User login
Anxiety and Depression in Chronic Obstructive Pulmonary Disease: Recognition and Management
Introduction
Anxiety and depression are common in patients with chronic obstructive pulmonary disease (COPD), occurring more frequently than in the general population1-4 or patients with other chronic diseases such as hypertension, diabetes, cancer, or musculoskeletal disorders.5,6 Their presence is associated with worse outcomes of COPD, and increased morbidity, mortality, disability, and health care expenditure.6-8 In spite of this, both anxiety and depression are frequently overlooked and undertreated in patients with COPD,9 and symptoms of anxiety and depression can overlap significantly, as well as overlap with COPD symptoms.7,10
Comorbid depressive disorders that may occur in patients with COPD include major depressive disorder, dysthymias (chronic depressive symptoms of mild severity), and minor depression.11 Depressive disorders are characterized by feelings of sadness, emptiness, and/or irritability, along with cognitive and somatic symptoms, which have a detrimental effect on the patient’s ability to function.11 Anxiety disorders include generalized anxiety disorder (GAD), phobias, and panic disorders.11 The main features of anxiety disorders, such as excessive fear and anxiety, may be accompanied by behavioral disturbances related to these symptoms, such as panic attacks and avoidance.11,12
The reported prevalence of depression in COPD varies widely between studies, owing to differences in sampling methods and degrees of illness severity used in assessment of depression6; rates have been reported to range from 10% to 42% in patients with stable COPD,6,13 and from 10% to 86% in patients with acute COPD exacerbation.14 Individuals with severe COPD are twice as likely to develop depression than patients with mild COPD.10
Prevalence rates for clinical anxiety in COPD range from 13% to 46% in outpatients and 10% to 55% among inpatients. GAD, panic disorders, and specific phobias are reported most frequently.15 Patients with COPD are 85% more likely to develop anxiety disorders compared with matched controls without COPD,4 and panic disorder is reported with a prevalence that is up to 10-fold higher than in the general population.16
Global prevalence rates of anxiety and depression are 1.8- and 1.4-fold higher in women than men, respectively17; the same gender difference is observed in patients with COPD.6 The higher prevalence rates of anxiety and depression in women are thought to be a result of sex differences in brain structure, function, and stress responses, as well as differences in exposure to reproductive hormones, social constraints, and experiences between women and men.18 However, psychologic comorbidity is an issue for both men and women with COPD, so it is important that clinicians are vigilant in recognizing anxiety and depression in both sexes, and are careful not to underestimate the burden in the male patient population.
It is also important to note that depression and anxiety often occur simultaneously in patients with COPD, with prevalence estimates of 26% to 43%.9,19,20 COPD patients with both depression and anxiety are at a heightened risk of suicidal ideation, increased physical disability, and chronic depressive symptoms versus those with either disorder alone.10,15 It is therefore important that comorbid anxiety and depression is not overlooked in patients with COPD.
Ensuring that anxiety and depression are recognized and treated effectively in patients with COPD is essential for optimizing outcomes. Primary care practitioners are well placed to diagnose anxiety and depression, and to ensure these conditions are suitably managed alongside treatments of COPD.
Potential mechanisms of anxiety and depression in COPD
Growing evidence suggests that the relationship between mood disorders—particularly depression—and COPD is bidirectional, meaning that mood disorders adversely impact prognosis in COPD, whereas COPD increases the risk of developing depression.21 For example, in a study of
60 stable patients with COPD, elevated dyspnea and reduced exercise capacity were the predominant mechanisms leading to anxiety and depression symptoms associated with the condition.22 In addition, the risk of new-onset depression was increased in COPD patients with moderate-to-severe dyspnea in a 3-year follow-up study.23 Conversely, depression has been shown to be a significant risk factor for disabling dyspnea (modified Medical Research Council score ≥2) in patients with COPD.24
COPD can lead to feelings of hopelessness, social isolation, reduced physical functioning, and sedentary lifestyle, all of which are associated with an increased level of depressive symptoms.25 Similarly, inadequate social support increases the risk of anxiety in patients with COPD.26 Therefore, ensuring that patients with COPD have high-quality support is very important for reducing anxiety and depressive symptoms.27
The exact mechanisms for the association between mood disorders and COPD remain unclear.7,10 Research to date indicates that the relationship between depression and impaired pulmonary function may be partly mediated by chronic inflammation7,10; systemic inflammation has been associated with other comorbidities of COPD (eg, muscle wasting and osteoporosis),28 and emerging data appear to show that proinflammatory cytokines partly mediate the association between depressive symptoms and pulmonary function.29 Smoking and hypoxemia may also influence the prevalence of depression in COPD, but symptom severity and impaired quality of life remain the most important determinants.6,30
Clinical studies have demonstrated that a number of patient-related factors, including female gender, younger age, current smoking, greater severity of airflow limitation, and lower socioeconomic status, are associated with a higher prevalence and/or increased risk of depression and/or anxiety in COPD.3,4,30,31 Frequent episodes of rehospitalization, and comorbidities such as hypertension, arthritis, cancer, and heart disease, have been found to increase the risk of anxiety and depression in patients with COPD.3,32 Risk of anxiety has been shown to increase with greater dyspnea severity.4 Pain, a frequently overlooked symptom in COPD, has been shown to be associated with symptoms of both anxiety and depression in patients with COPD.33 This is driven by worsened quality of life and sleep quality, decreased physical activity, and an increased fear of movement that occur as a result of pain.34
The impact of anxiety and depression in COPD
Comorbid anxiety and depression have a significant detrimental impact on morbidity and mortality in patients with COPD. Both disorders have been associated with an increased risk of death in COPD.13,35-37 Indeed, of 12 comorbidities proposed to be predictors of mortality in a cohort of 187 female outpatients with COPD, anxiety was associated with the highest risk of death.35,36
In addition, patients with COPD and anxiety and/or depression have a higher risk of COPD exacerbations,4,8,23,36,38-40 hospitalization,41,42 rehospitalization,14,36,43 longer hospital stays,37,41,44 and mortality after exacerbations,14,36,41 compared with patients without these comorbidities. Patients with COPD who have elevated anxiety symptoms also often experience their first hospitalization earlier in the natural course of COPD than those without anxiety.36
Psychologic comorbidities are also associated with worse lung function, dyspnea, and respiratory symptom burden in patients with COPD.37,40 Patients with COPD and anxiety are more likely to experience greater dyspnea at an earlier stage of disease than those without anxiety.36 Persistent smoking at 6 months after hospitalization for an acute exacerbation of COPD is also more likely to be seen in patients with depression.37
Patient-centered outcomes are worse in COPD patients with mood disorders. Both anxiety and depression have been shown to correlate with significantly reduced health-related quality of life (HRQoL), poorer physical health status, functional limitations, and reduced exercise capacity.4,23,37,40,45 The presence of either anxiety or depression at baseline has been shown to correlate with reduced HRQoL at 1-year follow-up, but depression appears to be the stronger predictor of low future HRQoL than anxiety.45
Additionally, mood disorders—particularly depression—reduce physical activity in patients with COPD.46,47 Emotional responses to COPD symptoms, such as dyspnea, can further decrease activity and worsen deconditioning, resulting in a downward spiral of reduced inactivity, social isolation, fear, anxiety, and depression.48
COPD patients with any comorbidity exhibit lower rates of medication adherence than those without comorbidities.49-51 Clinical studies have demonstrated that anxiety and depression are significant predictors of poor adherence to COPD interventions, including pulmonary rehabilitation (PR).51-55 Nonadherence to COPD therapies is associated with poor clinical outcomes, including higher hospitalization rates and increased emergency department visits, and increased costs.56,57 Health care expenditure, in terms of both specific COPD-related costs and general “all-cause” costs, is significantly higher in COPD patients with anxiety and/or depression than in those without.8
Diagnosis of anxiety and depression in patients with COPD
The underdiagnosis and undertreatment of anxiety and depression in this population is common and can adversely affect patient outcomes.6,7,9,10,58 Hence, it is crucial that anxiety and depression are identified and more effectively managed in clinical practice.10
Primary care practitioners are the main point of contact for many patients with COPD,6,59,60 and so can play a key role in screening for and early identification of anxiety and depression. However, detection of mood disorders by primary care practitioners is challenging for several reasons. These include the lack of a standardized approach in diagnosis, and inadequate knowledge or confidence in assessing psychological status (particularly given the number of strategies available for screening patients for mood disorders),6 as well as factors associated with time constraints, such as competing agendas, duration of visits, and high patient load.6,61 Furthermore, system-level barriers, such as lack of electronic medical records and adequate health insurance, as well as any communication gaps between primary care and mental health care, may hinder the detection and management of anxiety and depression.6 In addition, patients themselves may have a limited understanding of these comorbidities, or may be hesitant to discuss symptoms of anxiety or depression with their primary care practitioner owing to stigma around mental illness.6
Patients with COPD should be screened and assessed for anxiety and depression, and the United States Preventive Services Task Force recommends that clinicians screen for depression in all adults.6,62 There are several validated screening tools suitable for clinical use:
- Anxiety Inventory for Respiratory (AIR) Disease scale: a brief, easy-to-use tool for screening and measuring anxiety in COPD.63,64 It is a self-administered scale, and takes approximately 2 minutes to complete. The AIR scale is responsive to PR.64
- COPD Anxiety Questionnaire (CAF): a reliable tool for early identification of COPD-related anxiety.65
- Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire (PHQ; available at: http://www.phqscreeners.com/select-screener/): the PRIME-MD comprises 26 yes/no questions on the 5 most common psychiatric disorders, including depression and anxiety.66,67 This is not a diagnostic tool, but a high number of positive responses from a patient in any given module indicates that they require further clinical evaluation.
- PHQ-2 and PHQ-9 (Table 1; PHQ-9 available at http://www.phqscreeners.com/select-screener/): widely-used self-administered 2- and 9-item versions of the PRIME-MD, specific to depression; similarly, the 3-item PHQ-3 is available for anxiety assessment (Table 2).6,67,68 In a study investigating tools used by family physicians in England to assess depression, over 75% used PHQ-9.69
- Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire-version 20 (GHQ-20): both can be used to screen for psychologic distress in patients with COPD.71
- The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI): two 21-item self-report questionnaires that are widely used in the United States to evaluate anxiety and depression.72
In addition to specific anxiety and depression questionnaires (Tables 1 and 2), more general COPD assessments tools, such as the COPD Assessment Test and the COPD Clinical Questionnaire, also incorporate questions that may be indicative of symptoms of these comorbidities in patients with COPD.73
Management of anxiety and depression in COPD
Even though anxiety and depression are among the most common and burdensome comorbid conditions in COPD, less than one-third of patients with these comorbidities receive effective intervention.6,10 Primary care providers have an excellent opportunity to impact this care gap.
As in non-COPD patients, comorbid depression and anxiety may be treated with nonpharmacologic and/or pharmacologic interventions (Figure 1).76
Nonpharmacologic interventions
Evidence to date suggests that nonpharmacologic interventions such as behavioral therapy are as effective as antidepressants, and may be preferred by patients with mood disorders.12
Cognitive behavioral therapy (CBT), which is typically administered by psychologists/psychiatrists, may be effective in treating COPD-related anxiety and depression, especially in conjunction with exercise and education.12,76,77 Individualized or group CBT is the treatment of choice for addressing thinking patterns that contribute to anxiety and depression to change a patient’s behavior and emotional state.76 PR programs involve several components, including aerobic exercise, lung function training, and psycho-education.62,76 PR is suitable for most patients with COPD, and provides multiple benefits, including reduced hospitalizations in patients who have had a recent exacerbation, and improved dyspnea, exercise tolerance, and health status in patients with stable disease,62 as well as clinically and statistically significant improvements in depression and anxiety, irrespective of age.7,78,79 Exercise-based forms of PR appear to be the most effective for reducing mood symptoms,12,76 and incorporating psychotherapy may also improve psychologic outcomes.80 Stress reduction (relaxation) therapy aims to reduce anxiety-related physiologic changes, and includes a variety of techniques (eg, breathing exercises, sequential muscle relaxation, hypnosis, mindfulness meditation), some of which may be included in PR or used alongside other treatments (eg, CBT).76 Limited data indicate that such therapy may be beneficial for reducing anxiety and depression, as well as respiratory symptoms and dyspnea, in patients with COPD.12,76
Self-management techniques improve clinical outcomes in patients with COPD, but data on the management of depression or anxiety are inconclusive.7,12 A minimal, home-based, nurse-led, psycho-educational intervention was designed to encourage more open-ended, descriptive discussions of thoughts, emotions, behaviors, and bodily sensations in patients with COPD.81 The intervention, which involved nurses attending a 1-hour face-to-face session in the patients’ homes with a 15-minute telephone “booster” session 2 weeks later, helped patients with advanced COPD to self-manage their condition and provide relief from anxiety.81,82 However, it should be noted that there is currently a lack of high-quality data evaluating psychologic interventions in the COPD population.83
In addition, it is important that caregivers are supported in the management of patients with COPD and comorbid anxiety and/or depression; areas in which caregivers can be assisted in their role may include disease education and counseling, where appropriate.84
Given that smoking cessation is a key recommendation for patients with COPD,44,62 practitioners should be aware that patients with comorbid depression and anxiety may experience greater difficulty in smoking cessation, and worsened mood during nicotine withdrawal.44 Clinicians should therefore carefully monitor current smokers with COPD and comorbid depression/anxiety (using the tools described previously63,68,70,71) when they are attempting to quit smoking.
Pharmacologic interventions
Pharmacologic therapy of anxiety and depression has so far only been investigated in patients with COPD in small studies.76 However, the available evidence does not indicate that COPD patients with anxiety and depression should be managed any differently from individuals without COPD.62 As such, pharmacologic interventions are particularly important for patients with acute or severe anxiety or depression.
Antidepressant agents are categorized according to their mechanism of action, and most commonly include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors, bupropion (a norepinephrine- and dopamine-reuptake inhibitor; also approved for smoking cessation85), and mirtazapine (a norepinephrine and serotonin modulator), among others.86 SSRIs are the current firstline drug treatment for depression, and have been shown to significantly improve depression and anxiety in patients with COPD in some, but not all, trials published to date.76 However, it is important to note that a diagnosis of bipolar disorder must be ruled out before initiating standard antidepressant therapy.87 In addition to antidepressants, atypical antipsychotics have also been shown to be useful for treating anxiety, either as monotherapy or combination therapy, and possibly as an adjunctive therapy for the management of depression.88,89
Primary care practitioners can refer to existing guidelines on the management of anxiety and depression in patients with COPD,86,90 while taking certain factors into consideration. Any pharmacologic management strategy for the treatment of COPD may increase the risk of drug–drug or drug–disease interactions.76 For example, it is important to avoid medications that cause respiratory depression (eg, benzodiazepines [unless used with extreme caution], particularly in patients who are already CO2 retainers) or sedation; chosen drugs should have minimal other adverse effects.76 Moreover, SSRIs may also be associated with troublesome adverse effects during treatment initiation, such as gastrointestinal upset, headache, tremor, psychomotor activation, and sedation76; in addition, dry mouth is an adverse effect associated with both SSRI treatment and several inhaled therapies, so may be particularly problematic in patients with COPD.91,92 Currently, data are particularly scarce for the management of anxiety in patients with COPD, with inconclusive or contradictory findings reported for SSRIs, azapirones (including buspirone), and tricyclic antidepressants.76
In addition to monitoring adherence to COPD therapies, primary care practitioners should carefully monitor patients treated with antidepressants and anxiolytics for adherence. A meta-analysis of 18,245 individuals with chronic diseases showed that depressed patients had a 76% significantly higher risk of nonadherence to medication compared with those without depressive symptoms.93
Targeting dyspnea is key to the management of anxiety and depression in COPD, as breathlessness is frequently associated with the onset of both comorbidities.21,22 Therapeutic approaches to alleviating dyspnea include PR, optimizing respiratory mechanics and muscle function (with bronchodilator therapy), and reducing central neural drive to respiratory muscles with supplemental oxygen or opioid medication.94
Although bronchodilator therapy for COPD has not been shown to have significant direct effects on depression or anxiety,95 it can be assumed that the beneficial effects on dyspnea are likely to alleviate associated emotional and mood symptoms.
Further research into effective screening, diagnosis, and management of comorbid anxiety and depressive disorders in COPD is warranted, including evaluation of a broad range of nonpharmacologic and drug-based interventions, alone and in combination.76
Conclusions
Anxiety and depression are common, yet frequently overlooked, comorbidities in COPD. The impact of these psychologic comorbidities is significant; their consequences are evident in morbidity and mortality data, as well as in patient-reported outcomes. As key points of contact for patients with COPD, it is essential that primary care practitioners are vigilant in monitoring for anxiety and depression in their patients with COPD, making the most of the available tools that can support them in doing so, and maintain an ongoing line of communication with other members of the multidisciplinary team. Treatment of anxiety and depression in COPD should adopt a holistic approach that incorporates both nonpharmacologic and pharmacologic interventions. However, the impact of effective screening, diagnosis, and management of anxiety and depression on COPD burden in patients requires further investigation.
- Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016;33(2):174-178.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217-223.
- Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976.
- Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229-234.
- Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246-256.
- Maurer J, Rebbapragada V, Borson S, et al; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S-56S.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293-299.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- American Psychiatric Association. Depressive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
- Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33-39.
- Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):pii: 170026.
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858-866.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med. 2010;104(9):1246-1253.
- World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland; World Health Organization: 2017.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330.
- Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res. 2017;11(2):OC24-OC27.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15(12):1090-1096.
- Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766-777.
- Tetikkurt C, Ozdemir I, Tetikkurt S, Yilmaz N, Ertan T, Bayar N. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226-231.
- Yohannes AM, Müllerová H, Hanania NA, et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916-926.
- Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805-2812.
- Kirkil G, Deveci F, Deveci SE, Atmaca M. Anxiety and depression symptoms in patients with chronic obstructive pulmonary disease. Bulletin Clin Psychopharmacol. 2015;25(2):151-161.
- Fuller-Thomson E, Lacombe-Duncan A. Understanding the association between chronic obstructive pulmonary disease and current anxiety: a population-based study. COPD. 2016;13(5):622-631.
