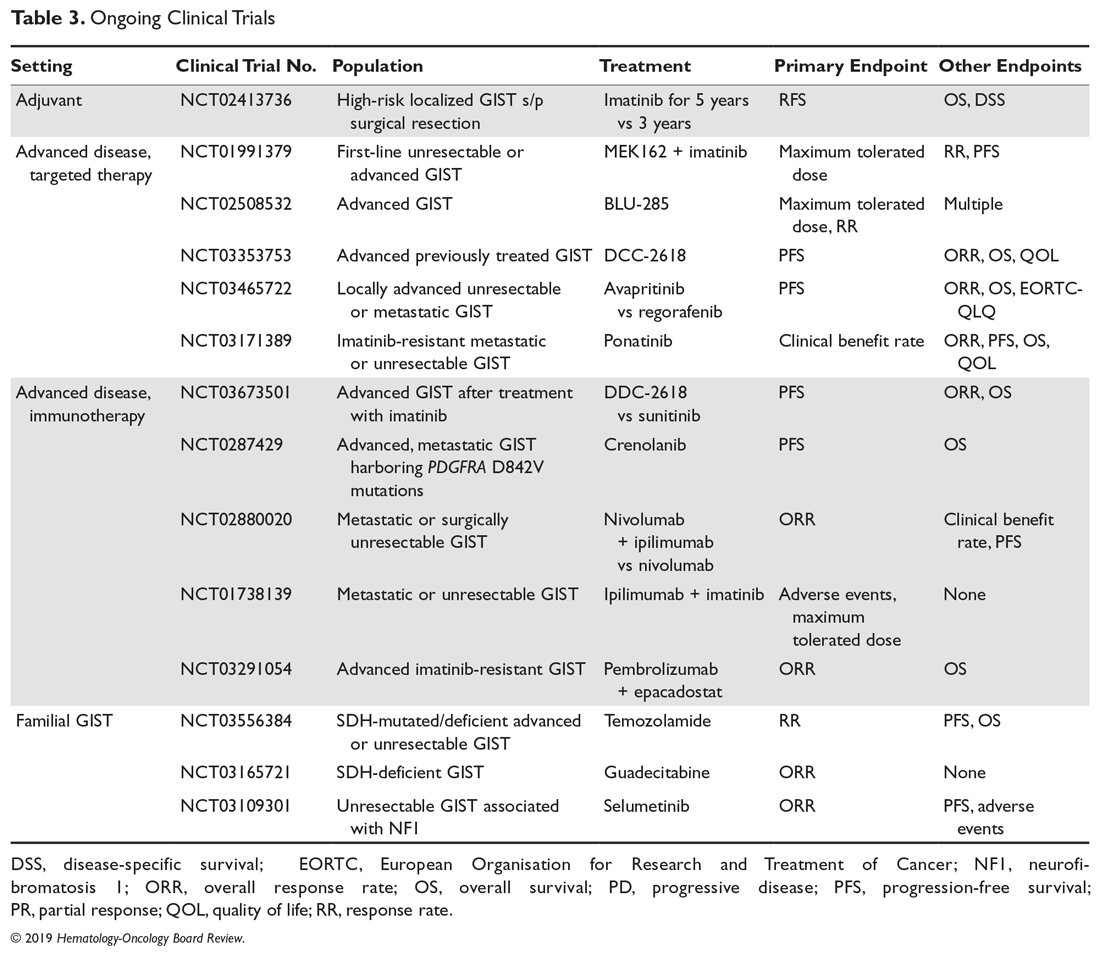
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.
Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.
A summary of FDA-approved drugs for treating GISTs is provided in Table 2.
Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.
Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
Most advanced gastrointestinal stromal tumors (GISTs) are due to a recurrence of localized disease, with only a small minority presenting with metastatic disease.1 Compared with chemotherapy, tyrosine kinase inhibitors (TKIs) have significantly improved the natural history of the disease, with median overall survival (OS) increasing from less than 1 year to about 5 years and approximately 1 in 5 patients achieving long-term survival.2 In addition, newer drugs in development and in clinical trials appear promising and have the potential to improve outcomes even further. This article reviews current evidence on options for treating metastatic or recurrent GISTs and GISTs that have progressed following initial therapy. The evaluation and diagnosis of GIST along with management of localized disease are reviewed in a separate article.
