User login
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
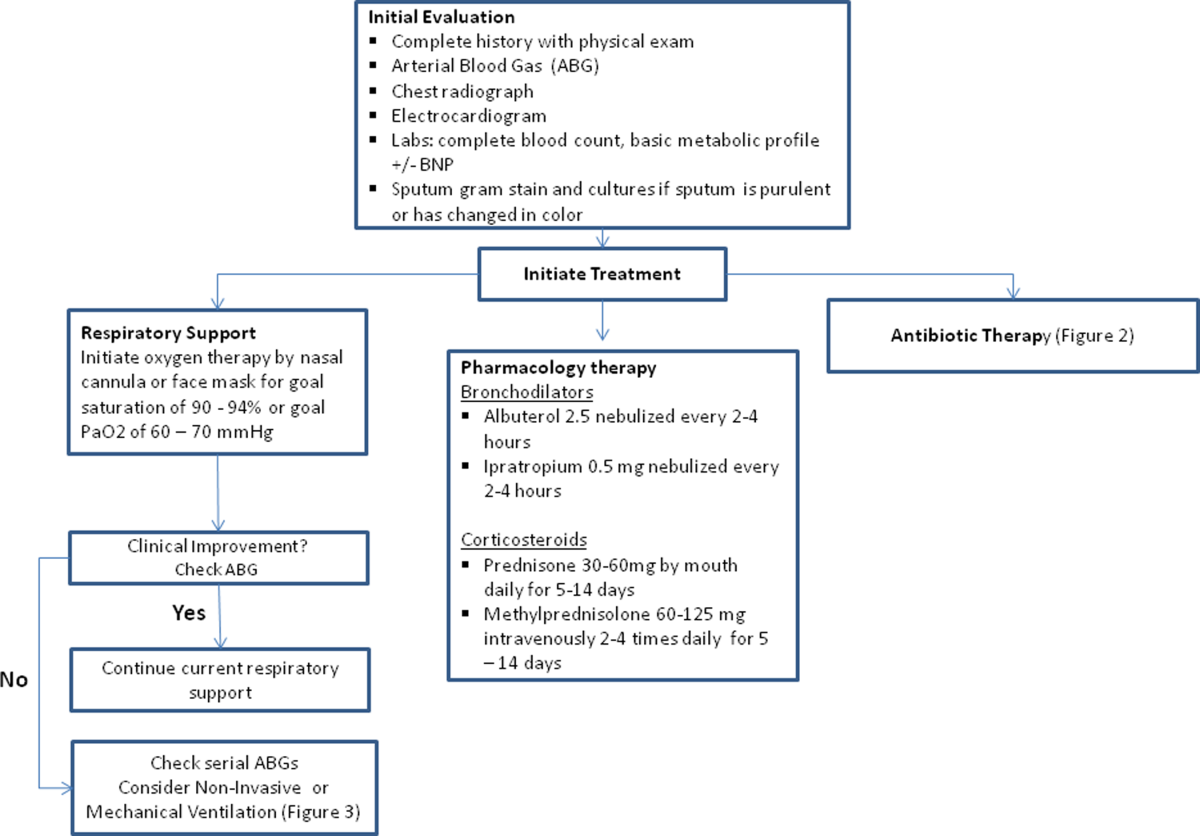
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
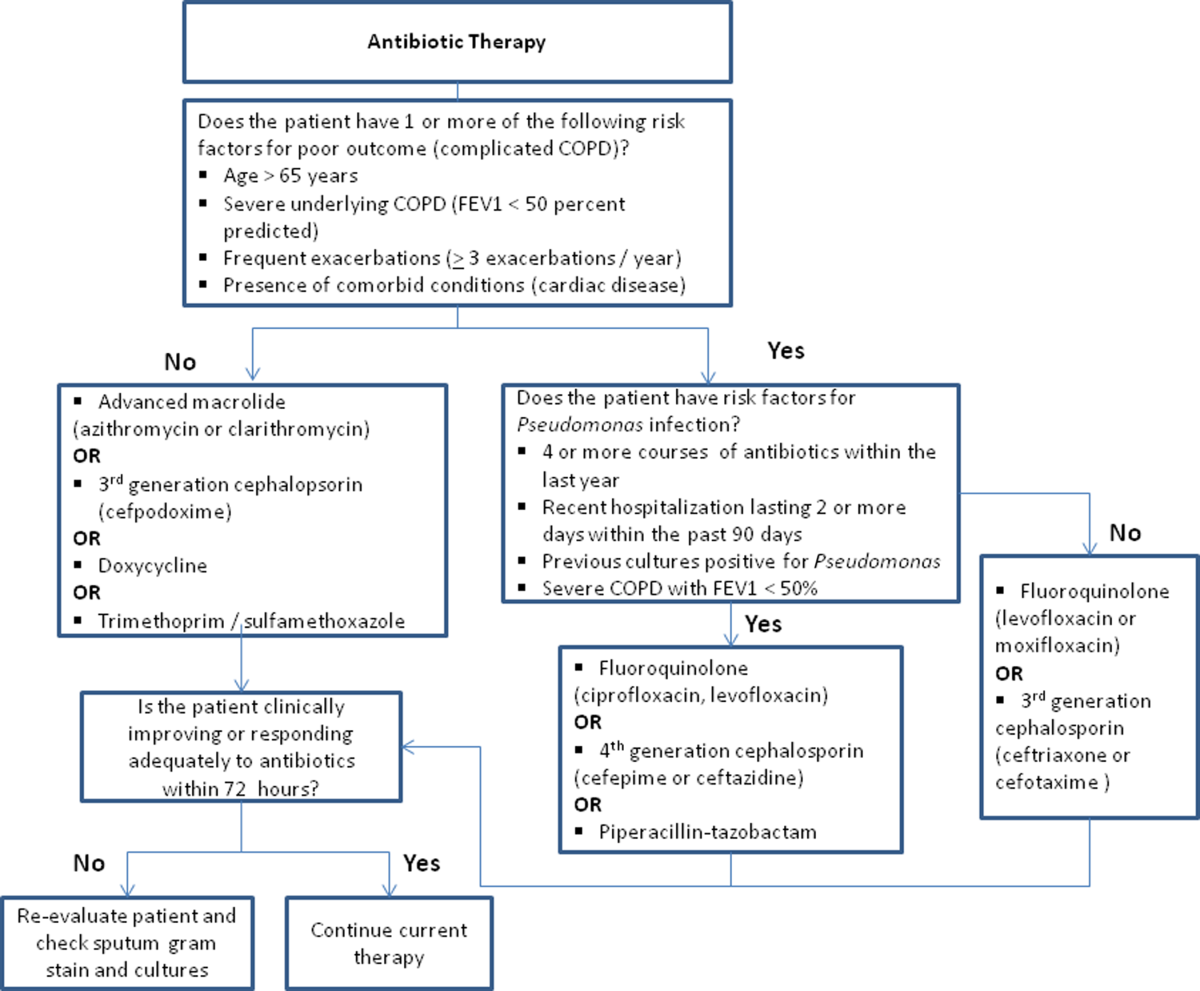
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]
Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
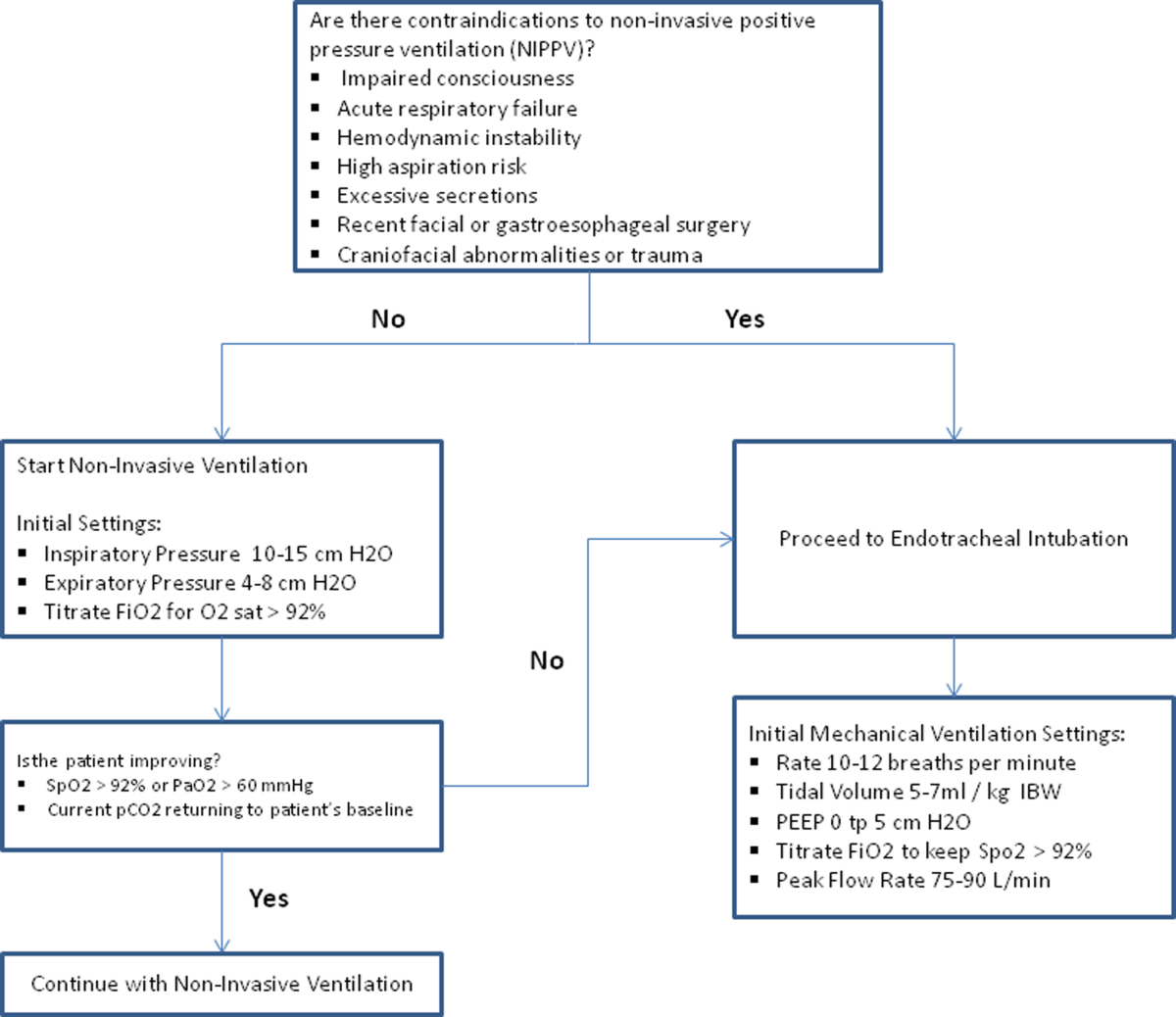
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.
Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]

Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.

Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]

Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.

Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.