- Yohannes AM. Is it quality or quantity of social support needed for patients with chronic medical illness? J Psychosom Res. 2013;74(2):87-88.
- Fabbri LM, Luppi F, Beghe B, Rabe KF. The multiple components of COPD. In: Hanania NA, Sharafkhaneh A, eds. COPD: a guide to diagnosis and clinical management. Humana Press; 2010.
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53.
- Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604-611.
- Zhang Q, Liao J, Liao X, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2014;14:92.
- Jose AK, Chelangara DP, Shaji KS. Factors associated with anxiety and depression in chronic obstructive pulmonary disease. Int J Res Med Sci. 2016;4(4):1074-1079.
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898.
- Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246-1258.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95-109.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60-67.
- Montserrat-Capdevila J, Godoy P, Marsal JR, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung. 2017;195(1):77-85.
- Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918-923.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335-343.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122(5):1633-1637.
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414-419.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65-70.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501-512.
- Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287-1295.
- Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park HK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease [published online ahead of print June 7, 2017]. Korean J Intern Med. 2017;doi: 10.3904/kjim.2016.090.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667-677.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204.
- Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043.
- Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365-1373.
- Busch AM, Scott-Sheldon LA, Pierce J, et al. Depressed mood predicts pulmonary rehabilitation completion among women, but not men. Respir Med. 2014;108(7):1007-1013.
- Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout? J Cardiopulm Rehabil Prev. 2013;33(6):419-426.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105-116.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- Dury R. COPD and emotional distress: not always noticed and therefore untreated. Br J Community Nurs. 2016;21(3):138-141.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5(2):115-128.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed June 2017.
- Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the anxiety inventory for respiratory disease. Chest. 2013;144(5):1587-1596.
- Yohannes AM, Dryden S, Hanania NA. The responsiveness of the anxiety inventory for respiratory disease scale following pulmonary rehabilitation. Chest. 2016;150(1):188-195.
- Kühl K, Kuhn C, Kenn K, Rief W. [The COPD-Anxiety-Questionnaire (CAF): a new instrument to assess illness specific anxiety in COPD patients]. Psychother Psychosom Med Psychol. 2011;61(1):e1-e9. German.
- Tamburrino MB, Lynch DJ, Nagel RW, Smith MK. Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry. 2009;11(6):339-343.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348-353.
- Yohannes AM, Hann M, Sibbald B. The management of depressive symptoms in patients with COPD: a postal survey of general practitioners. Prim Health Care Res Dev. 2011;12(3):237-244.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
- Bratås O, Grønning K, Forbord T. Psychometric properties of the Hospital Anxiety and Depression Scale and The General Health Questionnaire-20 in COPD inpatients. Scand J Caring Sci. 2014;28(2):413-420.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343.
- Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57-65.
- Cantor L, Jacobson R. COPD: How to manage comorbid depression and anxiety. Curr Psychiatry. 2003;2(11):45-54.
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2007;5:17.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297-328.
- Doyle C, Bhar S, Fearn M, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol. 2017;22(3):542-556.
- Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491-496.
- Bennett D, Bowen B, McCarthy P, Subramaniam A, O’Connor M, Henry MT. Outcomes of pulmonary rehabilitation for COPD in older patients: a comparative study. COPD. 2017;14(2):170-175.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open. 2015;5(7):e008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med. 2016;121:109-116.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3: CD010673. doi:10.002/14651858.CD010673.pub2.
- Cafarella P, Effing T, Frith P. An evaluation of the needs of carers of people with COPD. EurResp J. 2012;40(Suppl 56).
- Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zyban/pdf/ZYBAN-PI-MG.PDF. Accessed June 2017.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(10):1.
- Pary R, Matuschka PR, Lewis S, Lippmann S. Managing bipolar depression. Psychiatry (Edgmont). 2006;3(2):30-41.
- Blier P. Atypical antipsychotics for mood and anxiety disorders: safe and effective adjuncts? J Psychiatry Neurosci. 2005;30(4):232-233.
- Vulink NC, Figee M, Denys D. Review of atypical antipsychotics in anxiety. Eur Neuropsychopharmacol. 2011;21(6):429-449.
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi:10.1002/14651858.CD010844.pub2.
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175-1182.
- O’Donnell DE, Webb KA, Harle I, Neder JA. Pharmacological management of breathlessness in COPD: recent advances and hopes for the future. Expert Rev Respir Med. 2016;10(7):823-834.
- Hyun MK, Lee NR, Jang EJ, Yim JJ, Lee CH. Effect of inhaled drugs on anxiety and depression in patients with chronic obstructive pulmonary disease: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:747-754.
Introduction
Anxiety and depression are common in patients with chronic obstructive pulmonary disease (COPD), occurring more frequently than in the general population1-4 or patients with other chronic diseases such as hypertension, diabetes, cancer, or musculoskeletal disorders.5,6 Their presence is associated with worse outcomes of COPD, and increased morbidity, mortality, disability, and health care expenditure.6-8 In spite of this, both anxiety and depression are frequently overlooked and undertreated in patients with COPD,9 and symptoms of anxiety and depression can overlap significantly, as well as overlap with COPD symptoms.7,10
Comorbid depressive disorders that may occur in patients with COPD include major depressive disorder, dysthymias (chronic depressive symptoms of mild severity), and minor depression.11 Depressive disorders are characterized by feelings of sadness, emptiness, and/or irritability, along with cognitive and somatic symptoms, which have a detrimental effect on the patient’s ability to function.11 Anxiety disorders include generalized anxiety disorder (GAD), phobias, and panic disorders.11 The main features of anxiety disorders, such as excessive fear and anxiety, may be accompanied by behavioral disturbances related to these symptoms, such as panic attacks and avoidance.11,12
The reported prevalence of depression in COPD varies widely between studies, owing to differences in sampling methods and degrees of illness severity used in assessment of depression6; rates have been reported to range from 10% to 42% in patients with stable COPD,6,13 and from 10% to 86% in patients with acute COPD exacerbation.14 Individuals with severe COPD are twice as likely to develop depression than patients with mild COPD.10
Prevalence rates for clinical anxiety in COPD range from 13% to 46% in outpatients and 10% to 55% among inpatients. GAD, panic disorders, and specific phobias are reported most frequently.15 Patients with COPD are 85% more likely to develop anxiety disorders compared with matched controls without COPD,4 and panic disorder is reported with a prevalence that is up to 10-fold higher than in the general population.16
Global prevalence rates of anxiety and depression are 1.8- and 1.4-fold higher in women than men, respectively17; the same gender difference is observed in patients with COPD.6 The higher prevalence rates of anxiety and depression in women are thought to be a result of sex differences in brain structure, function, and stress responses, as well as differences in exposure to reproductive hormones, social constraints, and experiences between women and men.18 However, psychologic comorbidity is an issue for both men and women with COPD, so it is important that clinicians are vigilant in recognizing anxiety and depression in both sexes, and are careful not to underestimate the burden in the male patient population.
It is also important to note that depression and anxiety often occur simultaneously in patients with COPD, with prevalence estimates of 26% to 43%.9,19,20 COPD patients with both depression and anxiety are at a heightened risk of suicidal ideation, increased physical disability, and chronic depressive symptoms versus those with either disorder alone.10,15 It is therefore important that comorbid anxiety and depression is not overlooked in patients with COPD.
Ensuring that anxiety and depression are recognized and treated effectively in patients with COPD is essential for optimizing outcomes. Primary care practitioners are well placed to diagnose anxiety and depression, and to ensure these conditions are suitably managed alongside treatments of COPD.
Potential mechanisms of anxiety and depression in COPD
Growing evidence suggests that the relationship between mood disorders—particularly depression—and COPD is bidirectional, meaning that mood disorders adversely impact prognosis in COPD, whereas COPD increases the risk of developing depression.21 For example, in a study of
60 stable patients with COPD, elevated dyspnea and reduced exercise capacity were the predominant mechanisms leading to anxiety and depression symptoms associated with the condition.22 In addition, the risk of new-onset depression was increased in COPD patients with moderate-to-severe dyspnea in a 3-year follow-up study.23 Conversely, depression has been shown to be a significant risk factor for disabling dyspnea (modified Medical Research Council score ≥2) in patients with COPD.24
COPD can lead to feelings of hopelessness, social isolation, reduced physical functioning, and sedentary lifestyle, all of which are associated with an increased level of depressive symptoms.25 Similarly, inadequate social support increases the risk of anxiety in patients with COPD.26 Therefore, ensuring that patients with COPD have high-quality support is very important for reducing anxiety and depressive symptoms.27
The exact mechanisms for the association between mood disorders and COPD remain unclear.7,10 Research to date indicates that the relationship between depression and impaired pulmonary function may be partly mediated by chronic inflammation7,10; systemic inflammation has been associated with other comorbidities of COPD (eg, muscle wasting and osteoporosis),28 and emerging data appear to show that proinflammatory cytokines partly mediate the association between depressive symptoms and pulmonary function.29 Smoking and hypoxemia may also influence the prevalence of depression in COPD, but symptom severity and impaired quality of life remain the most important determinants.6,30
Clinical studies have demonstrated that a number of patient-related factors, including female gender, younger age, current smoking, greater severity of airflow limitation, and lower socioeconomic status, are associated with a higher prevalence and/or increased risk of depression and/or anxiety in COPD.3,4,30,31 Frequent episodes of rehospitalization, and comorbidities such as hypertension, arthritis, cancer, and heart disease, have been found to increase the risk of anxiety and depression in patients with COPD.3,32 Risk of anxiety has been shown to increase with greater dyspnea severity.4 Pain, a frequently overlooked symptom in COPD, has been shown to be associated with symptoms of both anxiety and depression in patients with COPD.33 This is driven by worsened quality of life and sleep quality, decreased physical activity, and an increased fear of movement that occur as a result of pain.34
The impact of anxiety and depression in COPD
Comorbid anxiety and depression have a significant detrimental impact on morbidity and mortality in patients with COPD. Both disorders have been associated with an increased risk of death in COPD.13,35-37 Indeed, of 12 comorbidities proposed to be predictors of mortality in a cohort of 187 female outpatients with COPD, anxiety was associated with the highest risk of death.35,36
In addition, patients with COPD and anxiety and/or depression have a higher risk of COPD exacerbations,4,8,23,36,38-40 hospitalization,41,42 rehospitalization,14,36,43 longer hospital stays,37,41,44 and mortality after exacerbations,14,36,41 compared with patients without these comorbidities. Patients with COPD who have elevated anxiety symptoms also often experience their first hospitalization earlier in the natural course of COPD than those without anxiety.36
Psychologic comorbidities are also associated with worse lung function, dyspnea, and respiratory symptom burden in patients with COPD.37,40 Patients with COPD and anxiety are more likely to experience greater dyspnea at an earlier stage of disease than those without anxiety.36 Persistent smoking at 6 months after hospitalization for an acute exacerbation of COPD is also more likely to be seen in patients with depression.37
Patient-centered outcomes are worse in COPD patients with mood disorders. Both anxiety and depression have been shown to correlate with significantly reduced health-related quality of life (HRQoL), poorer physical health status, functional limitations, and reduced exercise capacity.4,23,37,40,45 The presence of either anxiety or depression at baseline has been shown to correlate with reduced HRQoL at 1-year follow-up, but depression appears to be the stronger predictor of low future HRQoL than anxiety.45
Additionally, mood disorders—particularly depression—reduce physical activity in patients with COPD.46,47 Emotional responses to COPD symptoms, such as dyspnea, can further decrease activity and worsen deconditioning, resulting in a downward spiral of reduced inactivity, social isolation, fear, anxiety, and depression.48
COPD patients with any comorbidity exhibit lower rates of medication adherence than those without comorbidities.49-51 Clinical studies have demonstrated that anxiety and depression are significant predictors of poor adherence to COPD interventions, including pulmonary rehabilitation (PR).51-55 Nonadherence to COPD therapies is associated with poor clinical outcomes, including higher hospitalization rates and increased emergency department visits, and increased costs.56,57 Health care expenditure, in terms of both specific COPD-related costs and general “all-cause” costs, is significantly higher in COPD patients with anxiety and/or depression than in those without.8
Diagnosis of anxiety and depression in patients with COPD
The underdiagnosis and undertreatment of anxiety and depression in this population is common and can adversely affect patient outcomes.6,7,9,10,58 Hence, it is crucial that anxiety and depression are identified and more effectively managed in clinical practice.10
Primary care practitioners are the main point of contact for many patients with COPD,6,59,60 and so can play a key role in screening for and early identification of anxiety and depression. However, detection of mood disorders by primary care practitioners is challenging for several reasons. These include the lack of a standardized approach in diagnosis, and inadequate knowledge or confidence in assessing psychological status (particularly given the number of strategies available for screening patients for mood disorders),6 as well as factors associated with time constraints, such as competing agendas, duration of visits, and high patient load.6,61 Furthermore, system-level barriers, such as lack of electronic medical records and adequate health insurance, as well as any communication gaps between primary care and mental health care, may hinder the detection and management of anxiety and depression.6 In addition, patients themselves may have a limited understanding of these comorbidities, or may be hesitant to discuss symptoms of anxiety or depression with their primary care practitioner owing to stigma around mental illness.6
Patients with COPD should be screened and assessed for anxiety and depression, and the United States Preventive Services Task Force recommends that clinicians screen for depression in all adults.6,62 There are several validated screening tools suitable for clinical use:
- Anxiety Inventory for Respiratory (AIR) Disease scale: a brief, easy-to-use tool for screening and measuring anxiety in COPD.63,64 It is a self-administered scale, and takes approximately 2 minutes to complete. The AIR scale is responsive to PR.64
- COPD Anxiety Questionnaire (CAF): a reliable tool for early identification of COPD-related anxiety.65
- Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire (PHQ; available at: http://www.phqscreeners.com/select-screener/): the PRIME-MD comprises 26 yes/no questions on the 5 most common psychiatric disorders, including depression and anxiety.66,67 This is not a diagnostic tool, but a high number of positive responses from a patient in any given module indicates that they require further clinical evaluation.
- PHQ-2 and PHQ-9 (Table 1; PHQ-9 available at http://www.phqscreeners.com/select-screener/): widely-used self-administered 2- and 9-item versions of the PRIME-MD, specific to depression; similarly, the 3-item PHQ-3 is available for anxiety assessment (Table 2).6,67,68 In a study investigating tools used by family physicians in England to assess depression, over 75% used PHQ-9.69
- Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire-version 20 (GHQ-20): both can be used to screen for psychologic distress in patients with COPD.71
- The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI): two 21-item self-report questionnaires that are widely used in the United States to evaluate anxiety and depression.72
In addition to specific anxiety and depression questionnaires (Tables 1 and 2), more general COPD assessments tools, such as the COPD Assessment Test and the COPD Clinical Questionnaire, also incorporate questions that may be indicative of symptoms of these comorbidities in patients with COPD.73
Management of anxiety and depression in COPD
Even though anxiety and depression are among the most common and burdensome comorbid conditions in COPD, less than one-third of patients with these comorbidities receive effective intervention.6,10 Primary care providers have an excellent opportunity to impact this care gap.
As in non-COPD patients, comorbid depression and anxiety may be treated with nonpharmacologic and/or pharmacologic interventions (Figure 1).76
Nonpharmacologic interventions
Evidence to date suggests that nonpharmacologic interventions such as behavioral therapy are as effective as antidepressants, and may be preferred by patients with mood disorders.12
Cognitive behavioral therapy (CBT), which is typically administered by psychologists/psychiatrists, may be effective in treating COPD-related anxiety and depression, especially in conjunction with exercise and education.12,76,77 Individualized or group CBT is the treatment of choice for addressing thinking patterns that contribute to anxiety and depression to change a patient’s behavior and emotional state.76 PR programs involve several components, including aerobic exercise, lung function training, and psycho-education.62,76 PR is suitable for most patients with COPD, and provides multiple benefits, including reduced hospitalizations in patients who have had a recent exacerbation, and improved dyspnea, exercise tolerance, and health status in patients with stable disease,62 as well as clinically and statistically significant improvements in depression and anxiety, irrespective of age.7,78,79 Exercise-based forms of PR appear to be the most effective for reducing mood symptoms,12,76 and incorporating psychotherapy may also improve psychologic outcomes.80 Stress reduction (relaxation) therapy aims to reduce anxiety-related physiologic changes, and includes a variety of techniques (eg, breathing exercises, sequential muscle relaxation, hypnosis, mindfulness meditation), some of which may be included in PR or used alongside other treatments (eg, CBT).76 Limited data indicate that such therapy may be beneficial for reducing anxiety and depression, as well as respiratory symptoms and dyspnea, in patients with COPD.12,76
Self-management techniques improve clinical outcomes in patients with COPD, but data on the management of depression or anxiety are inconclusive.7,12 A minimal, home-based, nurse-led, psycho-educational intervention was designed to encourage more open-ended, descriptive discussions of thoughts, emotions, behaviors, and bodily sensations in patients with COPD.81 The intervention, which involved nurses attending a 1-hour face-to-face session in the patients’ homes with a 15-minute telephone “booster” session 2 weeks later, helped patients with advanced COPD to self-manage their condition and provide relief from anxiety.81,82 However, it should be noted that there is currently a lack of high-quality data evaluating psychologic interventions in the COPD population.83
In addition, it is important that caregivers are supported in the management of patients with COPD and comorbid anxiety and/or depression; areas in which caregivers can be assisted in their role may include disease education and counseling, where appropriate.84
Given that smoking cessation is a key recommendation for patients with COPD,44,62 practitioners should be aware that patients with comorbid depression and anxiety may experience greater difficulty in smoking cessation, and worsened mood during nicotine withdrawal.44 Clinicians should therefore carefully monitor current smokers with COPD and comorbid depression/anxiety (using the tools described previously63,68,70,71) when they are attempting to quit smoking.
Pharmacologic interventions
Pharmacologic therapy of anxiety and depression has so far only been investigated in patients with COPD in small studies.76 However, the available evidence does not indicate that COPD patients with anxiety and depression should be managed any differently from individuals without COPD.62 As such, pharmacologic interventions are particularly important for patients with acute or severe anxiety or depression.