Case Presentation
A 64-year-old African American man underwent surgical resection of a 10-cm gastric mass, which pathology reported was positive for CD117, DOG1, and CD34 and negative for smooth muscle actin and S-100, consistent with a diagnosis of GIST. There were 10 mitoses per 50 HPF, and there was no intraoperative or intraperitoneal tumor rupture. The patient was treated with adjuvant imatinib, which was discontinued after 3 years due to grade 2 myalgias, periorbital edema, and macrocytic anemia. Surveillance included office visits every 3 to 6 months and a contrast CT abdomen and pelvis every 6 months. For the past 5 years, he has not had any clinical or radiographic evidence of disease recurrence. New imaging reveals multiple liver metastases and peritoneal implants. He feels fatigued and has lost about 10 lb since his last visit. He is 5 years out from his initial diagnosis and 2 years out from last receiving imatinib. His original tumor harbored a KIT exon 11 deletion.
What treatment should you recommend now?
Imatinib for Advanced GISTs
Before the first report of the efficacy of imatinib for metastatic GISTs in 2002, patients with advanced unresectable or metastatic GISTs were routinely treated with doxorubicin-based chemotherapy regimens, which were largely ineffective, with response rates (RRs) of around 5% and a median overall survival (OS) of less than 1 year.3,4 In 2002 a landmark phase 2 study revealed imatinib’s significant efficacy profile in advanced or metastatic GISTs, resulting in its approval by the US Food and Drug Administration (FDA).5 In this study, 147 patients with CD117-positive GISTs were randomly assigned to receive daily imatinib 400 mg or 600 mg for up to 36 months. The RRs were similar between the 2 groups (68.5% vs 67.6%), with a median time to response of 12 weeks and median duration of response of 118 days. Results of this study were much more favorable when compared to doxorubicin, rendering imatinib the new standard of care for advanced GISTs. A long-term follow-up of this study after a median of 63 months confirmed near identical RRs, progression-free survival (PFS), and median survival of 57 months among the 2 groups.6
Imatinib Daily Dosing
Although 400 mg of daily imatinib proved to be efficacious, it was unclear if a dose-response relationship existed for imatinib. An EORTC phase 2 study demonstrated a benefit of using a higher dose of imatinib at 400 mg twice daily, producing a RR of 71% (4% complete , 67% partial) and 1-year PFS of 73%, which appeared favorable compared with once-daily dosing and set the framework for larger phase 3 studies.7 Two phase 3 studies compared imatinib 400 mg once daily versus twice daily (until disease progression or unacceptable toxicity) among patients with CD117-positive advanced or metastatic GISTs. These studies were eventually combined into a meta-analysis (metaGIST) to compare RR, PFS and OS between the treatment groups. Both studies allowed cross-over to the 800 mg dose for patients who progressed on 400 mg daily.
The first study, conducted jointly by the EORTC, Italian Sarcoma Group, and Australasian Gastro-Intestinal Trials Group (EU-AUS),8 randomly assigned 946 patients to 400 mg once daily or twice daily. There were no differences in response rates between the groups, but the twice-daily group had a predicted 18% reduction in the hazard for progression compared with the once-daily group (estimated HR, 0.82; P = 0.026), which came at the expense of greater toxicities warranting dose reductions (60%) and treatment interruptions (64%). Cross-over to high-dose imatinib was feasible and safe, producing a partial response in 2%, stable disease in 27%, and a median PFS of 81 days. The second study was an intergroup study conducted jointly by SWOG, CALGB, NCI-C, and ECOG (S0033, US-CDN), with a nearly identical study design as the EU-AUS trial.9 The trial enrolled 746 patients. After a median follow up of 4.5 years, the median PFS and OS were not statistically different (18 vs 20 months and 55 vs 51 months, respectively). There were also no differences in response rates. One third of patients initially placed on the once-daily arm who crossed over after progression achieved a treatment response or stable disease.
