User login
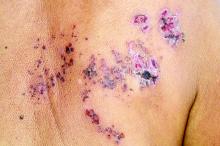
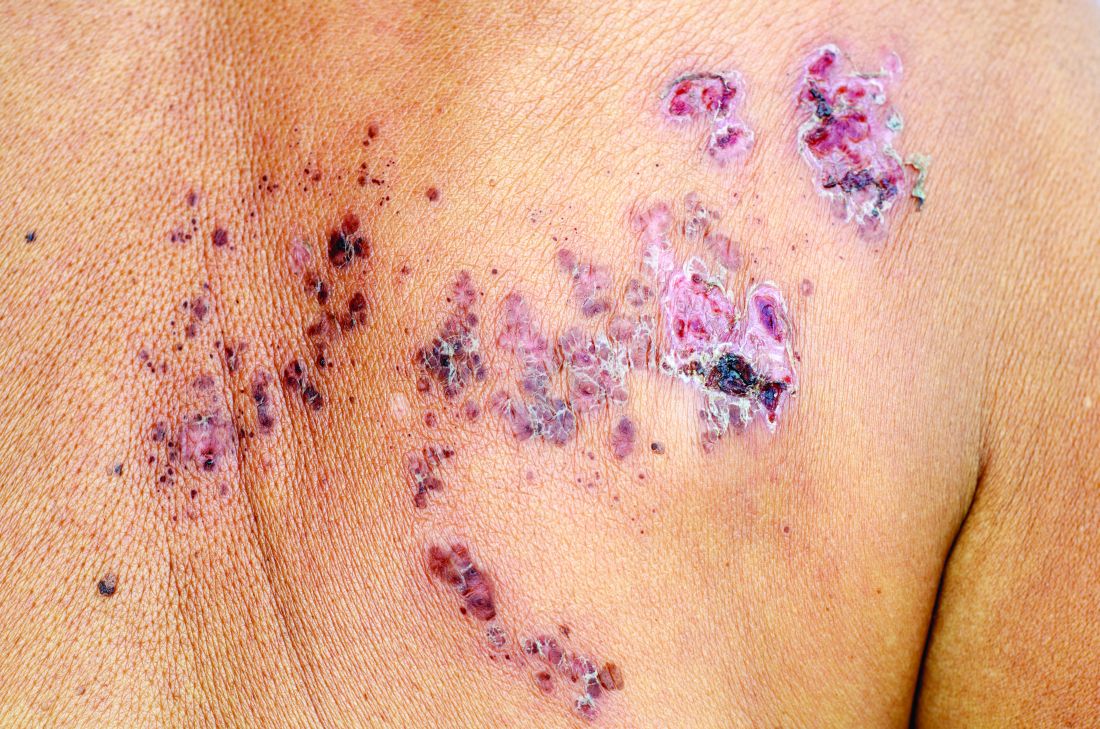
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
All individuals with psoriasis or psoriatic arthritis aged over 50 years should receive the recombinant herpes zoster vaccine, according to a systematic review and consensus recommendations from the National Psoriasis Foundation.
Emily Baumrin, MD, of Brigham and Women’s Hospital, Boston, and her coauthors reviewed 41 studies of herpes zoster in people with psoriasis or psoriatic arthritis according to treatment modality. Their report is in the Journal of the American Academy of Dermatology.
Overall, psoriasis was associated with an increased rate of herpes zoster when compared with the general population: 13.3 cases per 1,000 patient-years for psoriasis and 15.9 for psoriatic arthritis, compared with 8.5 in healthy controls after adjustment for age, sex, and systemic medications. Most of this increased incidence was seen in patients with more severe disease: Those with mild disease who were not receiving systemic therapy had a risk similar to that of healthy controls.
However, one study suggested much of the increased risk of herpes zoster in psoriasis was accounted for by immunosuppressive therapy; when those patients were excluded, there was an 8% increase in risk.
The authors found that people whose psoriasis was treated with tofacitinib (Xeljanz) had a two- to threefold increased risk of herpes zoster, compared with those treated with tumor necrosis factor (TNF) inhibitors or conventional synthetic disease-modifying antirheumatic drugs (DMARDs).
Corticosteroids – either alone or in combination with DMARDs – were also associated with significant increases in the risk of herpes zoster. Patients treated with TNF inhibitor monotherapy had a risk of herpes zoster similar to that of those treated with conventional synthetic DMARDs or no synthetic therapy.
On the question of immunization, the authors pointed to guidelines recommending use of the live attenuated zoster vaccine (Zostavax) in immunocompetent patients or those on low-dose immunosuppression, although they noted that the vaccine is currently contraindicated for patients on biologic DMARDs.
They also examined the evidence for the use of the recently-released non-live recombinant herpes zoster vaccine (Shingrix) in immunocompromised patients, which found no evidence of vaccine-related serious adverse events in individuals with HIV and low CD4 cell counts and in autologous hematopoietic stem cell transplant recipients.
Given this, they recommended that the recombinant vaccine be administered to all patients aged over 50 years with psoriasis or psoriatic arthritis, and to those aged under 50 years who were being treated with tofacitinib, systemic corticosteroids, or combination systemic therapy.
There were insufficient data to draw conclusions about the impact of treatment with the interleukin-12/23 blocker ustekinumab (Stelara) on herpes zoster risk, but the authors noted that there was a trend toward an increased risk. They found no increase in the risk of herpes zoster with interleukin-17 inhibitors (ixekizumab [Taltz], secukinumab [Cosentyx], and brodalumab [Siliq]) and interleukin-23 (p19 subunit) inhibitors (guselkumab [Tremfya], tildrakizumab [Ilumya], and risankizumab) but noted an absence of long-term safety data for these drugs.
Four authors declared advisory, consultancy, or speaker positions with the pharmaceutical sector.
SOURCE: Baumrin E et al. J Am Acad Dermatol. 2019 March 15. doi: 10.1016/j.jaad.2019.03.017.
FROM THE JOURNAL OF THE AMERICAN ACADEMY OF DERMATOLOGY