Antidepressant agents are categorized according to their mechanism of action, and most commonly include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors, bupropion (a norepinephrine- and dopamine-reuptake inhibitor; also approved for smoking cessation85), and mirtazapine (a norepinephrine and serotonin modulator), among others.86 SSRIs are the current firstline drug treatment for depression, and have been shown to significantly improve depression and anxiety in patients with COPD in some, but not all, trials published to date.76 However, it is important to note that a diagnosis of bipolar disorder must be ruled out before initiating standard antidepressant therapy.87 In addition to antidepressants, atypical antipsychotics have also been shown to be useful for treating anxiety, either as monotherapy or combination therapy, and possibly as an adjunctive therapy for the management of depression.88,89
Primary care practitioners can refer to existing guidelines on the management of anxiety and depression in patients with COPD,86,90 while taking certain factors into consideration. Any pharmacologic management strategy for the treatment of COPD may increase the risk of drug–drug or drug–disease interactions.76 For example, it is important to avoid medications that cause respiratory depression (eg, benzodiazepines [unless used with extreme caution], particularly in patients who are already CO2 retainers) or sedation; chosen drugs should have minimal other adverse effects.76 Moreover, SSRIs may also be associated with troublesome adverse effects during treatment initiation, such as gastrointestinal upset, headache, tremor, psychomotor activation, and sedation76; in addition, dry mouth is an adverse effect associated with both SSRI treatment and several inhaled therapies, so may be particularly problematic in patients with COPD.91,92 Currently, data are particularly scarce for the management of anxiety in patients with COPD, with inconclusive or contradictory findings reported for SSRIs, azapirones (including buspirone), and tricyclic antidepressants.76
In addition to monitoring adherence to COPD therapies, primary care practitioners should carefully monitor patients treated with antidepressants and anxiolytics for adherence. A meta-analysis of 18,245 individuals with chronic diseases showed that depressed patients had a 76% significantly higher risk of nonadherence to medication compared with those without depressive symptoms.93
Targeting dyspnea is key to the management of anxiety and depression in COPD, as breathlessness is frequently associated with the onset of both comorbidities.21,22 Therapeutic approaches to alleviating dyspnea include PR, optimizing respiratory mechanics and muscle function (with bronchodilator therapy), and reducing central neural drive to respiratory muscles with supplemental oxygen or opioid medication.94
Although bronchodilator therapy for COPD has not been shown to have significant direct effects on depression or anxiety,95 it can be assumed that the beneficial effects on dyspnea are likely to alleviate associated emotional and mood symptoms.
Further research into effective screening, diagnosis, and management of comorbid anxiety and depressive disorders in COPD is warranted, including evaluation of a broad range of nonpharmacologic and drug-based interventions, alone and in combination.76
Conclusions
Anxiety and depression are common, yet frequently overlooked, comorbidities in COPD. The impact of these psychologic comorbidities is significant; their consequences are evident in morbidity and mortality data, as well as in patient-reported outcomes. As key points of contact for patients with COPD, it is essential that primary care practitioners are vigilant in monitoring for anxiety and depression in their patients with COPD, making the most of the available tools that can support them in doing so, and maintain an ongoing line of communication with other members of the multidisciplinary team. Treatment of anxiety and depression in COPD should adopt a holistic approach that incorporates both nonpharmacologic and pharmacologic interventions. However, the impact of effective screening, diagnosis, and management of anxiety and depression on COPD burden in patients requires further investigation.
Introduction
Anxiety and depression are common in patients with chronic obstructive pulmonary disease (COPD), occurring more frequently than in the general population1-4 or patients with other chronic diseases such as hypertension, diabetes, cancer, or musculoskeletal disorders.5,6 Their presence is associated with worse outcomes of COPD, and increased morbidity, mortality, disability, and health care expenditure.6-8 In spite of this, both anxiety and depression are frequently overlooked and undertreated in patients with COPD,9 and symptoms of anxiety and depression can overlap significantly, as well as overlap with COPD symptoms.7,10
Comorbid depressive disorders that may occur in patients with COPD include major depressive disorder, dysthymias (chronic depressive symptoms of mild severity), and minor depression.11 Depressive disorders are characterized by feelings of sadness, emptiness, and/or irritability, along with cognitive and somatic symptoms, which have a detrimental effect on the patient’s ability to function.11 Anxiety disorders include generalized anxiety disorder (GAD), phobias, and panic disorders.11 The main features of anxiety disorders, such as excessive fear and anxiety, may be accompanied by behavioral disturbances related to these symptoms, such as panic attacks and avoidance.11,12
The reported prevalence of depression in COPD varies widely between studies, owing to differences in sampling methods and degrees of illness severity used in assessment of depression6; rates have been reported to range from 10% to 42% in patients with stable COPD,6,13 and from 10% to 86% in patients with acute COPD exacerbation.14 Individuals with severe COPD are twice as likely to develop depression than patients with mild COPD.10
Prevalence rates for clinical anxiety in COPD range from 13% to 46% in outpatients and 10% to 55% among inpatients. GAD, panic disorders, and specific phobias are reported most frequently.15 Patients with COPD are 85% more likely to develop anxiety disorders compared with matched controls without COPD,4 and panic disorder is reported with a prevalence that is up to 10-fold higher than in the general population.16
Global prevalence rates of anxiety and depression are 1.8- and 1.4-fold higher in women than men, respectively17; the same gender difference is observed in patients with COPD.6 The higher prevalence rates of anxiety and depression in women are thought to be a result of sex differences in brain structure, function, and stress responses, as well as differences in exposure to reproductive hormones, social constraints, and experiences between women and men.18 However, psychologic comorbidity is an issue for both men and women with COPD, so it is important that clinicians are vigilant in recognizing anxiety and depression in both sexes, and are careful not to underestimate the burden in the male patient population.
It is also important to note that depression and anxiety often occur simultaneously in patients with COPD, with prevalence estimates of 26% to 43%.9,19,20 COPD patients with both depression and anxiety are at a heightened risk of suicidal ideation, increased physical disability, and chronic depressive symptoms versus those with either disorder alone.10,15 It is therefore important that comorbid anxiety and depression is not overlooked in patients with COPD.
Ensuring that anxiety and depression are recognized and treated effectively in patients with COPD is essential for optimizing outcomes. Primary care practitioners are well placed to diagnose anxiety and depression, and to ensure these conditions are suitably managed alongside treatments of COPD.
Potential mechanisms of anxiety and depression in COPD
Growing evidence suggests that the relationship between mood disorders—particularly depression—and COPD is bidirectional, meaning that mood disorders adversely impact prognosis in COPD, whereas COPD increases the risk of developing depression.21 For example, in a study of
60 stable patients with COPD, elevated dyspnea and reduced exercise capacity were the predominant mechanisms leading to anxiety and depression symptoms associated with the condition.22 In addition, the risk of new-onset depression was increased in COPD patients with moderate-to-severe dyspnea in a 3-year follow-up study.23 Conversely, depression has been shown to be a significant risk factor for disabling dyspnea (modified Medical Research Council score ≥2) in patients with COPD.24
COPD can lead to feelings of hopelessness, social isolation, reduced physical functioning, and sedentary lifestyle, all of which are associated with an increased level of depressive symptoms.25 Similarly, inadequate social support increases the risk of anxiety in patients with COPD.26 Therefore, ensuring that patients with COPD have high-quality support is very important for reducing anxiety and depressive symptoms.27
The exact mechanisms for the association between mood disorders and COPD remain unclear.7,10 Research to date indicates that the relationship between depression and impaired pulmonary function may be partly mediated by chronic inflammation7,10; systemic inflammation has been associated with other comorbidities of COPD (eg, muscle wasting and osteoporosis),28 and emerging data appear to show that proinflammatory cytokines partly mediate the association between depressive symptoms and pulmonary function.29 Smoking and hypoxemia may also influence the prevalence of depression in COPD, but symptom severity and impaired quality of life remain the most important determinants.6,30
Clinical studies have demonstrated that a number of patient-related factors, including female gender, younger age, current smoking, greater severity of airflow limitation, and lower socioeconomic status, are associated with a higher prevalence and/or increased risk of depression and/or anxiety in COPD.3,4,30,31 Frequent episodes of rehospitalization, and comorbidities such as hypertension, arthritis, cancer, and heart disease, have been found to increase the risk of anxiety and depression in patients with COPD.3,32 Risk of anxiety has been shown to increase with greater dyspnea severity.4 Pain, a frequently overlooked symptom in COPD, has been shown to be associated with symptoms of both anxiety and depression in patients with COPD.33 This is driven by worsened quality of life and sleep quality, decreased physical activity, and an increased fear of movement that occur as a result of pain.34
The impact of anxiety and depression in COPD
Comorbid anxiety and depression have a significant detrimental impact on morbidity and mortality in patients with COPD. Both disorders have been associated with an increased risk of death in COPD.13,35-37 Indeed, of 12 comorbidities proposed to be predictors of mortality in a cohort of 187 female outpatients with COPD, anxiety was associated with the highest risk of death.35,36
In addition, patients with COPD and anxiety and/or depression have a higher risk of COPD exacerbations,4,8,23,36,38-40 hospitalization,41,42 rehospitalization,14,36,43 longer hospital stays,37,41,44 and mortality after exacerbations,14,36,41 compared with patients without these comorbidities. Patients with COPD who have elevated anxiety symptoms also often experience their first hospitalization earlier in the natural course of COPD than those without anxiety.36
Psychologic comorbidities are also associated with worse lung function, dyspnea, and respiratory symptom burden in patients with COPD.37,40 Patients with COPD and anxiety are more likely to experience greater dyspnea at an earlier stage of disease than those without anxiety.36 Persistent smoking at 6 months after hospitalization for an acute exacerbation of COPD is also more likely to be seen in patients with depression.37
Patient-centered outcomes are worse in COPD patients with mood disorders. Both anxiety and depression have been shown to correlate with significantly reduced health-related quality of life (HRQoL), poorer physical health status, functional limitations, and reduced exercise capacity.4,23,37,40,45 The presence of either anxiety or depression at baseline has been shown to correlate with reduced HRQoL at 1-year follow-up, but depression appears to be the stronger predictor of low future HRQoL than anxiety.45
Additionally, mood disorders—particularly depression—reduce physical activity in patients with COPD.46,47 Emotional responses to COPD symptoms, such as dyspnea, can further decrease activity and worsen deconditioning, resulting in a downward spiral of reduced inactivity, social isolation, fear, anxiety, and depression.48
COPD patients with any comorbidity exhibit lower rates of medication adherence than those without comorbidities.49-51 Clinical studies have demonstrated that anxiety and depression are significant predictors of poor adherence to COPD interventions, including pulmonary rehabilitation (PR).51-55 Nonadherence to COPD therapies is associated with poor clinical outcomes, including higher hospitalization rates and increased emergency department visits, and increased costs.56,57 Health care expenditure, in terms of both specific COPD-related costs and general “all-cause” costs, is significantly higher in COPD patients with anxiety and/or depression than in those without.8
Diagnosis of anxiety and depression in patients with COPD
The underdiagnosis and undertreatment of anxiety and depression in this population is common and can adversely affect patient outcomes.6,7,9,10,58 Hence, it is crucial that anxiety and depression are identified and more effectively managed in clinical practice.10
Primary care practitioners are the main point of contact for many patients with COPD,6,59,60 and so can play a key role in screening for and early identification of anxiety and depression. However, detection of mood disorders by primary care practitioners is challenging for several reasons. These include the lack of a standardized approach in diagnosis, and inadequate knowledge or confidence in assessing psychological status (particularly given the number of strategies available for screening patients for mood disorders),6 as well as factors associated with time constraints, such as competing agendas, duration of visits, and high patient load.6,61 Furthermore, system-level barriers, such as lack of electronic medical records and adequate health insurance, as well as any communication gaps between primary care and mental health care, may hinder the detection and management of anxiety and depression.6 In addition, patients themselves may have a limited understanding of these comorbidities, or may be hesitant to discuss symptoms of anxiety or depression with their primary care practitioner owing to stigma around mental illness.6
Patients with COPD should be screened and assessed for anxiety and depression, and the United States Preventive Services Task Force recommends that clinicians screen for depression in all adults.6,62 There are several validated screening tools suitable for clinical use:
- Anxiety Inventory for Respiratory (AIR) Disease scale: a brief, easy-to-use tool for screening and measuring anxiety in COPD.63,64 It is a self-administered scale, and takes approximately 2 minutes to complete. The AIR scale is responsive to PR.64
- COPD Anxiety Questionnaire (CAF): a reliable tool for early identification of COPD-related anxiety.65
- Primary Care Evaluation of Mental Disorders (PRIME-MD) Patient Health Questionnaire (PHQ; available at: http://www.phqscreeners.com/select-screener/): the PRIME-MD comprises 26 yes/no questions on the 5 most common psychiatric disorders, including depression and anxiety.66,67 This is not a diagnostic tool, but a high number of positive responses from a patient in any given module indicates that they require further clinical evaluation.
- PHQ-2 and PHQ-9 (Table 1; PHQ-9 available at http://www.phqscreeners.com/select-screener/): widely-used self-administered 2- and 9-item versions of the PRIME-MD, specific to depression; similarly, the 3-item PHQ-3 is available for anxiety assessment (Table 2).6,67,68 In a study investigating tools used by family physicians in England to assess depression, over 75% used PHQ-9.69
- Hospital Anxiety and Depression Scale (HADS) and General Health Questionnaire-version 20 (GHQ-20): both can be used to screen for psychologic distress in patients with COPD.71
- The Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI): two 21-item self-report questionnaires that are widely used in the United States to evaluate anxiety and depression.72
In addition to specific anxiety and depression questionnaires (Tables 1 and 2), more general COPD assessments tools, such as the COPD Assessment Test and the COPD Clinical Questionnaire, also incorporate questions that may be indicative of symptoms of these comorbidities in patients with COPD.73
Management of anxiety and depression in COPD
Even though anxiety and depression are among the most common and burdensome comorbid conditions in COPD, less than one-third of patients with these comorbidities receive effective intervention.6,10 Primary care providers have an excellent opportunity to impact this care gap.
As in non-COPD patients, comorbid depression and anxiety may be treated with nonpharmacologic and/or pharmacologic interventions (Figure 1).76
Nonpharmacologic interventions
Evidence to date suggests that nonpharmacologic interventions such as behavioral therapy are as effective as antidepressants, and may be preferred by patients with mood disorders.12
Cognitive behavioral therapy (CBT), which is typically administered by psychologists/psychiatrists, may be effective in treating COPD-related anxiety and depression, especially in conjunction with exercise and education.12,76,77 Individualized or group CBT is the treatment of choice for addressing thinking patterns that contribute to anxiety and depression to change a patient’s behavior and emotional state.76 PR programs involve several components, including aerobic exercise, lung function training, and psycho-education.62,76 PR is suitable for most patients with COPD, and provides multiple benefits, including reduced hospitalizations in patients who have had a recent exacerbation, and improved dyspnea, exercise tolerance, and health status in patients with stable disease,62 as well as clinically and statistically significant improvements in depression and anxiety, irrespective of age.7,78,79 Exercise-based forms of PR appear to be the most effective for reducing mood symptoms,12,76 and incorporating psychotherapy may also improve psychologic outcomes.80 Stress reduction (relaxation) therapy aims to reduce anxiety-related physiologic changes, and includes a variety of techniques (eg, breathing exercises, sequential muscle relaxation, hypnosis, mindfulness meditation), some of which may be included in PR or used alongside other treatments (eg, CBT).76 Limited data indicate that such therapy may be beneficial for reducing anxiety and depression, as well as respiratory symptoms and dyspnea, in patients with COPD.12,76
Self-management techniques improve clinical outcomes in patients with COPD, but data on the management of depression or anxiety are inconclusive.7,12 A minimal, home-based, nurse-led, psycho-educational intervention was designed to encourage more open-ended, descriptive discussions of thoughts, emotions, behaviors, and bodily sensations in patients with COPD.81 The intervention, which involved nurses attending a 1-hour face-to-face session in the patients’ homes with a 15-minute telephone “booster” session 2 weeks later, helped patients with advanced COPD to self-manage their condition and provide relief from anxiety.81,82 However, it should be noted that there is currently a lack of high-quality data evaluating psychologic interventions in the COPD population.83
In addition, it is important that caregivers are supported in the management of patients with COPD and comorbid anxiety and/or depression; areas in which caregivers can be assisted in their role may include disease education and counseling, where appropriate.84
Given that smoking cessation is a key recommendation for patients with COPD,44,62 practitioners should be aware that patients with comorbid depression and anxiety may experience greater difficulty in smoking cessation, and worsened mood during nicotine withdrawal.44 Clinicians should therefore carefully monitor current smokers with COPD and comorbid depression/anxiety (using the tools described previously63,68,70,71) when they are attempting to quit smoking.
Pharmacologic interventions
Pharmacologic therapy of anxiety and depression has so far only been investigated in patients with COPD in small studies.76 However, the available evidence does not indicate that COPD patients with anxiety and depression should be managed any differently from individuals without COPD.62 As such, pharmacologic interventions are particularly important for patients with acute or severe anxiety or depression.