The combined EU-AUS and US-CDN analysis (metaGIST) included 1640 patients with a median age of 60 years and 58% of whom were men; 818 and 822 patients were assigned to the 400 mg and 800 mg total daily doses, respectively.10 The median follow-up was 37.5 months. There were no differences in OS (49 vs 48.7 months), median PFS (18.9 vs 23.2 months), or overall response rates (51.4% vs 53.9%). Patients who had crossed over (n = 347) to the 800 mg total daily dose arm had a 7.7-month average PFS while on the higher daily dose. An analysis was performed on 377 patients in the EU-AUS trial assessing the impact of mutational status on clinical outcomes among imatinib-treated patients. KIT exon 9 activating mutations were found to be a significant independent prognostic factor for death when compared with KIT exon 11 mutations. However, the adverse prognostic value of KIT exon 9 mutations was partially overcome with higher doses of imatinib, as those who received 800 mg total had a significantly better PFS, with a 61% relative risk reduction, than those who received 400 mg. Altogether, it was concluded that imatinib 400 mg once daily should be the standard-of-care first-line treatment for advanced or metastatic GISTs, unless a KIT exon 9 mutation is present, in which case imatinib 800 mg should be considered, if 400 mg is well tolerated. In addition, patients treated with frontline imatinib at 400 mg once daily, if tolerated well, should be considered for imatinib 800 mg upon progression of disease.
Despite there being problems with secondary resistance, significant progress has occurred in the treatment of metastatic disease over a short period of time. Prior to 2000, median OS for patients with metastatic GISTs was 9 months. With the introduction of imatinib and other TKIs, the median OS has increased to 5 years, with an estimated 10-year OS rate of approximately 20%.2
Imatinib Interruption
Since at this point, imatinib was a well-established standard of care for advanced GISTs, it was questioned whether imatinib therapy could be interrupted. At this time, treatment interruption in a stop-and-go fashion was deemed feasible in other metastatic solid tumors such as colorectal cancer (OPTIMOX1).11 The BFR French trial showed that stopping imatinib therapy in patients who had a response or stable disease after 1, 3, or 5 years was generally followed by relatively rapid tumor progression (approximately 50% of patients within 6 months), even when tumors were previously removed.12 Therefore, it is recommended that treatment in the metastatic setting should be continued indefinitely, unless there is disease progression. Hence, unlike with colorectal cancer or chronic myelogenous leukemia, as of now there is no role for imatinib interruption in metastatic GISTs.
Case Continued
The patient is started on imatinib 400 mg daily, and overall he tolerates therapy well. Interval CT imaging reveals a treatment response. Two years later, imaging reveals an increase in the tumor size and density with a new nodule present within a preexisting mass. There are no clinical trials in the area.
What defines tumor progression?
Disease Progression
When GISTs are responding to treatment, on imaging the tumors can become more cystic and less dense but with an increase in size. In addition, tumor progression may not always be associated with increased size—increased density of the tumor or a nodule within a mass that may indicate progression. If CT imaging is equivocal for progression, positron emission tomography (PET) can play a role in identifying true progression. It is critically important that tumor size and density are carefully assessed when performing interval imaging. Of note, radiofrequency ablation, cryotherapy, or chemoembolization can be used for symptomatic liver metastases or oligometastatic disease. When evaluating for progression, one needs to ask patients about compliance (ie, maintaining dose intensity related to side effects of therapy as well as the financial burden of treatment—copay toxicity).
What are mechanisms of secondary imatinib resistance?
Imatinib resistance can be subtle in patients with GISTs, manifesting with new nodular, enhancing foci enclosed within a preexisting mass (resistant clonal nodule), or can be clinically or radiographically overt.13 Imatinib resistance occurs through multiple mechanisms including acquisition of secondary activating KIT mutations in the intracellular ATP-binding domain (exons 13 and 14) and the activation loop (exons 17 and 18).14
What are the treatment options for this patient?
Second-line Therapy
Sunitinib malate is a multitargeted TKI that not only targets c-Kit and PDGFRA, but also has anti-angiogenic activity through inhibition of vascular endothelial growth factor receptors (VEGFR). Sunitinib gained FDA approval for the second-line treatment of advanced GISTs based on an international double-blind trial that randomized 312 patients with imatinib-resistant metastatic GISTs in a 2:1 fashion to receive sunitinib 50 mg daily for 4 weeks on and 2 weeks off or placebo.15,16 The trial was unblinded early at the planned interim analysis, which revealed a marked benefit, producing a 66% reduction in the hazard risk of progression (27.3 vs 6.4 weeks, HR, 0.33; P < 0.001). The most common treatment-related adverse events were fatigue, diarrhea, skin discoloration, nausea, and hand-foot syndrome. Another open-label phase 2 study assessed a continuous dosing schema of sunitinib 37.5 mg daily, which has been shown to be effective with less toxicity.17 Among the 60 patients enrolled, the primary endpoint of clinical benefit rate at 24 weeks was reached in 53%, which consisted of 13% partial responses and 40% stable disease. Most toxicities were grade 1 or 2 and easily manageable through standard interventions. This has been recommended as an alternative to the initial scheduled regimen.18 Part of sunitinib’s success is its activity against GISTs harboring secondary KIT exon 13 and 14 mutations, and possibly its anti-angiogenic activity.19 Sunitinib is particularly efficacious among GISTs harboring KIT exon 9 mutations.