Antidepressant agents are categorized according to their mechanism of action, and most commonly include selective serotonin-reuptake inhibitors (SSRIs), selective norepinephrine-reuptake inhibitors, bupropion (a norepinephrine- and dopamine-reuptake inhibitor; also approved for smoking cessation85), and mirtazapine (a norepinephrine and serotonin modulator), among others.86 SSRIs are the current firstline drug treatment for depression, and have been shown to significantly improve depression and anxiety in patients with COPD in some, but not all, trials published to date.76 However, it is important to note that a diagnosis of bipolar disorder must be ruled out before initiating standard antidepressant therapy.87 In addition to antidepressants, atypical antipsychotics have also been shown to be useful for treating anxiety, either as monotherapy or combination therapy, and possibly as an adjunctive therapy for the management of depression.88,89
Primary care practitioners can refer to existing guidelines on the management of anxiety and depression in patients with COPD,86,90 while taking certain factors into consideration. Any pharmacologic management strategy for the treatment of COPD may increase the risk of drug–drug or drug–disease interactions.76 For example, it is important to avoid medications that cause respiratory depression (eg, benzodiazepines [unless used with extreme caution], particularly in patients who are already CO2 retainers) or sedation; chosen drugs should have minimal other adverse effects.76 Moreover, SSRIs may also be associated with troublesome adverse effects during treatment initiation, such as gastrointestinal upset, headache, tremor, psychomotor activation, and sedation76; in addition, dry mouth is an adverse effect associated with both SSRI treatment and several inhaled therapies, so may be particularly problematic in patients with COPD.91,92 Currently, data are particularly scarce for the management of anxiety in patients with COPD, with inconclusive or contradictory findings reported for SSRIs, azapirones (including buspirone), and tricyclic antidepressants.76
In addition to monitoring adherence to COPD therapies, primary care practitioners should carefully monitor patients treated with antidepressants and anxiolytics for adherence. A meta-analysis of 18,245 individuals with chronic diseases showed that depressed patients had a 76% significantly higher risk of nonadherence to medication compared with those without depressive symptoms.93
Targeting dyspnea is key to the management of anxiety and depression in COPD, as breathlessness is frequently associated with the onset of both comorbidities.21,22 Therapeutic approaches to alleviating dyspnea include PR, optimizing respiratory mechanics and muscle function (with bronchodilator therapy), and reducing central neural drive to respiratory muscles with supplemental oxygen or opioid medication.94
Although bronchodilator therapy for COPD has not been shown to have significant direct effects on depression or anxiety,95 it can be assumed that the beneficial effects on dyspnea are likely to alleviate associated emotional and mood symptoms.
Further research into effective screening, diagnosis, and management of comorbid anxiety and depressive disorders in COPD is warranted, including evaluation of a broad range of nonpharmacologic and drug-based interventions, alone and in combination.76
Conclusions
Anxiety and depression are common, yet frequently overlooked, comorbidities in COPD. The impact of these psychologic comorbidities is significant; their consequences are evident in morbidity and mortality data, as well as in patient-reported outcomes. As key points of contact for patients with COPD, it is essential that primary care practitioners are vigilant in monitoring for anxiety and depression in their patients with COPD, making the most of the available tools that can support them in doing so, and maintain an ongoing line of communication with other members of the multidisciplinary team. Treatment of anxiety and depression in COPD should adopt a holistic approach that incorporates both nonpharmacologic and pharmacologic interventions. However, the impact of effective screening, diagnosis, and management of anxiety and depression on COPD burden in patients requires further investigation.
- Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016;33(2):174-178.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217-223.
- Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976.
- Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229-234.
- Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246-256.
- Maurer J, Rebbapragada V, Borson S, et al; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S-56S.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293-299.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- American Psychiatric Association. Depressive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
- Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33-39.
- Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):pii: 170026.
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858-866.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med. 2010;104(9):1246-1253.
- World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland; World Health Organization: 2017.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330.
- Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res. 2017;11(2):OC24-OC27.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15(12):1090-1096.
- Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766-777.
- Tetikkurt C, Ozdemir I, Tetikkurt S, Yilmaz N, Ertan T, Bayar N. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226-231.
- Yohannes AM, Müllerová H, Hanania NA, et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916-926.
- Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805-2812.
- Kirkil G, Deveci F, Deveci SE, Atmaca M. Anxiety and depression symptoms in patients with chronic obstructive pulmonary disease. Bulletin Clin Psychopharmacol. 2015;25(2):151-161.
- Fuller-Thomson E, Lacombe-Duncan A. Understanding the association between chronic obstructive pulmonary disease and current anxiety: a population-based study. COPD. 2016;13(5):622-631.
- Yohannes AM. Is it quality or quantity of social support needed for patients with chronic medical illness? J Psychosom Res. 2013;74(2):87-88.
- Fabbri LM, Luppi F, Beghe B, Rabe KF. The multiple components of COPD. In: Hanania NA, Sharafkhaneh A, eds. COPD: a guide to diagnosis and clinical management. Humana Press; 2010.
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53.
- Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604-611.
- Zhang Q, Liao J, Liao X, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2014;14:92.
- Jose AK, Chelangara DP, Shaji KS. Factors associated with anxiety and depression in chronic obstructive pulmonary disease. Int J Res Med Sci. 2016;4(4):1074-1079.
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898.
- Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246-1258.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95-109.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60-67.
- Montserrat-Capdevila J, Godoy P, Marsal JR, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung. 2017;195(1):77-85.
- Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918-923.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335-343.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122(5):1633-1637.
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414-419.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65-70.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501-512.
- Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287-1295.
- Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park HK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease [published online ahead of print June 7, 2017]. Korean J Intern Med. 2017;doi: 10.3904/kjim.2016.090.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667-677.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204.
- Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043.
- Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365-1373.
- Busch AM, Scott-Sheldon LA, Pierce J, et al. Depressed mood predicts pulmonary rehabilitation completion among women, but not men. Respir Med. 2014;108(7):1007-1013.
- Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout? J Cardiopulm Rehabil Prev. 2013;33(6):419-426.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105-116.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- Dury R. COPD and emotional distress: not always noticed and therefore untreated. Br J Community Nurs. 2016;21(3):138-141.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5(2):115-128.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed June 2017.
- Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the anxiety inventory for respiratory disease. Chest. 2013;144(5):1587-1596.
- Yohannes AM, Dryden S, Hanania NA. The responsiveness of the anxiety inventory for respiratory disease scale following pulmonary rehabilitation. Chest. 2016;150(1):188-195.
- Kühl K, Kuhn C, Kenn K, Rief W. [The COPD-Anxiety-Questionnaire (CAF): a new instrument to assess illness specific anxiety in COPD patients]. Psychother Psychosom Med Psychol. 2011;61(1):e1-e9. German.
- Tamburrino MB, Lynch DJ, Nagel RW, Smith MK. Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry. 2009;11(6):339-343.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348-353.
- Yohannes AM, Hann M, Sibbald B. The management of depressive symptoms in patients with COPD: a postal survey of general practitioners. Prim Health Care Res Dev. 2011;12(3):237-244.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
- Bratås O, Grønning K, Forbord T. Psychometric properties of the Hospital Anxiety and Depression Scale and The General Health Questionnaire-20 in COPD inpatients. Scand J Caring Sci. 2014;28(2):413-420.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343.
- Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57-65.
- Cantor L, Jacobson R. COPD: How to manage comorbid depression and anxiety. Curr Psychiatry. 2003;2(11):45-54.
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2007;5:17.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297-328.
- Doyle C, Bhar S, Fearn M, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol. 2017;22(3):542-556.
- Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491-496.
- Bennett D, Bowen B, McCarthy P, Subramaniam A, O’Connor M, Henry MT. Outcomes of pulmonary rehabilitation for COPD in older patients: a comparative study. COPD. 2017;14(2):170-175.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open. 2015;5(7):e008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med. 2016;121:109-116.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3: CD010673. doi:10.002/14651858.CD010673.pub2.
- Cafarella P, Effing T, Frith P. An evaluation of the needs of carers of people with COPD. EurResp J. 2012;40(Suppl 56).
- Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zyban/pdf/ZYBAN-PI-MG.PDF. Accessed June 2017.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(10):1.
- Pary R, Matuschka PR, Lewis S, Lippmann S. Managing bipolar depression. Psychiatry (Edgmont). 2006;3(2):30-41.
- Blier P. Atypical antipsychotics for mood and anxiety disorders: safe and effective adjuncts? J Psychiatry Neurosci. 2005;30(4):232-233.
- Vulink NC, Figee M, Denys D. Review of atypical antipsychotics in anxiety. Eur Neuropsychopharmacol. 2011;21(6):429-449.
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi:10.1002/14651858.CD010844.pub2.
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175-1182.
- O’Donnell DE, Webb KA, Harle I, Neder JA. Pharmacological management of breathlessness in COPD: recent advances and hopes for the future. Expert Rev Respir Med. 2016;10(7):823-834.
- Hyun MK, Lee NR, Jang EJ, Yim JJ, Lee CH. Effect of inhaled drugs on anxiety and depression in patients with chronic obstructive pulmonary disease: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:747-754.
- Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016;33(2):174-178.
- Zhang MW, Ho RC, Cheung MW, Fu E, Mak A. Prevalence of depressive symptoms in patients with chronic obstructive pulmonary disease: a systematic review, meta-analysis and meta-regression. Gen Hosp Psychiatry. 2011;33(3):217-223.
- Tsai TY, Livneh H, Lu MC, Tsai PY, Chen PC, Sung FC. Increased risk and related factors of depression among patients with COPD: a population-based cohort study. BMC Public Health. 2013;13:976.
- Eisner MD, Blanc PD, Yelin EH, et al. Influence of anxiety on health outcomes in COPD. Thorax. 2010;65(3):229-234.
- Marsh S, Guck TP. Anxiety and depression: easing the burden in COPD patients. J Fam Pract. 2016;65(4):246-256.
- Maurer J, Rebbapragada V, Borson S, et al; ACCP Workshop Panel on Anxiety and Depression in COPD. Anxiety and depression in COPD: current understanding, unanswered questions, and research needs. Chest. 2008;134(4 Suppl):43S-56S.
- Pumar MI, Gray CR, Walsh JR, Yang IA, Rolls TA, Ward DL. Anxiety and depression—important psychological comorbidities of COPD. J Thorac Dis. 2014;6(11):1615-1631.
- Dalal AA, Shah M, Lunacsek O, Hanania NA. Clinical and economic burden of depression/anxiety in chronic obstructive pulmonary disease patients within a managed care population. COPD. 2011;8(4):293-299.
- Kunik ME, Roundy K, Veazey C, et al. Surprisingly high prevalence of anxiety and depression in chronic breathing disorders. Chest. 2005;127(4):1205-1211.
- Yohannes AM, Alexopoulos GS. Depression and anxiety in patients with COPD. Eur Respir Rev. 2014;23(133):345-349.
- American Psychiatric Association. Depressive Disorders. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
- Panagioti M, Scott C, Blakemore A, Coventry PA. Overview of the prevalence, impact, and management of depression and anxiety in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2014;9:1289-1306.
- Laforest L, Roche N, Devouassoux G, et al. Frequency of comorbidities in chronic obstructive pulmonary disease, and impact on all-cause mortality: a population-based cohort study. Respir Med. 2016;117:33-39.
- Lecheler L, Richter M, Franzen DP, et al. The frequent and underrecognised co-occurrence of acute exacerbated COPD and depression warrants screening: a systematic review. Eur Respir Rev. 2017;26(144):pii: 170026.
- Willgoss TG, Yohannes AM. Anxiety disorders in patients with COPD: a systematic review. Respir Care. 2013;58(5):858-866.
- Livermore N, Sharpe L, McKenzie D. Panic attacks and panic disorder in chronic obstructive pulmonary disease: a cognitive behavioral perspective. Respir Med. 2010;104(9):1246-1253.
- World Health Organization. Depression and other common mental disorders: global health estimates. Geneva, Switzerland; World Health Organization: 2017.
- Altemus M, Sarvaiya N, Neill Epperson C. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320-330.
- Biswas D, Mukherjee S, Chakroborty R, et al. Occurrence of anxiety and depression among stable COPD patients and its impact on functional capability. J Clin Diagn Res. 2017;11(2):OC24-OC27.
- Yohannes AM, Baldwin RC, Connolly MJ. Depression and anxiety in elderly outpatients with chronic obstructive pulmonary disease: prevalence, and validation of the BASDEC screening questionnaire. Int J Geriatr Psychiatry. 2000;15(12):1090-1096.
- Atlantis E, Fahey P, Cochrane B, Smith S. Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta-analysis. Chest. 2013;144(3):766-777.
- Tetikkurt C, Ozdemir I, Tetikkurt S, Yilmaz N, Ertan T, Bayar N. Anxiety and depression in COPD patients and correlation with sputum and BAL cytology. Multidiscip Respir Med. 2011;6(4):226-231.
- Yohannes AM, Müllerová H, Hanania NA, et al. Long-term course of depression trajectories in patients with COPD: a 3-year follow-up analysis of the evaluation of COPD longitudinally to identify predictive surrogate endpoints cohort. Chest. 2016;149(4):916-926.
- Sundh J, Ekström M. Persistent disabling breathlessness in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2016;11:2805-2812.
- Kirkil G, Deveci F, Deveci SE, Atmaca M. Anxiety and depression symptoms in patients with chronic obstructive pulmonary disease. Bulletin Clin Psychopharmacol. 2015;25(2):151-161.
- Fuller-Thomson E, Lacombe-Duncan A. Understanding the association between chronic obstructive pulmonary disease and current anxiety: a population-based study. COPD. 2016;13(5):622-631.
- Yohannes AM. Is it quality or quantity of social support needed for patients with chronic medical illness? J Psychosom Res. 2013;74(2):87-88.
- Fabbri LM, Luppi F, Beghe B, Rabe KF. The multiple components of COPD. In: Hanania NA, Sharafkhaneh A, eds. COPD: a guide to diagnosis and clinical management. Humana Press; 2010.
- Lu Y, Feng L, Feng L, Nyunt MS, Yap KB, Ng TP. Systemic inflammation, depression and obstructive pulmonary function: a population-based study. Respir Res. 2013;14:53.
- Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604-611.
- Zhang Q, Liao J, Liao X, et al. Disease knowledge level is a noteworthy risk factor of anxiety and depression in patients with chronic obstructive pulmonary disease: a cross-sectional study. BMC Pulm Med. 2014;14:92.
- Jose AK, Chelangara DP, Shaji KS. Factors associated with anxiety and depression in chronic obstructive pulmonary disease. Int J Res Med Sci. 2016;4(4):1074-1079.
- van Dam van Isselt EF, Groenewegen-Sipkema KH, Spruit-van Eijk M, et al. Pain in patients with COPD: a systematic review and meta-analysis. BMJ Open. 2014;4(9):e005898.
- Lee AL, Harrison SL, Goldstein RS, Brooks D. Pain and its clinical associations in individuals with COPD: a systematic review. Chest. 2015;147(5):1246-1258.
- Divo M, Cote C, de Torres JP, et al; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;186(2):155-161.
- Hillas G, Perlikos F, Tsiligianni I, Tzanakis N. Managing comorbidities in COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:95-109.
- Ng TP, Niti M, Tan WC, Cao Z, Ong KC, Eng P. Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007;167(1):60-67.
- Montserrat-Capdevila J, Godoy P, Marsal JR, et al. Overview of the impact of depression and anxiety in chronic obstructive pulmonary disease. Lung. 2017;195(1):77-85.
- Laurin C, Moullec G, Bacon SL, Lavoie KL. Impact of anxiety and depression on chronic obstructive pulmonary disease exacerbation risk. Am J Respir Crit Care Med. 2012;185(9):918-923.
- Martinez Rivera C, Costan Galicia J, Alcázar Navarrete B, et al. Factors associated with depression in COPD: a multicenter study. Lung. 2016;194(3):335-343.
- Pooler A, Beech R. Examining the relationship between anxiety and depression and exacerbations of COPD which result in hospital admission: a systematic review. Int J Chron Obstruct Pulmon Dis. 2014;9:315-330.
- Dahlén I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122(5):1633-1637.
- Gudmundsson G, Gislason T, Janson C, et al. Risk factors for rehospitalisation in COPD: role of health status, anxiety and depression. Eur Respir J. 2005;26(3):414-419.
- Mikkelsen RL, Middelboe T, Pisinger C, Stage KB. Anxiety and depression in patients with chronic obstructive pulmonary disease (COPD). A review. Nord J Psychiatry. 2004;58(1):65-70.
- Blakemore A, Dickens C, Guthrie E, et al. Depression and anxiety predict health-related quality of life in chronic obstructive pulmonary disease: systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2014;9:501-512.
- Dueñas-Espín I, Demeyer H, Gimeno-Santos E, et al. Depression symptoms reduce physical activity in COPD patients: a prospective multicenter study. Int J Chron Obstruct Pulmon Dis. 2016;11:1287-1295.
- Lee SH, Kim KU, Lee H, Kim YS, Lee MK, Park HK. Factors associated with low-level physical activity in elderly patients with chronic obstructive pulmonary disease [published online ahead of print June 7, 2017]. Korean J Intern Med. 2017;doi: 10.3904/kjim.2016.090.
- Hill K, Geist R, Goldstein RS, Lacasse Y. Anxiety and depression in end-stage COPD. Eur Respir J. 2008;31(3):667-677.
- George J, Kong DC, Thoman R, Stewart K. Factors associated with medication nonadherence in patients with COPD. Chest. 2005;128(5):3198-3204.
- Morrison D, Agur K, Mercer S, Eiras A, González-Montalvo JI, Gruffydd-Jones K. Managing multimorbidity in primary care in patients with chronic respiratory conditions. NPJ Prim Care Respir Med. 2016;26:16043.
- Khdour MR, Hawwa AF, Kidney JC, Smyth BM, McElnay JC. Potential risk factors for medication non-adherence in patients with chronic obstructive pulmonary disease (COPD). Eur J Clin Pharmacol. 2012;68(10):1365-1373.
- Busch AM, Scott-Sheldon LA, Pierce J, et al. Depressed mood predicts pulmonary rehabilitation completion among women, but not men. Respir Med. 2014;108(7):1007-1013.
- Heerema-Poelman A, Stuive I, Wempe JB. Adherence to a maintenance exercise program 1 year after pulmonary rehabilitation: what are the predictors of dropout? J Cardiopulm Rehabil Prev. 2013;33(6):419-426.
- DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101-2107.
- Fan VS, Giardino ND, Blough DK, Kaplan RM, Ramsey SD; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008;5(2):105-116.
- Bourbeau J, Bartlett SJ. Patient adherence in COPD. Thorax. 2008;63(9):831-838.
- van Boven JF, Chavannes NH, van der Molen T, Rutten-van Mölken MP, Postma MJ, Vegter S. Clinical and economic impact of non-adherence in COPD: a systematic review. Respir Med. 2014;108(1):103-113.
- Dury R. COPD and emotional distress: not always noticed and therefore untreated. Br J Community Nurs. 2016;21(3):138-141.
- Price D, Crockett A, Arne M, et al. Spirometry in primary care case-identification, diagnosis and management of COPD. Prim Care Respir J. 2009;18(3):216-223.
- van Boven JF, Ryan D, Eakin MN, Canonica GW, Barot A, Foster JM; Respiratory Effectiveness Group. Enhancing respiratory medication adherence: the role of health care professionals and cost-effectiveness considerations. J Allergy Clin Immunol Pract. 2016;4(5):835-846.
- Wittchen HU, Mühlig S, Beesdo K. Mental disorders in primary care. Dialogues Clin Neurosci. 2003;5(2):115-128.
- Global Initiative for Chronic Obstructive Lung Disease. GOLD 2017 Global Strategy for the Diagnosis, Management and Prevention of COPD. http://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd. Accessed June 2017.
- Willgoss TG, Goldbart J, Fatoye F, Yohannes AM. The development and validation of the anxiety inventory for respiratory disease. Chest. 2013;144(5):1587-1596.
- Yohannes AM, Dryden S, Hanania NA. The responsiveness of the anxiety inventory for respiratory disease scale following pulmonary rehabilitation. Chest. 2016;150(1):188-195.
- Kühl K, Kuhn C, Kenn K, Rief W. [The COPD-Anxiety-Questionnaire (CAF): a new instrument to assess illness specific anxiety in COPD patients]. Psychother Psychosom Med Psychol. 2011;61(1):e1-e9. German.
- Tamburrino MB, Lynch DJ, Nagel RW, Smith MK. Primary care evaluation of mental disorders (PRIME-MD) screening for minor depressive disorder in primary care. Prim Care Companion J Clin Psychiatry. 2009;11(6):339-343.
- Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999;282(18):1737-1744.
- Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348-353.
- Yohannes AM, Hann M, Sibbald B. The management of depressive symptoms in patients with COPD: a postal survey of general practitioners. Prim Health Care Res Dev. 2011;12(3):237-244.
- Spitzer RL, Kroenke K, Williams JB, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med. 2006;166(10):1092-1097.
- Bratås O, Grønning K, Forbord T. Psychometric properties of the Hospital Anxiety and Depression Scale and The General Health Questionnaire-20 in COPD inpatients. Scand J Caring Sci. 2014;28(2):413-420.
- Lovibond PF, Lovibond SH. The structure of negative emotional states: comparison of the Depression Anxiety Stress Scales (DASS) with the Beck Depression and Anxiety Inventories. Behav Res Ther. 1995;33(3):335-343.
- Sundh J, Ställberg B, Lisspers K, Kämpe M, Janson C, Montgomery S. Comparison of the COPD Assessment Test (CAT) and the Clinical COPD Questionnaire (CCQ) in a clinical population. COPD. 2016;13(1):57-65.
- Cantor L, Jacobson R. COPD: How to manage comorbid depression and anxiety. Curr Psychiatry. 2003;2(11):45-54.
- Yohannes AM. Palliative care provision for patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes. 2007;5:17.
- Tselebis A, Pachi A, Ilias I, et al. Strategies to improve anxiety and depression in patients with COPD: a mental health perspective. Neuropsychiatr Dis Treat. 2016;12:297-328.
- Doyle C, Bhar S, Fearn M, et al. The impact of telephone-delivered cognitive behaviour therapy and befriending on mood disorders in people with chronic obstructive pulmonary disease: a randomized controlled trial. Br J Health Psychol. 2017;22(3):542-556.
- Alsaraireh FA, Aloush SA. Does pulmonary rehabilitation alleviate depression in older patients with chronic obstructive pulmonary disease. Saudi Med J. 2017;38(5):491-496.
- Bennett D, Bowen B, McCarthy P, Subramaniam A, O’Connor M, Henry MT. Outcomes of pulmonary rehabilitation for COPD in older patients: a comparative study. COPD. 2017;14(2):170-175.
- Smith SM, Sonego S, Ketcheson L, Larson JL. A review of the effectiveness of psychological interventions used for anxiety and depression in chronic obstructive pulmonary disease. BMJ Open Respir Res. 2014;1(1):e000042.
- Bove DG, Overgaard D, Lomborg K, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention versus usual care for managing anxiety and dyspnoea in patients with severe chronic obstructive pulmonary disease: a randomised controlled trial protocol. BMJ Open. 2015;5(7):e008031.
- Bove DG, Lomborg K, Jensen AK, Overgaard D, Lindhardt BØ, Midtgaard J. Efficacy of a minimal home-based psychoeducative intervention in patients with advanced COPD: a randomised controlled trial. Respir Med. 2016;121:109-116.
- Usmani ZA, Carson KV, Heslop K, Esterman AJ, De Soyza A, Smith BJ. Psychological therapies for the treatment of anxiety disorders in chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;3: CD010673. doi:10.002/14651858.CD010673.pub2.
- Cafarella P, Effing T, Frith P. An evaluation of the needs of carers of people with COPD. EurResp J. 2012;40(Suppl 56).
- Zyban [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. Available at: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Zyban/pdf/ZYBAN-PI-MG.PDF. Accessed June 2017.
- Gelenberg AJ, Freeman MP, Markowitz JC, et al. Practice guideline for the treatment of patients with major depressive disorder. Am J Psychiatry. 2010;167(10):1.
- Pary R, Matuschka PR, Lewis S, Lippmann S. Managing bipolar depression. Psychiatry (Edgmont). 2006;3(2):30-41.
- Blier P. Atypical antipsychotics for mood and anxiety disorders: safe and effective adjuncts? J Psychiatry Neurosci. 2005;30(4):232-233.
- Vulink NC, Figee M, Denys D. Review of atypical antipsychotics in anxiety. Eur Neuropsychopharmacol. 2011;21(6):429-449.
- Locke AB, Kirst N, Shultz CG. Diagnosis and management of generalized anxiety disorder and panic disorder in adults. Am Fam Physician. 2015;91(9):617-624.
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta-analysis. Cochrane Database Syst Rev. 2014;(3):CD010844. doi:10.1002/14651858.CD010844.pub2.
- Scully C. Drug effects on salivary glands: dry mouth. Oral Dis. 2003;9(4):165-176.
- Grenard JL, Munjas BA, Adams JL, et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med. 2011;26(10):1175-1182.
- O’Donnell DE, Webb KA, Harle I, Neder JA. Pharmacological management of breathlessness in COPD: recent advances and hopes for the future. Expert Rev Respir Med. 2016;10(7):823-834.
- Hyun MK, Lee NR, Jang EJ, Yim JJ, Lee CH. Effect of inhaled drugs on anxiety and depression in patients with chronic obstructive pulmonary disease: a prospective observational study. Int J Chron Obstruct Pulmon Dis. 2016;11:747-754.
Hospital Management of AECOPD
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]
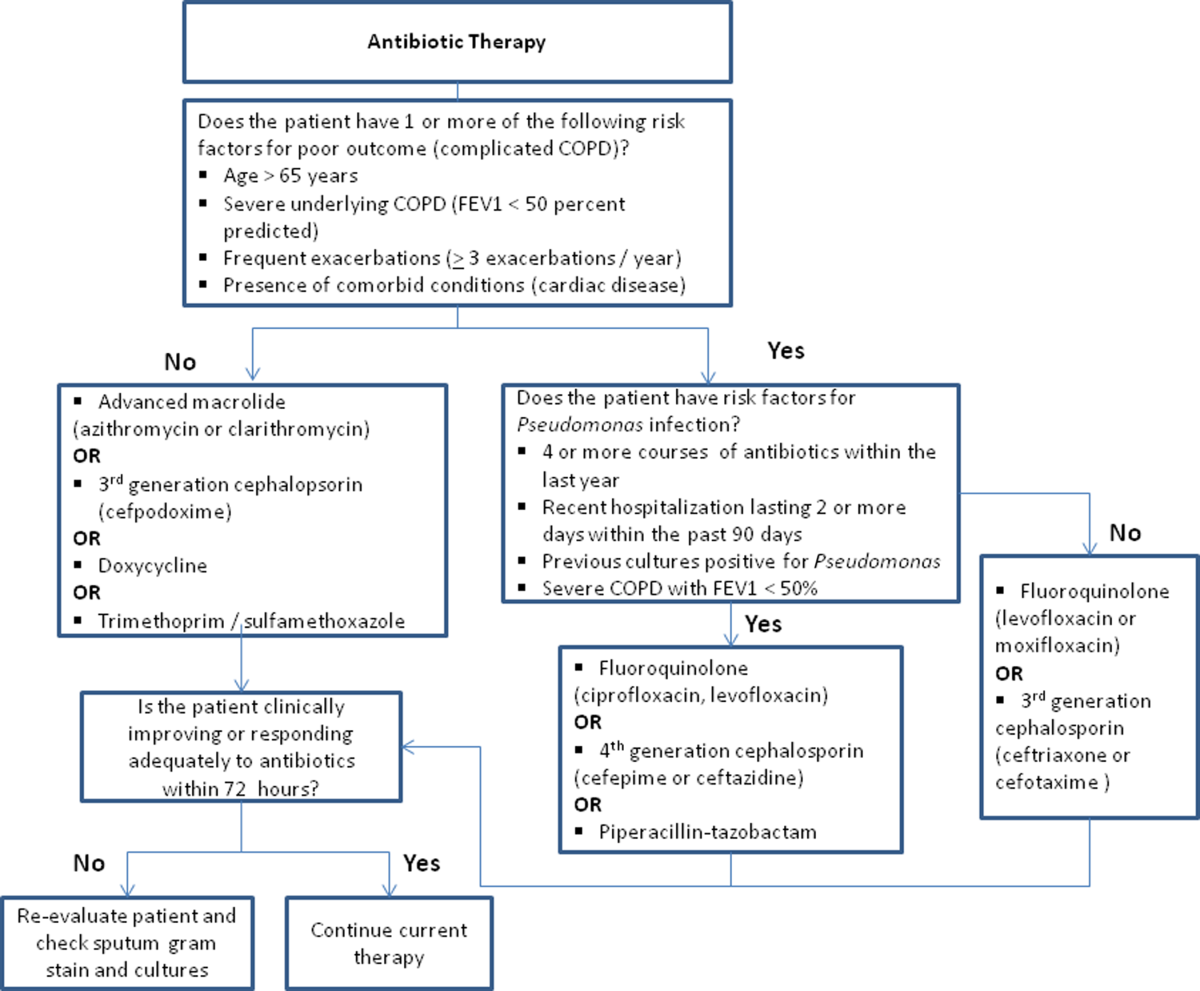
Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.
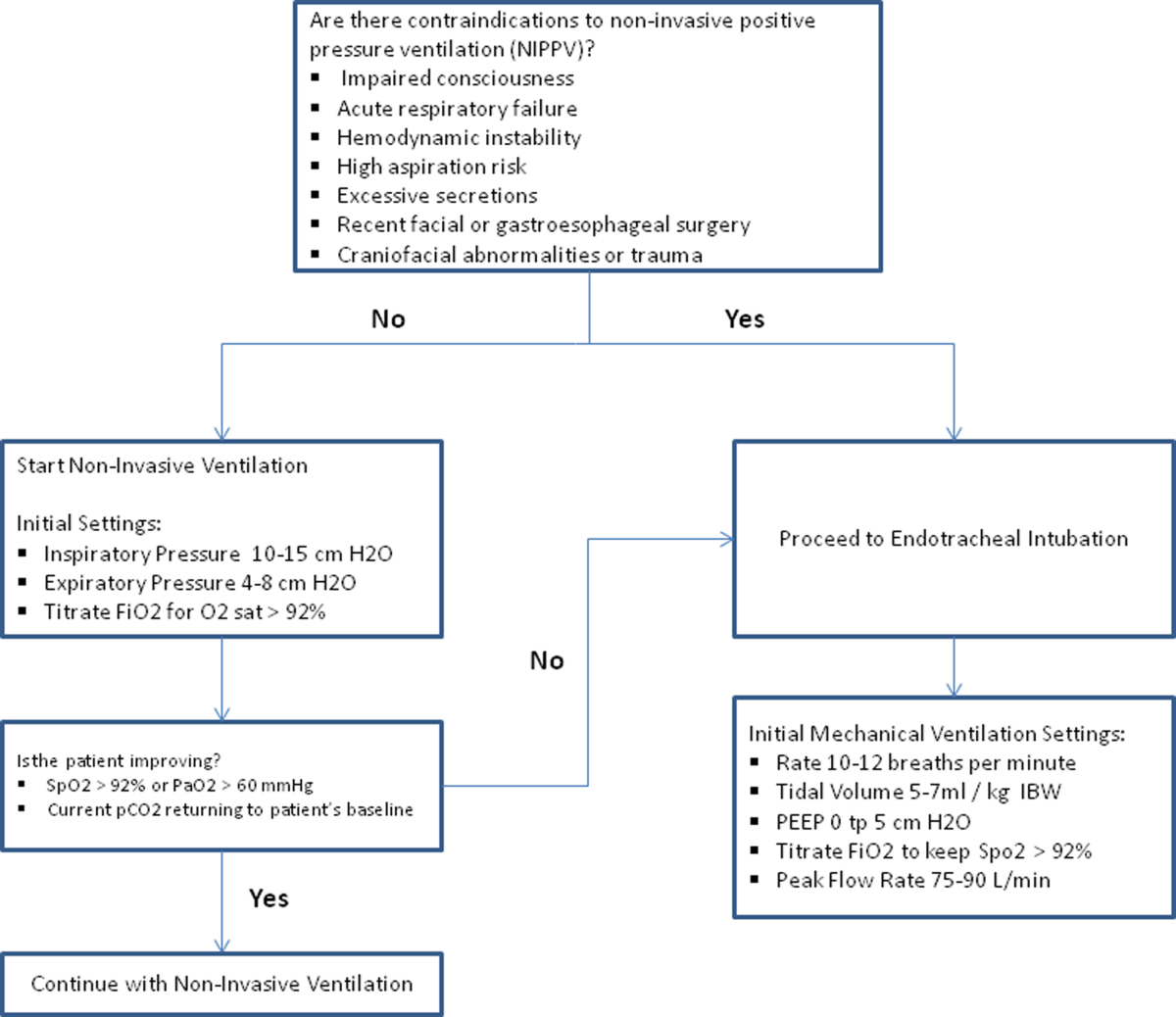
Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]

Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.

Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
Chronic obstructive pulmonary disease (COPD) is currently the third leading cause of death in the United States, accounting for over 140,000 deaths in 2009.[1] The economic burden of COPD is felt at all levels of the healthcare system with hospitalizations making up a large proportion of these costs.[2] As the US population ages, the prevalence of this disease is expected to rise, as will its impact on healthcare utilization and healthcare costs. The total estimated US healthcare costs attributable to COPD were $32.1 billion in 2010, with a projected 53% increase to $49.0 billion in 2020.[3] The Global Initiative for Chronic Obstructive Lung Disease (GOLD) defines an exacerbation as an acute event characterized by a worsening of the patient's respiratory symptoms that is beyond normal day‐to‐day variations.[4] Although there are no well‐established criteria, 3 cardinal symptoms suggest an exacerbation: worsening of dyspnea, increase in sputum volume, and increase in sputum purulence. Additionally, constitutional symptoms and a variable decrease in pulmonary function are also typically encountered in patients with an acute exacerbation.
Exacerbations have a major impact on the course of COPD. They have been shown to negatively affect quality of life, accelerate decline of lung function, and increase risk of mortality. Although the majority of exacerbations are managed in the outpatient setting, severe exacerbations will warrant emergency department visits and often hospital admission. Such exacerbations may often be complicated by respiratory failure and result in death.[4] Indeed, exacerbations requiring hospital admission have an estimated in‐hospital mortality of anywhere from 4% to 30% and are associated with poor long‐term outcomes and increased risk of rehospitalization.[5] Furthermore, the increased risk of mortality from a severe exacerbation remains elevated for approximately 90 days after the index hospitalization.[6] This review will provide an overview of the etiology, assessment, management, and follow‐up care of patients with COPD exacerbation in the hospital setting.
ETIOLOGY
Approximately 70% to 80% of exacerbations can be attributed to respiratory infections, with the remaining 20% to 30% due to environmental pollution or an unknown etiology.[7] Both viral and bacterial infections have been implicated in COPD exacerbations. Rhinoviruses are the most common viruses associated with acute exacerbations of COPD (AECOPD). Common bacteria implicated in triggering AECOPD include Haemophilus influenzae, Streptococcus pneumoniae, and Moraxella catarrhalis.[8, 9] Coinfection with multiple organisms can worsen severity of exacerbations.[10]
Exacerbations may also occur in the absence of an infectious trigger. Environmental factors may play a role, and increased risk of exacerbations has been reported during periods of higher air pollution. Increased concentrations of pollutants such as black smoke, sulphur dioxide, ozone, and nitrogen dioxide are associated with worsening in respiratory symptoms, increased risk of hospital admissions, and COPD‐associated mortality.[11] Exacerbations can also be precipitated or complicated by the presence of certain comorbid conditions such as aspiration or congestive heart failure (CHF). Other factors associated with increased risk for exacerbations include increased age, severity of airway obstruction, gastroesophageal reflux, chronic mucous hypersecretion, longer duration of COPD, productive cough and wheeze, increases in cough and sputum, and poor health‐related quality of life.[12, 13, 14, 15] Most importantly, a past history of exacerbation is a very good predictor of a subsequent episode.
CLINICAL ASSESSMENT
Initial evaluation of a severe exacerbation should include a comprehensive medical history, physical exam, and occasionally laboratory tests. A chest radiograph is often performed to rule out alternative diagnoses such as pneumonia or CHF.[4] Arterial blood gas (ABG) analysis is almost always needed when managing severe exacerbations to evaluate the presence of respiratory failure, which may require noninvasive or mechanical ventilation.[16, 17] Initial laboratory tests for hospitalized patients should include a complete blood cell count to help identify the presence of polycythemia, anemia, or leukocytosis, and a basic metabolic profile to identify any electrolyte abnormalities. Additional testing, such as an electrocardiogram (ECG), should be performed in the appropriate clinical context. Common ECG findings seen in COPD patients include right ventricular hypertrophy, right atrial enlargement, and low voltage QRS complexes.[18] Arrhythmias, such as multifocal atrial tachycardia, atrial fibrillation, and ventricular tachycardia, can also be observed.[19] Although pulmonary function tests performed during an acute exacerbation will have limited diagnostic or prognostic utility because the patient is not at clinical baseline, spirometry testing prior to hospital discharge may be helpful for confirming the diagnosis of COPD in patients who have not had pulmonary function testing before.