Third-line Therapy
Patients who have progressed on prior imatinib and sunitinib can receive third-line regorafenib, a multi-TKI that differs chemically from sorafenib by a fluorouracil group (fluoro-sorafenib). FDA approval of regorafenib was based on the phase 3 GRID (GIST Regorafenib In progressive Disease) multicenter international trial.20 This trial randomly assigned 199 patients in a 2:1 fashion to receive regorafenib 160 mg daily for 21 days out of 28-day cycles plus best supportive care (BSC) versus placebo plus BSC. Cross-over was allowed. Regorafenib significantly reduced the hazard risk of progression by 73% compared with placebo (4.8 vs 0.9 months; HR, 0.27; P < 0.001). There was no difference in OS, which may be because of cross-over (median OS, 17.4 months in both arms). As a result, regorafenib is now considered standard third-line treatment for patients with metastatic GISTs. It has a less favorable toxicity profile than imatinib, with hand-foot syndrome, transaminitis, hypertension and fatigue being the most common treatment toxicities. In order to avoid noncompliance, it is recommended to start at 80 mg and carefully titrate upwards to the 160 mg dose.
A list of landmark studies for advanced GISTs is provided in Table 1.
A summary of FDA-approved drugs for treating GISTs is provided in Table 2.
Clinical Trials
Clinical trial enrollment should be considered for all patients with advanced or unresectable GISTs throughout their treatment continuum. Owing to significant advances in genomic profiling through next-generation sequencing, multiple driver mutations have recently been identified, and targeted therapies are being explored in clinical trials.21 For example, the neurotrophic receptor tyrosine kinase (NTRK) gene appears to be mutated in a small number of advanced GISTs, and these can respond to the highly selective TRK inhibitor larotrectinib.22 Additionally, ongoing studies are assessing immunotherapies for sporadic GISTs and treatment for familial GISTs (Table 3). Some notable studies include those assessing the efficacy of agents that target KIT and PDGFR secondary mutations, including avapritinib (BLU-285) and DCC-2618, MEK inhibitors, and the multi-kinase inhibitor crenolanib for GISTs harboring the imatinib-resistant PDGFRA D842V mutation. There are also studies utilizing checkpoint inhibitors alone or in combination with imatinib.
Case Conclusion
Given the patient’s progression on imatinib, he is started on second-line sunitinib malate. He experiences grade 1 fatigue and hand-foot syndrome, which are managed supportively. After he has been on sunitinib for approximately 8 months, his disease progresses. He subsequently undergoes genomic profiling of his tumor and starts BLU-285 on a clinical trial.
Key Points
- For advanced and metastatic disease, TKIs have substantially improved the prognosis of KIT mutated GISTs, with 3 FDA-approved drugs: imatinib, sunitinib, and regorafenib. Imatinib 400 mg is the standard-of-care frontline therapy for locally advanced, unresectable, or metastatic imatinib-sensitive GISTs. If a patient has a KIT exon 9 mutation and 400 mg is well-tolerated, increasing to 800 mg is recommended. Imatinib should be continued indefinitely unless there is intolerance, a specific patient request for interruption, or progression of disease.
- When there is progression of disease in a patient with a sensitive mutation on 400 mg of imatinib, the dose can be increased to 800 mg.
- For patients who are imatinib-intolerant or have progression, standard second line is sunitinib.
- For patients who further progress or are sunitinib-intolerant, regorafenib is the standard third-line treatment.
- There needs to be close attention to side effects, drug and food interactions, and patient copay costs in order to maintain patient compliance while on TKI therapy.