Pulmonary embolism (PE) may mimic the clinical presentation of a COPD exacerbation with features such as acute dyspnea, tachycardia, and pleuritic chest pain. Workup for PE should be considered if a clear cause for the exacerbation is not identified.[20] A meta‐analysis of 5 observational studies determined that the prevalence of PE was nearly 25% in hospitalized patients with COPD exacerbation.[21] However, significant heterogeneity in the data examined in this analysis was noted, with a wide range of reported PE incidence in the studies included.
The use of certain biomarkers such as brain natriuretic peptide (BNP) and procalcitonin may be helpful in guiding therapy by ruling out other concomitant disorders such as CHF (BNP) or ruling in a respiratory infection as a trigger (procalcitonin). BNP levels have been found to be significantly higher in patients with diastolic heart failure compared to patients with obstruction lung disease (224 240 pg/mL vs 14 12 pg/mL, P < 0.0001).[22] Furthermore, an increase in BNP levels of 100 pg/mL in patients with AECOPD was found to independently predict the need for intensive care unit admission (hazard ratio [HR], 1.13; 95% confidence interval [CI], 1.03 to 1.24).[23] Procalcitonin may be helpful in deciding when to use antibiotics in bacterial infection[24]; however, further studies are needed to characterize its use in guiding antibiotic therapy for COPD exacerbations.
Sputum Gram stain and cultures should be considered in patients with purulence or change in sputum color. Additional indications for collecting sputum include frequent exacerbations, severe airflow limitation, and exacerbations requiring mechanical ventilation due to the possibility of antibiotic‐resistant pathogens. The risk for certain organisms such as Pseudomonas include: (1) recent hospitalization with duration of at least 2 days within the past 90 days, (2) frequent antibiotic therapy of >4 courses within the past year, (3) Severe or very severe airflow obstruction (GOLD stage III or IV), (4) isolation of Pseudomonas aeruginosa during a previous exacerbation, and (5) recent systemic glucocorticoid use. Routine use of Gram stain and culture in patients without the above features may be of little yield, as common bacterial pathogens may be difficult to isolate in sputum or may have already been present as a colonizing organism.[25, 26, 27]
Patients who may warrant hospital admission have some of the following features: marked increase in intensity of symptoms, severe underlying COPD, lack of response to initial medical management, presence of serious comorbidities such as heart failure, history of frequent exacerbations, older age, and insufficient home support.[4] Indications for hospital admission and for intensive care unit admission are listed in Table 1.[16, 28]
|
| Consider hospital admission |
| Failure to respond to initial medical management |
| New severe or progressive symptoms (eg, dyspnea at rest, accessory muscle use) |
| Severe COPD |
| History of frequent exacerbations |
| New physical exam findings (eg, cyanosis, peripheral edema) |
| Older age |
| Comorbidities (eg, heart arrhythmias, heart failure) |
| Lack of home support |
| Consider ICU admission |
| Severe dyspnea that responds inadequately to initial treatment |
| Persistent hypoxemia or acidosis not responsive to O2 therapy and NIPPV |
| Impending or active respiratory failure |
| Changes in mental status such as confusion, lethargy, or coma |
| Hemodynamic instability |
MANAGEMENT
The initial goals of inpatient management of AECOPD are to correct the underlying respiratory dysfunction and hypoxemia, minimize progression of symptoms, and manage underlying triggers and comorbid conditions. Figure 1 outlines initial assessment and management actions to perform once a patient is admitted.[4] Once the patient has been stabilized, objectives change to prevention of subsequent exacerbations through a number of methods including optimization of outpatient pharmacotherapy, establishment of adequate home care, and close hospital follow‐up.

Pharmacologic Therapy
The major components of pharmacologic therapy used in the management of acute exacerbation of COPD in the hospital setting include bronchodilators, systemic corticosteroids, and antibiotics.
Bronchodilators
Short‐acting 2‐adrenergic agonists (eg, albuterol) with or without short‐acting anticholinergic agents (eg, ipratropium bromide) are the mainstay initial bronchodilators in an exacerbation. Short‐acting agents are preferred because of their rapid onset of action and efficacy in achieving bronchodilation. The 2 agents are often used together based on findings in studies that found combination therapy produced bronchodilation beyond what could be achieved with either agent alone.[29] Although a systematic review demonstrated comparable efficacy of bronchodilator delivery with nebulized therapy and meter‐dosed inhaler therapy, nebulization is often the preferred modality due to improved tolerance of administration in acute exacerbations.[30] Typical doses for albuterol are 2.5 mg by nebulizer every 2 to 4 hours as needed. Ipratropium bromide is usually dosed at 0.5 mg by nebulizer every 4 hours as needed. More frequent bronchodilator therapy than every 2 hours, possibly even continuous nebulized treatment, may be considered for severe symptoms. The use of long‐acting bronchodilators is restricted to maintenance therapy and should not be used in the treatment of an acute exacerbation.
Methylxanthines such as aminophylline and theophylline are not recommended for the initial management of acute exacerbations, and should only be considered as second line therapy in the setting of insufficient response to short‐acting bronchodilators.[4] In a review of randomized controlled trials, adding methylxanthines to conventional therapy did not readily reveal a significant improvement in lung function or symptoms.[31] Furthermore, therapy was associated with significantly more nausea and vomiting, tremors, palpitations, and arrhythmias compared to placebo.[31, 32]
Systemic Corticosteroids
Systemic glucocorticoids have an essential role in the management of patients hospitalized for COPD exacerbation. Studies have demonstrated that systemic corticosteroid use shortens recovery time, reduces hospital stays, reduces early treatment failure, and improves lung function. One of the most comprehensive trials establishing the clinical efficacy of systemic corticosteroids is the Veterans Affairs Cooperative Study of Systemic Corticosteroids in COPD Exacerbation.[33] In this study, 271 patients were randomly assigned to receive placebo, an 8‐week course of systemic corticosteroid therapy, or a 2‐week course of systemic corticosteroids. The primary endpoint of analysis was treatment failure as evidenced by an intensification of pharmacologic therapy, readmission, intubation, or death. The groups treated with systemic corticosteroids were found to have lower rates of treatment failure, shorter initial hospital stay, and more rapid improvement in forced expiratory volume in 1 second (FEV1). Recent studies have not found significant differences in outcome between patients treated with a shorter duration of systemic corticosteroids (57 days) and those using a longer duration of (1014 days).[34, 35] Furthermore, COPD patients admitted to the intensive care unit (ICU) may potentially have worse outcomes and adverse events when given higher doses of steroids. One cohort study assessing hospital mortality in COPD patients admitted to the ICU and treated with corticosteroids within the first 2 days of admission found that patients who received low doses of steroids (240 mg/d on hospital day 1 or 2) did not have significant reduction in mortality (odds ratio [OR] 0.85; 95% CI, 0.71 to 1.01;P= 0.06) but was associated with reduction in hospital (OR 0.44 d; 95% CI, 0.67 to 0.21; P< 0.01) and ICU length of stays (OR 0.31 d; 95% CI, 0.46 to 0.16;P< 0.01), hospital costs (OR $2559; 95% CI, $4508 to $609;P= 0.01), length of mechanical ventilation (OR 0.29 d; 95% CI, 0.52 to 0.06;P= 0.01), need for insulin therapy (22.7% vs 25.1%;P< 0.01), and fungal infections (3.3% vs 4.4%;P< 0.01).[36] Additionally, oral corticosteroids do not appear to be inferior to intravenous therapy.[37] Most patients admitted to the hospital with COPD exacerbation should be treated with a short course of low‐dose systemic corticosteroids such as 40 mg of prednisone daily for 5 days. Patients without adequate initial response to therapy may deserve alteration of dose or duration of steroid treatment. Although the use of a 40‐mg daily dose of prednisone is a suggested regimen of treatment in the majority of cases, the dosing and duration of steroids may need to be increased in more severe cases. The use of inhaled corticosteroids is limited to the maintenance therapy of COPD in conjunction with long‐acting bronchodilators.
Mucoactive Agents
Current literature does not support the routine use of mucoactive agents in the management of AECOPD.[38, 39, 40]
Antibiotics
There is a clear benefit for the use of antibiotics to treat exacerbations of COPD in an inpatient setting, especially given that most exacerbations are triggered by a respiratory infection. A 2012 systematic review of 16 placebo‐controlled studies demonstrated high‐quality evidence that antibiotics significantly reduced risk of treatment failure in hospitalized with severe exacerbations not requiring ICU admission (number needed to treat [NNT] = 10; relative risk [RR] 0.77; 95% CI, 0.65 to 0.91; I2= 47%).[41] However, there was no statistically significant effect on mortality or hospital length of stay. Patient groups treated with antibiotics were more likely to experience adverse events, with diarrhea being the most common side effect.
Of those studies, only 1 addressed antibiotic use in the ICU. In this study, patients with severe exacerbation requiring mechanical ventilation were treated with either ofloxacin 400 mg daily or placebo for 10 days.[42] The treatment group had significantly lower mortality (NNT = 6; absolute risk reduction [ARR] 17.5%; 95% CI, 4.3 to 30.7; P = 0.01) and a decreased need for additional courses of antibiotics (NNT = 4; ARR 28.4%; 95% CI, 12.9 to 43.9; P = 0.0006). Both the duration of mechanical ventilation and duration of hospital stay were significantly shorter in the treatment group (absolute difference 4.2 days; 95% CI, 2.5 to 5.9; and absolute difference 9.6 days; 95% CI, 3.4 to 12.8, respectively). Mortality benefit and reduced length of stay were seen only in patients admitted to the ICU.[42]
Despite the multitude of studies demonstrating significant benefits of antibiotic use for moderate to severe exacerbations, optimal antibiotic regimens for treatment have not been established. A risk stratification approach to antibiotic therapy has been proposed. In this approach, patients who are diagnosed with moderate or severe exacerbations (defined as having at least 2 of the 3 cardinal symptoms of exacerbation) are differentiated into simple or complicated patients. An algorithm that helps in choosing antibiotics is outlined in Figure 2.[43] Complicated patients are those who had at least 1 or more of the following risk factors for poor outcome: age >65 years, FEV1 <50%, comorbid disease such as cardiac disease, or 3 more exacerbations in the previous 12 months. If a specific antibiotic had been used within the last 3 months, a different class of agents is generally recommended. Additionally, patients treated according to this approach should be reassessed in 48 to 72 hours.[16, 43, 44]

Respiratory Support
Oxygen therapy plays an important part in the inpatient management of exacerbations. Correction of hypoxemia takes priority over correction of hypercapnea. Several devices such as nasal cannulas, Venturi masks, and nonrebreathing masks can be utilized to ensure adequate delivery of supplemental oxygen. Controlled oxygen therapy should target an oxygen saturation of >92%, allowing for the treatment of hypoxemia while reducing the risk of hypercapnia and respiratory acidosis related to worsening of ventilation perfusion mismatch.[45] ABGs should ideally be checked 30 to 60 minutes after the initiation of oxygen to assess for adequate oxygenation without interval worsening of carbon dioxide retention or respiratory acidosis.[4]
The use of noninvasive or invasive mechanical ventilation should be considered if acidemia (pH 7.35) occurs either on presentation or with continued oxygen therapy, or if symptoms worsen with evidence of respiratory muscle fatigue. The use of noninvasive ventilation has been shown to reduce the work of breathing and tachypnea. More importantly, it significantly improves pH within the first hour of treatment and reduces mortality (NNT 10), need for intubation (NNT 4), and hospital length of stay (reduction of 3.2 days [95% CI, 2.1 to 4.4 days]).[46, 47, 48, 49] Noninvasive positive pressure ventilation (NIPPV) is usually administered in a combination of continuous positive airway pressure (CPAP) and pressure support ventilation (PSV). Initial settings for CPAP and PSV are 4 to 8 cm H2O and 10 to 15 cm H2O, respectively. Serial ABGs repeated every 30 to 60 minutes after initiating NIPPV or other clinical changes are necessary to correctly assess and guide therapy. Contraindications to NIPPV include significantly altered mental status, respiratory arrest, cardiovascular instability, presence of copious secretions with high aspiration risk, recent facial or gastroesophageal surgery, and facial trauma or anatomic abnormality.[16, 50]
Invasive mechanical ventilation should be considered if a trial of noninvasive ventilation is unsuccessful. Additional indications are outlined in Figure 3.[4] Ventilatory strategies are geared toward correcting gas exchange abnormalities and minimizing lung injury. Minute ventilation should be titrated with the goal of normalizing the pH and returning partial pressure of CO2 back to the patient's baseline. COPD patients can have chronic hypercapnea and may have difficulty weaning from the ventilator if they are ventilated to a normal CO2. Additional considerations in the management of respiratory failure from AECOPD with mechanical ventilation include minimizing regional overdistension and management of dynamic hyperinflation. Overdistension injury or volutrauma can occur when high tidal volumes delivered by the ventilator force the already open alveoli to overdistend and develop stretch injury. Excessive volumes can also increase the risk of hyperinflation and barotrauma. Therefore, lower tidal volumes (eg, 57 mL/kg) have increasingly been utilized in the initial ventilatory management of these patients. Incomplete expiration of an inspired breath prior to initiation of the next breath causes air trapping, which in turn increases the alveolar pressure at the end of expiration or autopeak end expiratory pressure (auto‐PEEP). Increased auto‐PEEP can cause significant negative effects including increased work of breathing, barotrauma, and decreased systemic venous return.[51] Strategies to reduce auto‐PEEP include the following: reducing patient minute ventilation and ventilatory demand, lengthening the expiratory time, and reducing airflow resistance by pharmacologic agents. If auto‐PEEP persists despite management, applying external PEEP may reduce the threshold load for inspiratory effort caused by auto‐PEEP, and thus may decrease the work of breathing. Initial ventilator settings and mode used is dependent on operator and local practices. Suggested appropriate initial settings include the use of volume assist control ventilation with a rate of 10 to 12 breaths/minute, low tidal volumes of 5 to 7 mL/kg, PEEP of 5 cmH2O, and FiO2 needed to keep saturations >92% and/or a PaO2 > 60 mm Hg. Settings can be adjusted based on serial ABG analysis and the patient's tolerance of mechanical ventilation.[51, 52] Sedation may be needed to help patients tolerate ventilatory support.

Management of Comorbidities
Many comorbidities are associated with COPD. Common comorbidities include anxiety, depression, lung cancer, hypertension, diabetes, and cardiovascular disease.[50] Comorbid conditions complicate the management of COPD by increasing risk of hospitalization and mortality and significantly increasing healthcare costs.[53, 54] The clinical manifestations of these comorbid conditions and COPD are associated by means of the inflammation pathway either as a result of a spillover of inflammatory mediators occurring in the lungs or as a result of a systemic inflammatory state.[55, 56] Although there are no randomized controlled studies evaluating the effects of treating comorbidities in patients with COPD, observational studies have suggested that treating some of these conditions may be beneficial COPD.[50, 57, 58, 59, 60] Treatment of comorbidities should be optimized once the acute problems warranting admission have been stabilized. As a general rule, treatment of comorbidities should not affect the management of COPD and should be treated according to the guidelines for the comorbidity.[4] The management of cardiovascular disease and anxiety and depression will be addressed here.