- There are still major limitations in the systemic treatment of GISTs marked by their inherent genetic heterogeneity and secondary resistance. Continued translational and clinical research is needed in order to improve treatment for patients who develop secondary resistance or who have less common primary resistant mutations. Patients are encouraged to participate in clinical trials of new therapies.
Summary
GISTs are the most common mesenchymal tumors of the GI tract. They comprise an expanding landscape of tumors that are heterogenous in terms of natural history, mutations, and response to systemic treatments. The mainstay of treatment for localized GISTs is surgical resection followed by at least 3-years of adjuvant imatinib for patients with high-risk features who are imatinib-sensitive. Patients with GISTs harboring resistance mutations such as PDGFRA D842V or with SDH-deficient or NF1-associated GISTs should not receive adjuvant imatinib. Patients with more advanced GISTs and/or in difficult to resect sites harboring a sensitive mutation can be considered for neoadjuvant imatinib. Those with metastatic GISTs can receive first-, second-, and third-line imatinib, sunitinib, or regorafenib, respectively. Clinical trial enrollment should be encouraged for patients whose GISTs harbor primary imatinib-resistant mutations, and those with advanced or unresectable GISTs with secondary resistance.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.
1. Ma GL, Murphy JD, Martinez ME et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298-302.
2. Heinrich MC, Rankin C, Blanke CD, et al. Correlation of long-term results of imatinib in advanced gastrointestinal stromal tumors with next-generation sequencing results: analysis of phase 3 SWOG Intergroup Trial S0033. JAMA Oncol. 2017;3:944-952.
3. DeMatteo RP, Lewis JJ, Leung D, et al. Two hundred gastrointestinal stromal tumors recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231:51-58.
4. Goss GA, Merriam P, Manola J, et al. Clinical and pathological characteristics of gastrointestinal stromal tumors (GIST). Prog Proc Am Soc Clin Oncol. 2000;19:599a.
5. Demetri GD, von Mehren M, Blanke CD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472-480.
6. Blanke CD, Demetri GD, von Mehren M, et al. Long-term results from a randomized phase ii trial of standard- versus higher-dose imatinib mesylate for patients with unresectable or metastatic gastrointestinal stromal tumors expressing KIT. J Clin Oncol. 2008;26:620-625.
7. Verweij J, van Oosterom A, Blay JY, et al. Imatinib mesylate (STI-571 Glivec, Gleevac) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target. Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Eur J Cancer. 2003;39:2006-2011.
8. Verweij J, Casali PG, Zalcberg J, et al. Progression-free survival in gastrointestinal stromal tumours with high-dose imatinib: randomized trial. Lancet. 2004;364:1127-1134.
9. Blanke CD, Rankin C, Demetri GD, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008;26:626-632.
10. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010;28:1247-1253.
11. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer –a GERCOR study. J Clin Oncol. 2006;24:394-400.
12. Blay JV, Cesne AL, Ray-Coquard I, et al. Prospective multicentric randomized phase iii study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: The French Sarcoma Group. J Clin Oncol. 2007;25:1107-1113.
13. Desai J, Shankar S, Heinrich MC, et al. Clonal evolution of resistance to imatinib in patients with metastatic gastrointestinal stromal tumors. Clin Cancer Res. 2007;13(18 Pt 1): 5398-5405.
14. Gramza AW, Corless CL, Heinrich MC. Resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumors. Clin Cancer Res. 2009;15:7510-7518.
15. Sutent (sunitinib malate) [package insert]. New York, NY: Pfizer Labs; 2017.
16. Demetri GD, van Oosterom AT, Garrett CR, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomized controlled trial. Lancet. 2006;368:1329-1338.
17. George S, Blay JY, Casali PG, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer. 2009;45:1959-1968.
18. Brennan MF, Antonescu CR, Maki RG. Management of Soft Tissue Sarcomas. Switzerland: Springer International Publishing; 2013.
19. Heinrich MC, Maki RG, Corless CL, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumors. J Clin Oncol. 2008;26:5352-5359.
20. Demetri GD, Reichardt P, Kang YK, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:295-302.
21. Wilky BA, Villalobos VM. Emerging role for precision therapy through next-generation sequencing for sarcomas. JCO Precision Oncology. 2018;2:1-4.
22. Drilon A, Laetsch TW, Kummar S, et al. Efficacy of larotrectinib in trk fusion-positive cancers in adults and children. N Engl J Med. 2018;378:731-739.