Cardiovascular Disease
Cardiovascular disease is a major comorbidity in COPD. Several studies have observed the coexistence of the 2 conditions. COPD and cardiovascular disease share tobacco abuse as a risk factor.[61] Common entities in cardiovascular disease include ischemic heart disease, CHF, atrial fibrillation, and hypertension. Treatment of these conditions should generally adhere to current guidelines, as there is no evidence to suggest treatment should negatively impact COPD.[4] If considering the use of ‐blockers as part of a cardiac management regimen, cardioselective ‐blockers such as atenolol or metoprolol are recommended over nonselective blockade due to potential precipitation of bronchospasm in predisposed patients. A systematic review assessing the effect of short‐term and long‐term cardioselective ‐blocker use on the respiratory function of patients with COPD did not reveal significant adverse effects.[62] Regarding inhaled pharmacotherapy in patients with both COPD and cardiovascular disease, treatment should adhere to current GOLD guidelines. There has been concern for adverse cardiovascular effects associated with inhaled long‐acting agonist and long‐acting anticholinergic agents, but data from large long‐term studies have not shown a significant negative effect.[63, 64]
Anxiety and Depression
Comorbid anxiety or depression may complicate management in patients with COPD by worsening prognosis or interfering with therapy. The presence of these comorbid conditions has predicted poor adherence to treatment, lower health‐related quality of life, decreased exercise capacity, increased disability, and increased risk of exacerbation and mortality.[65, 66, 67, 68] A recent meta‐analysis found that the presence of comorbid depression increased the risk of mortality by 83%, and comorbid anxiety increased the risk of exacerbation and mortality by 28%. Additionally, patients with COPD were found to be at 55% to 69% increased risk of developing depression.[69]
Although further study is needed to clearly define screening and management, treatment of these co‐morbid conditions in patients with COPD should adhere to usual guidelines. During an admission for exacerbation, screening for depression and anxiety with a referral to psychiatry should be considered on a case‐by‐case basis. No changes to pharmacologic management for COPD are necessary while a patient is under treatment for anxiety or depression.[4] Exercise training during hospitalization for acute exacerbation of COPD can be considered, as recent data revealed beneficial effects on depression symptoms and overall mood.[70]
Palliative Care
The focus of palliative care in a COPD patient is to provide care aimed at improving symptom control, communication, physical activity, and emotional support to overall better the patient's quality of life.[71] Palliative care in pulmonary disease can be divided into 3 main areas of concentration: support for patient and family, care of the patient, and responsibility of the professional caregiver. Discussions with patients regarding initiation of palliative care should begin at time of diagnosis of COPD.[4] However, there are significant barriers to planning end‐of‐life care in these patients including difficulty with establishing prognosis in end‐stage COPD, patients' lack of awareness regarding progression of disease, and lack of communication between care teams. Given these obstacles, patients admitted with AECOPD often have no care plan in place.[71]
Responsibility of the caregiver during an admission for AECOPD includes advance care planning and medical management for relief of distressing symptoms such as dyspnea, anxiety, or depression. Palliative care teams are becoming more available for consultation on hospitalized patients, and they will help facilitate the palliative care discussion in multiple areas including goals of care, optimization of quality of life, and identification of community/palliative care resources that may be available once the patient is discharged.[4, 72]
DISCHARGE PLANNING
Patients admitted for AECOPD can be considered for discharge once symptoms are improved and their condition is stable enough to permit outpatient management. A discharge checklist is suggested in Table 2 to ensure proper follow‐up and that teaching has been performed prior to discharge.[4] Risk factors for rehospitalization include the following: previous hospital admissions for exacerbation, continuous dyspnea, oral corticosteroid use, long‐term oxygen therapy, poor health‐related quality of life, and lack of routine physical activity.[73, 74] An optimal length of stay has not been established, and more research is needed to identify predictive factors associated with hospitalization/rehospitalization.[75, 76]
|
| Patient and/or caregiver must demonstrate the ability to follow an outpatient regimen for the treatment of COPD |
| Reassess inhaler technique |
| Educate patient on the role of maintenance therapy and completion of steroid and/or antibiotic therapy |
| Establish a care plan for patient's medical problems |
| Patient must be evaluated for and if needed set for oxygen therapy |
| Patient must be scheduled for outpatient follow up in 4 |
There are interventions that can shorten length of stay and expedite recovery from symptoms in the outpatient setting. Establishing home health visits by a nurse has allowed patients to be discharged earlier without significantly increasing readmission rates.[77, 78] Additionally, the use of a written action plan has allowed for more appropriate treatment for exacerbations, which may shorten recovery time, although there was no change in healthcare resource utilization.[79, 80, 81] Prior to discharge, patients should start or restart long‐acting bronchodilator maintenance medications, which usually include long‐acting 2 agonists, long‐acting anticholinergics, or both. In addition, the use of inhaled corticosteroids and phosphodiesterase 4 (PDE‐4) inhibitors should also be considered if appropriate for the severity of the underlying disease. Patients should also have the following performed at time of discharge: optimization of home maintenance pharmacologic therapy, reassessment of inhaler technique, education regarding role of maintenance therapy, instructions regarding antibiotic and steroid use, management plan of comorbidities, scheduled hospital follow‐up, and evaluation of long‐term oxygen use.
There are insufficient data to establish a specific schedule postdischarge that will maximize positive outcomes. One retrospective cohort study found that patients who had a follow‐up visit with their primary care provider or pulmonologist within 30 days of discharge had significantly reduced risk of an emergency room (ER) visit (HR 0.86; 95% CI, 0.83 to 0.9) and reduced readmission rates (HR 0.91; 95% CI, 0.87 to 0.96).[82] Nonetheless, current guidelines recommend follow‐up to occur within 4 to 6 weeks after discharge from the hospital. A shorter follow‐up interval of 1 to 2 weeks after discharge may be needed for patients at higher risk for relapse such as those who have frequent exacerbations or those admitted to the ICU for respiratory failure.[16, 28]
PREVENTION
After hospitalization, most patients are not discharged with appropriate support and medications, which in turn, increases their risk for hospital readmission.[83] Several modalities including vaccination, action plans, long‐acting inhaled bronchodilators, and antibiotics have been shown to be effective in prevention of COPD exacerbations. However, there has been little guidance available to help clinicians choose therapies from the currently available options that would be most appropriate for their patients. This year, the American College of Chest Physicians and the Canadian Thoracic Society published an evidence‐based guideline on the prevention of COPD exacerbations.[84] Recommended therapies (those with level 1 evidence) will be discussed here.
Vaccinations
Annual influenza vaccinations are recommended for COPD patients. A meta‐analysis of 11 trials, with 6 of those trials specifically performed in patients with COPD, demonstrated a reduction in total number of exacerbations per vaccinated patient compared to patients who received placebo (mean difference of 0.037, 0.64 to 0.11; P = 0.006).[85]
Pneumococcal vaccines should also be administered, especially because COPD exacerbations related to pneumococcal infection have had been associated with longer hospitalizations and worsening impairment of lung function compared to noninfectious exacerbations. However, there is insufficient evidence to indicate that pneumococcal vaccination can prevent AECOPD, although a Cochrane systematic review of 7 studies examining this suggests a borderline statistically significant improvement in pneumonia rates in those with COPD versus controls (OR 0.72; 95% CI, 0.51 to 1.01).[86]
Pulmonary Rehabilitation
Pulmonary rehabilitation is a comprehensive program based on exercise training, education, and behavior change that is designed to improve the physical and psychological condition of people with chronic respiratory disease as well as promote long‐term adherence to health enhancing behaviors. Although a pooled analysis of 623 patients from 9 studies demonstrated a significant reduction in hospitalizations in patients who participated in pulmonary rehabilitation compared to those who pursued conventional care (OR 0.4; 95% CI, 0.22 to 0.91; P = 0.03), the overall quality of evidence was low with significant heterogeneity also observed (P = 0.03; I2 = 52%). However, when the studies were categorized by timing of rehabilitation, patients who participated in a rehabilitation program initiated within 1 month after a COPD hospitalization had a reduction in rehospitalizations after completion of rehabilitation (OR 0.24; 95% CI, 0.07 to 0.88; P = 0.03). No reduction was seen in patients without a recent history of AECOPD (>1 month) who underwent rehabilitation (OR 0.79; 95% CI, 0.42 to 1.5; P = 0.47). Based on these findings, pulmonary rehabilitation should be initiated in patients within 4 weeks of an AECOPD.[84]
Education, Action Plans, and Case Management
Education, action plans, and case management are all interventions that focus on enabling patients to be knowledgeable about COPD, equipping them with the necessary skills to manage their chronic disease, and motivating them to be proactive with their healthcare. There are no formal definitions describing these modalities. Patient education is usually a formal delivery of COPD topics in forms such as nurse teaching or classes with the objective of improving knowledge and understanding of the disease process. Action plans are usually written plans created by a clinician for individual patients aiming to teach them how to identify and self‐manage AECOPD. Case management consists of patients either receiving formal follow‐up or consistent communication such as scheduled telephone calls with a healthcare professional allowing for closer monitoring of symptoms, better availability of medical staff, prompt coordination of care, and early identification and treatment of AECOPD.
Although several studies have evaluated the impact on hospitalization rates after implementation of the above interventions as an individual modality or in combination with each other, only the combination of patient education and case management that included direct access to a healthcare specialist at least monthly demonstrated a significant decrease in hospitalization rate with a pooled opportunity risk of 0.82 (95% CI, 0.17 to 3.99) and significant heterogeneity between studies (P = 0.003, I2 = 89%). There was insufficient evidence to recommend use of all 3 interventions together. Use of any of these interventions individually after a COPD hospitalization was not recommended.[84]
Maintenance Pharmacotherapies
The use of long‐acting inhaled bronchodilators with or without inhaled corticosteroids (ICS) as maintenance therapy has been shown to decrease exacerbations. Efficacy of long‐acting 2 agonists (LABAs), long acting muscarinic antagonists (LAMAs), and combination therapies with or without ICS will be discussed here.
A systematic review of LABAs demonstrated a reduced exacerbation rate with long‐acting 2 agonist use versus placebo.[87] Data from 7 studies with a total of 2859 patients enrolled demonstrated an OR for severe exacerbation requiring admissions of 0.73 (95% CI, 0.56 to 0.95). Data from 7 studies with 3375 patients evaluating rates of moderate exacerbations demonstrated an OR of 0.73 (95% CI, 0.61 to 0.87).[84]
Tiotropium is the best studied inhaled LAMA in the treatment of COPD. Two major trials helped establish role of tiotropium in COPD management. The first by Niewoehner et al. demonstrated that the addition of tiotropium to standard treatment significantly decreased the proportion of patients who experienced 1 or more exacerbations during the 6‐month duration of treatment (27.9% vs 32.3%; P = 0.037).[88] The UPLIFT (Understanding Potential Long‐term Impacts on Function with Tiotropium) trial was published soon after, and found a 14% reduction in exacerbations over 4 years in patients treated with tiotropium compared to those receiving usual care (0.73 vs 0.85 exacerbations per year; RR 0.86; 95% CI, 0.81 to 0.91).[89] A recently published systematic review assessing the effectiveness of tiotropium versus placebo demonstrated a reduction in the rate of acute exacerbations with tiotropium by 22%. The OR was 0.78 (95% CI, 0.70 to 0.87) with a NNT of 16. Additional analysis of 21 studies enrolling 22,852 patients found that tiotropium treatment was associated with fewer hospitalizations due to exacerbations, with an OR of 0.85 (95% CI, 0.72 to 1.00).[90] Studies comparing LAMAs to short‐acting muscarinic antagonist ipratroprium showed that tiotropium was superior in exacerbation prevention (OR 0.71; 95% CI, 0.52 to 0.95).[91] LAMAs have also demonstrated a lower rate of exacerbation when compared to LABAs. In a systematic review of 6 studies enrolling 12,123 patients, those using tiotropium alone had an OR of 0.86 (95% CI, 0.79 to 0.93) compared to patients using LABAs. Further analysis of the 4 studies in this review that reported COPD hospitalization as an outcome showed that rates of hospitalization in subjects receiving tiotropium was significantly lower in subjects who received tiotropium compared to LABA (OR 0.87; 95% CI, 0.77 to 0.99).[92]
The largest clinical trial to date for ICS/LABA combination therapy was the TORCH (Towards a Revolution in COPD Health) study. In this 3‐year study, 6112 patients were randomized to treatment with fluticasone‐salmeterol or placebo. Patients treated with the combination therapy had a 25% reduction in exacerbations when compared to placebo.[64] However, there are few long‐term studies comparing combination ICS/LABA versus single drugs with exacerbations as the primary outcome. A recent Cochrane meta‐analysis found 14 studies that met inclusion criteria that randomized a total of 11,794 patients with severe COPD. Results indicate combination ICS/LABA reduced the number of exacerbations but did not significantly affect the rate of hospitalizations when compared with LABA monotherapy. Additionally, there was a 4% increased risk of pneumonia in the combination therapy group compared with the LABA alone.[93]
There are also little data comparing triple therapy (LABA/ICS and LAMA) to double or single therapy. A recent systematic review compared the efficacy of 3 therapeutic approaches: tiotropium plus LABA (dual therapy), LABA/ICS (combined therapy), and tiotropium plus ICS/LABA. The review consisted of 20 trials with a total of 6803 patients included. Both dual therapy and triple therapy did not have significant impact on risk of exacerbations in comparison to tiotropium monotherapy.[94]
There are no guidelines regarding the step up of maintenance inhaler therapy immediately after COPD‐related hospitalization. That being said, any patient with COPD who is hospitalized for AECOPD is already considered to be at high risk for exacerbation and can therefore be classified as group C or D according to the GOLD combined assessment. Per GOLD guidelines for management of stable COPD, recommended first choice for maintenance therapy in a group C patient would be ICS/LABA or LAMA and in a group D patient would be ICS/LABA LAMA. Further titration of maintenance therapy should be performed on an outpatient basis.[4]
Additional Therapies
There are several additional therapies including long‐term macrolides and PDE4 inhibitors such as roflumilast that have demonstrated significant reduction in exacerbations[95]; more data are needed before these modalities can be fully recommended.[84]
CONCLUSIONS
COPD exacerbations are important events that complicate the course of the disease. They are significant contributors to the morbidity and mortality. In patients with severe exacerbations resulting in hospitalization, a detailed assessment is important to identify those who may need intensive care or mechanical ventilation. Immediate management of these patients includes correcting hypoxemia, respiratory support, and pharmacologic therapy with short‐acting bronchodilators, antibiotics, and systemic corticosteroids. Comorbid conditions should be evaluated and treated as well. Prior to discharge, outpatient pharmacotherapy needs to be optimized and patient education is needed to ensure that the affected individuals understand the importance of maintenance therapy and identify factors that may contribute to their exacerbations. Close outpatient follow‐up is necessary to prevent exacerbation relapses.
Disclosure
N.A.H. received research grant support (to institution) and served as a consultant for GSK, Boehringer Ingelheim, Sunovion, Mylan, Pearl, Pfizer and Novartis, and served on the ACCP/CTS COPD Exacerbation Guidelines' Panel. The authors have no other funding, financial relationships, or conflicts of interest to disclose.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
- , , , , . Deaths: preliminary data for 2009. Natl Vital Stat Rep. 2011; 59(4): 1–51.
- , , . Delivering cost‐effective care for COPD in the USA: recent progress and current challenges. Expert Rev Pharmacoecon Outcomes Res. 2012; 12(6): 725–731.
- , , , , , . Total and state‐specific medical and absenteeism costs of chronic obstructive pulmonary disease among adults aged >/=18 years in the United States for 2010 and projections through 2020. Chest. 2015; 147(1): 31–45.
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2014. Available at: http://www.goldcopd.org. Accessed December 1, 2014.
- , , , . In‐hospital mortality following acute exacerbations of chronic obstructive pulmonary disease. Arch Intern Med. 2003; 163(10): 1180–1186.
- , , . Long‐term natural history of chronic obstructive pulmonary disease: severe exacerbations and mortality. Thorax. 2012; 67(11): 957–963.
- , . COPD exacerbations 2: aetiology. Thorax. 2006; 61(3): 250–258.
- , , , et al. Respiratory viruses, symptoms, and inflammatory markers in acute exacerbations and stable chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001; 164(9): 1618–1623.
- , . Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(22): 2355–2365.
- , , , et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006; 173(10): 1114–1121.
- , , , et al. Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J. 1997; 10(5): 1064–1071.
- , , , , , . Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998; 157(5 pt 1): 1418–1422.
- , , , et al. Risk indexes for exacerbations and hospitalizations due to COPD. Chest. 2007; 131(1): 20–28.
- , , , , , . Factors associated with increased risk of exacerbation and hospital admission in a cohort of ambulatory COPD patients: a multiple logistic regression analysis. The EOLO Study Group. Respiration. 2000; 67(5): 495–501.
- , , ; Initiatives Bronchopneumopathie Chronique Obstructive Scientific Committee. Cough and sputum production are associated with frequent exacerbations and hospitalizations in COPD subjects. Chest. 2009; 135(4): 975–982.
- , , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004; 23(6): 932–946.
- , , , , . Relationship between arterial blood gases and spirometry in acute exacerbations of chronic obstructive pulmonary disease. Ann Emerg Med. 1989; 18(5): 523–527.
- , , , et al.; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009; 53(11): 992–1002.
- , , , , , . Frequency and significance of cardiac arrhythmias in chronic obstructive lung disease. Chest. 1988; 94(1): 44–48.
- , , , et al. The diagnosis of acute pulmonary embolism in patients with chronic obstructive pulmonary disease. Chest. 1992; 102(1): 17–22.
- , , . Prevalence of pulmonary embolism in acute exacerbations of COPD: a systematic review and metaanalysis. Chest. 2009; 135(3): 786–793.
- , , , et al. Brain natriuretic peptide blood levels in the differential diagnosis of dyspnea. Chest. 2001; 120(6): 2047–2050.
- , , , et al. Use of B‐type natriuretic peptide in the risk stratification of acute exacerbations of COPD. Chest. 2008; 133(5): 1088–1094.
- , , , et al. Procalcitonin to initiate or discontinue antibiotics in acute respiratory tract infections. Cochrane Database Syst Rev. 2012; 9: CD007498.
- , , , . Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004; 170(3): 266–272.
- , , , , , . Haemophilus haemolyticus: a human respiratory tract commensal to be distinguished from Haemophilus influenzae. J Infect Dis. 2007; 195(1): 81–89.
- , , , , , . Role of infection in chronic bronchitis. Am Rev Respir Dis. 1976; 113(4): 465–474.
- , , , et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013; 187(4): 347–365.
- , . Effects of combined treatment with glycopyrrolate and albuterol in acute exacerbation of chronic obstructive pulmonary disease. Ann Emerg Med. 1995; 25(4): 470–473.
- , , , . Bronchodilator delivery in acute airflow obstruction. A meta‐analysis. Arch Intern Med. 1997; 157(15): 1736–1744.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2003(2): CD002168.
- , , . Methylxanthines for exacerbations of chronic obstructive pulmonary disease: meta‐analysis of randomised trials. BMJ. 2003; 327(7416): 643.
- , , . Systemic Corticosteroids in Chronic Obstructive Pulmonary Disease Exacerbations (SCCOPE): rationale and design of an equivalence trial. Veterans Administration Cooperative Trials SCCOPE Study Group. Control Clin Trials. 1998; 19(4): 404–417.
- , , , , . Different durations of corticosteroid therapy for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011(10): CD006897.
- , , , et al. Short‐term vs conventional glucocorticoid therapy in acute exacerbations of chronic obstructive pulmonary disease: the REDUCE randomized clinical trial. JAMA. 2013; 309(21): 2223–2231.
- , , , , . Outcomes associated with corticosteroid dosage in critically ill patients with acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2014; 189(9): 1052–1064.
- , , , , , . Oral or IV prednisolone in the treatment of COPD exacerbations: a randomized, controlled, double‐blind study. Chest. 2007; 132(6): 1741–1747.
- , , ; Joint Expert Panel on Chronic Obstructive Pulmonary Disease of the American College of Chest Physicians and the American College of Physicians‐American Society of Internal Medicine. Evidence base for management of acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2001; 134(7): 595–599.
- , , , ; American College of Physicians‐American Society of Internal Medicine; American College of Chest Physicians. Management of acute exacerbations of chronic obstructive pulmonary disease: a summary and appraisal of published evidence. Ann Intern Med. 2001; 134(7): 600–620.
- , , , , . Randomised, controlled trial of N‐acetylcysteine for treatment of acute exacerbations of chronic obstructive pulmonary disease [ISRCTN21676344]. BMC Pulm Med. 2004; 4: 13.
- , , , , . Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 12: CD010257.
- , , , , , . Once daily oral ofloxacin in chronic obstructive pulmonary disease exacerbation requiring mechanical ventilation: a randomised placebo‐controlled trial. Lancet. 2001; 358(9298): 2020–2025.
- , . Optimizing antibiotic selection in treating COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2008; 3(1): 31–44.
- . Clinical practice. Acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 2002; 346(13): 988–994.
- , , , , . Effect of high flow oxygen on mortality in chronic obstructive pulmonary disease patients in prehospital setting: randomised controlled trial. BMJ. 2010; 341: c5462.
- , , , , . Randomized, prospective trial of noninvasive positive pressure ventilation in acute respiratory failure. Am J Respir Crit Care Med. 1995; 151(6): 1799–1806.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000; 355(9219): 1931–1935.
- , . Noninvasive positive pressure ventilation to treat respiratory failure. Ann Intern Med. 1994; 120(9): 760–770.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database Syst Rev. 2005;(3): CD004360.
- , . Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009; 33(5): 1165–1185.
- , . Review of ventilatory techniques to optimize mechanical ventilation in acute exacerbation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2007; 2(4): 441–452.
- , . Acute exacerbations and respiratory failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(4): 530–535.
- , , , . Mortality in COPD: role of comorbidities. Eur Respir J. 2006; 28(6): 1245–1257.
- , , , et al.; BODE Collaborative Group. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012; 186(2): 155–161.
- , . Chronic obstructive pulmonary disease, inflammation and co‐morbidity—a common inflammatory phenotype? Respir Res. 2006; 7: 70.
- , . From COPD to chronic systemic inflammatory syndrome? Lancet. 2007; 370(9589): 797–799.
- , . Chronic obstructive pulmonary disease and comorbidities. Lancet Respir Med. 2013; 1(1): 73–83.
- , , , , , . Reduction of morbidity and mortality by statins, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers in patients with chronic obstructive pulmonary disease. J Am Coll Cardiol. 2006; 47(12): 2554–2560.
- , , , . Statin use is associated with reduced mortality in COPD. Eur Respir J. 2007; 29(2): 279–283.
- , , , , , . The use of statins and lung function in current and former smokers. Chest. 2007; 132(6): 1764–1771.
- , , , et al. Coronary artery disease is under‐diagnosed and under‐treated in advanced lung disease. Am J Med. 2012; 125(12): 1228.e13–1228 .e22.
- , , . Cardioselective beta‐blockers for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2005(4): CD003566.
- , , , et al.; TORCH Investigators. Cardiovascular events in patients with COPD: TORCH study results. Thorax. 2010; 65(8): 719–725.
- , , , et al.; TORCH investigators. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007; 356(8): 775–789.
- , , , et al.; WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004; 291(21): 2581–2590.
- , , , , ; Nett Research Group. Costs of pulmonary rehabilitation and predictors of adherence in the National Emphysema Treatment Trial. COPD. 2008; 5(2): 105–116.
- , , , et al.; National Emphysema Treatment Trial (NETT) Research Group. Sex, depression, and risk of hospitalization and mortality in chronic obstructive pulmonary disease. Arch Intern Med. 2007; 167(21): 2345–2353.
- , , , , , . Depressive symptoms and chronic obstructive pulmonary disease: effect on mortality, hospital readmission, symptom burden, functional status, and quality of life. Arch Intern Med. 2007; 167(1): 60–67.
- , , , . Bidirectional associations between clinically relevant depression or anxiety and COPD: a systematic review and meta‐analysis. Chest. 2013; 144(3): 766–777.
- , , , et al. Exercise prescription for hospitalized people with chronic obstructive pulmonary disease and comorbidities: a synthesis of systematic reviews. Int J Chron Obstruct Pulmon Dis. 2012; 7: 297–320.
- , , , . Recommendations for end‐of‐life care in patients with chronic obstructive pulmonary disease [in Spanish]. Arch Bronconeumol. 2009; 45(6): 297–303.
- , , , , , ; American College of Chest Physicians. Palliative and end‐of‐life care for patients with cardiopulmonary diseases: American College of Chest Physicians position statement. Chest. 2005; 128(5): 3599–3610.
- , , , , , ; Estudi del Factors de Risc d'Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003; 58(2): 100–105.
- , . Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007; 2(3): 241–251.
- , , , , . The necessary length of hospital stay for chronic pulmonary disease. JAMA. 1991; 266(1): 80–83.
- , , , , . Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999; 159(1): 158–164.
- , , , , , . Randomised controlled trial of home based care of patients with chronic obstructive pulmonary disease. BMJ. 2002; 325(7370): 938.
- , , , et al. Early discharge for patients with exacerbations of chronic obstructive pulmonary disease: a randomized controlled trial. Thorax. 2000; 55(11): 902–906.
- . Action plans and case manager support may hasten recovery of symptoms following an acute exacerbation in patients with chronic obstructive pulmonary disease (COPD). J Physiother. 2012; 58(1): 60.
- , , , . Written action plans in chronic obstructive pulmonary disease increase appropriate treatment for acute exacerbations. Respirology. 2006; 11(5): 619–626.
- , , , et al. Effects of written action plan adherence on COPD exacerbation recovery. Thorax. 2011; 66(1): 26–31.
- , , , , . Outpatient follow‐up visit and 30‐day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med. 2010; 170(18): 1664–1670.
- , , , et al. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010; 7(2): 85–92.
- , , , et al. Prevention of acute exacerbations of chronic obstructive pulmonary disease: American College of Chest Physicians and Canadian Thoracic Society Guideline [published online ahead of print October 16, 2014]. Chest. doi: 10.1378/chest.14‐1676.
- , , , . Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;(1): CD002733.
- , , , , . Injectable vaccines for preventing pneumococcal infection in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010(11): CD001390.
- , , . Long‐acting beta2‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 10: CD010177.
- , , , et al. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once‐daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005; 143(5): 317–326.
- , , , et al.; UPLIFT Study Investigators. A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008; 359(15): 1543–1554.
- , , . Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 7: CD009285.
- , , . Tiotropium versus ipratropium bromide for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013; 9: CD009552.
- , , . Tiotropium versus long‐acting beta‐agonists for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD009157.
- , , . Combined corticosteroid and long‐acting beta(2)‐agonist in one inhaler versus long‐acting beta(2)‐agonists for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012; 9: CD006829.
- , , . Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulm Pharmacol Ther. 2012; 25(1): 40–47.
- , , . Preventing acute exacerbations and hospital admissions in COPD. Chest. 2013; 143(5): 1444–1454.
Effectiveness of NIV vs. IMV in AECOPD
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States,[1] and its rising mortality trend is unique among the top 5 causes of death.[2] Acute exacerbations of COPD (AECOPD) are important events in the natural history of COPD, accounting for 1.5 million emergency department (ED) visits and 726,000 hospitalizations each year in the United States.[3, 4] Given the significant morbidity and mortality from AECOPD, Healthy People 2020 lists reducing deaths, hospitalizations, and ED visits as the key objectives for COPD.[5]
Over the past 2 decades, noninvasive ventilation (NIV) has emerged as a potentially useful treatment modality in AECOPD patients with acute respiratory failure. Noninvasive ventilation commonly refers to positive‐pressure ventilatory support delivered through a nasal or full‐face mask, such as bilevel positive airway pressure.[6] A number of randomized controlled trials[7, 8, 9] and meta‐analyses[10] have suggested a mortality‐reduction benefit with NIV use compared with standard medical care in AECOPD. To our knowledge, however, very few small randomized controlled trials compared NIV vs invasive mechanical ventilation (IMV) head‐to‐head,[11, 12, 13] and a recent evidence review found only 5 studies (405 subjects) on this topic.[14] Collectively, the limited evidence from randomized trials showed that NIV use resulted in similar intensive care unit (ICU) and in‐hospital mortality, fewer complications (eg, ventilator‐associated pneumonia and sepsis), and shorter hospital length of stays (LOS). Given that these trials have a smaller sample size and tend to exclude older patient (age >75 years) or patients with multiple comorbidities, there is a need to better understand the adoption and effectiveness of NIV treatment for AECOPD in a much larger patient population in the real‐world setting using observational data.
To address these knowledge gaps in the literature, we analyzed data from a large, nationally representative ED and inpatient sample. The objective of the present analysis was 2‐fold: (1) to characterize the use of NIV and IMV in AECOPD patients with acute respiratory failure at a national level; and (2) to compare the effectiveness of NIV vs IMV in the real‐world setting.
METHODS
Study Design and Setting
We conducted a retrospective cohort study using data from the 20062008 Nationwide Emergency Department Sample (NEDS),[15] a component of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The NEDS is nationally representative of all community hospitalbased EDs in the United States, defined by the American Hospital Association as all nonfederal, short‐term, general, and other specialty hospitals.[16] Community hospitals include academic medical centers if they are nonfederal short‐term hospitals. The NEDS was constructed using administrative records from the State Emergency Department Databases and the State Inpatient Databases. The former captures information on ED visits that do not result in an admission (ie, treat‐and‐release visits or transfers to another hospital); the latter contains information on patients initially seen in the ED and then admitted to the same hospital. Taken together, the resulting NEDS represents all ED visits regardless of disposition and contains information on short‐term outcomes for patients admitted through the ED. In other words, the NEDS is the largest all‐payer ED and inpatient database in the United States. The NEDS represents an approximately 20% stratified sample of US hospital‐based EDs, containing more than 28 million records of ED visits from approximately 1000 hospitals each year. Additional details of the NEDS can be found elsewhere.[15, 17] We received a waiver for this analysis from our institutional review board.
Study Population
Patient visits were included in this analysis if they carried any COPD‐related diagnostic code (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 491.xx [chronic bronchitis], 492.xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) as their primary ED diagnosis and any acute respiratory failure code (ie, 518.81 [acute respiratory failure], 518.82 [pulmonary insufficiency not elsewhere classified, 518.84 [acute and chronic respiratory failure], or 799.1 [respiratory arrest]) as their secondary diagnosis. Patient visits with a primary diagnosis of acute respiratory failure and a secondary diagnosis of COPD were also included. Patients age <40 years were excluded, because they are much less likely to have COPD.[18]
Modes of Mechanical Ventilation
The primary exposure variable was mode of mechanical ventilation. To compare the effectiveness of different ventilatory modes, patients were divided into 3 groups according to the ventilation mode they received: (1) NIV alone, (2) IMV alone, and (3) combined modes of NIV and IMV. The use of NIV was identified by using Current Procedural Terminology (CPT) code of 94660 or ICD‐9 procedure code 93.90, whereas the use of IMV was identified by using CPT code of 31500 or ICD‐9 procedure code 96.04 or 96.7x.
Patient‐Level and Emergency DepartmentLevel Variables
The NEDS contains information on patient demographics, national quartiles for median household income based on the patient's ZIP code, payment sources, ICD‐9‐CM diagnoses and procedures, ED disposition, hospital LOS, and hospital disposition. Hospital characteristics include annual visit volume, urban‐rural status, ownership, teaching status, and US region. Geographic regions (Northeast, South, Midwest, and West) were defined according to Census Bureau boundaries.[19] To adjust for confounding by patient mix, Elixhauser comorbidity measures were derived based on the ICD‐9 codes, using the Agency for Healthcare Research and Quality's Comorbidity Software.[20] This risk‐adjustment tool has been derived and validated extensively.[21]
Outcome Measures
The outcome measures were all‐cause inpatient mortality, hospital LOS, hospital charges, and ventilator‐related complications. Three ventilator‐related complications were identified using ICD‐9 procedure codes: ventilator‐associated pneumonia (997.31), facial injury (910.x), and iatrogenic pneumothorax (512.1).
Statistical Analysis
Summary statistics are presented as proportions (with 95% confidence intervals [CI]), means (with standard deviations [SD]), or medians (with interquartile ranges). Bivariate associations were examined using Student t tests, Kruskal‐Wallis tests, and [2] tests, as appropriate. Emergency department and discharge weights were used to obtain national estimates at the ED and visit level. At all other times (eg, the propensity score and instrumental variable analyses), the unweighted cohort was analyzed, because survey weights are generally not advised for propensity score analysis using complex survey data.[22]
Propensity Score Analysis
To adjust for baseline patient and ED characteristics that may have confounded the relationship between ventilation mode and clinical outcomes, we performed propensity score and instrumental variable analyses. To compare the effectiveness of NIV vs IMV, a propensity score or predicted probability of NIV was estimated using a logistic‐regression model with all patient characteristics (age, sex, quartiles for median household income, weekend admission, insurance status, season, calendar year, and comorbid conditions) and ED characteristics (urban/rural and teaching status, US region, annual ED volume, and annual volume of AECOPD with respiratory failure) as the independent variables. We then performed 1:1 propensity score matching based on a nearest‐neighbor algorithm with caliper distance of 0.01. Although propensity score matching may result in a smaller sample, it provides a clinically relevant estimate of treatment effect because subjects in the matched sample are potential candidates for either treatment option.[23, 24] An absolute standardized difference between characteristics of <10% was considered as adequate balance.[25]
Instrumental Variable Analysis
When hospitals always or nearly always use NIV or IMV, this suggests the choice is largely independent of patient characteristics, and it is possible to use the hospital preference as a proxy for the actual treatment choice (ie, an instrument variable). The instrumental variable analysis simulates a natural randomization of patients to 2 hospital groups with high and low NIV use.
The main difference between instrumental variable and propensity score analysis is that the former could potentially adjust for unmeasured confounders.[26] We used Stata procedure IVREG to estimate the outcome differences between NIV‐preferring hospitals (NIV use in 90% of patients) and IMV‐preferring hospitals (NIV use in 10% of patients).
All odds ratios (ORs) and ‐coefficients are presented with 95% CIs. All analyses were performed using Stata 12.0 software (StataCorp, College Station, TX). All P values are 2‐sided, with P<0.05 considered statistically significant.
Sensitivity Analyses
We conducted a sensitivity analysis to determine whether it was plausible that an unmeasured confounder could completely explain the observed results. The risk ratio of a hypothetical unmeasured confounder on study outcome and the exposure‐confounder imbalance were both varied to see at what point the observed association was reduced to 1.0.[27]
RESULTS
Patient and ED Characteristics
The 20062008 NEDS sample contained 67,651 ED visits for AECOPD with acute respiratory failure from 1594 US EDs. After the weighting procedure, there were an estimated 101,000 visits annually for AECOPD with acute respiratory failure from approximately 4700 US EDs. In the weighted analysis, the mean patient age of these visits was 68 years, and 56% were made by women. Ninety‐six percent were admitted to the hospital. Of these, the mortality rate was 9% and the mean hospital LOS was 7 days. Figure 1 shows the secular trends in NIV, IMV, and the combined use over the 3‐year study period. Use of IMV decreased from 28% in 2006 to 19% in 2008 (P<0.001), whereas NIV use increased slightly from 14% in 2006 to 16% in 2008 (P=0.049); the combined use of both ventilation modalities remained stable (4%). Inpatient mortality decreased from 10% in 2006 to 7% in 2008 (P<0.001).
Figure 2 shows that the frequency of NIV use (including combined use of NIV and IMV) varied widely between hospitals, ranging from 0% to 100% with a median of 11%. In the unweighted cohort of AECOPD with acute respiratory failure, 43% received some forms of ventilatory support. Table 1 shows the patient and hospital characteristics of the patients receiving ventilatory support: 36% received NIV, 56% received IMV, and 8% received combined use. In general, patients receiving combined use of NIV and IMV tended to have more comorbidities (eg, congestive heart failure and pneumonia) compared with the NIV‐alone or IMV‐alone groups. With respect to hospital characteristics, NIV was used more often in hospitals with higher volumes of COPD exacerbation and respiratory failure, in nonmetropolitan hospitals, and in hospitals in the Northeast.
| NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value, A vs B | P Value, B vs C | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age, y, | <0.001 | 0.64 | |||
| 4049 | 5 | 5 | 5 | ||
| 5059 | 17 | 18 | 19 | ||
| 6069 | 31 | 33 | 33 | ||
| 7079 | 30 | 29 | 29 | ||
| 80 | 17 | 15 | 13 | ||
| Female sex, % | 57 | 53 | 54 | <0.001 | 0.87 |
| Quartile for median household income of patient ZIP code, $, % | <0.001 | <0.001 | |||
| 138,999 | 30 | 34 | 29 | ||
| 39,00047,999 | 28 | 28 | 28 | ||
| 48,00062,999 | 24 | 22 | 24 | ||
| 63,000 | 18 | 15 | 19 | ||
| Weekend admission, % | 27 | 28 | 28 | 0.07 | 0.80 |
| Insurance status, % | <0.001 | 0.91 | |||
| Medicare | 74 | 70 | 70 | ||
| Medicaid | 9 | 12 | 12 | ||
| Private | 12 | 13 | 13 | ||
| Self‐pay | 2 | 3 | 2 | ||
| Other | 2 | 2 | 2 | ||
| Season, % | <0.001 | 0.16 | |||
| Winter (January 1March 31) | 29 | 32 | 31 | ||
| Spring (April 1June 30) | 24 | 25 | 26 | ||
| Summer (July 1September 30) | 22 | 20 | 19 | ||
| Fall (October 1December 31) | 25 | 22 | 24 | ||
| No. of comorbidities, median (IQR) | 4 (35) | 4 (35) | 4 (36) | <0.001 | <0.001 |
| Selected comorbidities, % | |||||
| Hypertension | 56 | 55 | 55 | 0.01 | 0.65 |
| CHF | 38 | 40 | 44 | 0.001 | <0.001 |
| Fluid and electrolyte disorders | 37 | 44 | 49 | <0.001 | <0.001 |
| Diabetes, uncomplicated | 27 | 26 | 29 | 0.04 | 0.002 |
| Pneumonia | 19 | 34 | 39 | <0.001 | <0.001 |
| Deficiency anemia | 16 | 19 | 19 | <0.001 | 0.39 |
| Obesity | 18 | 12 | 17 | <0.001 | <0.001 |
| Depression | 15 | 11 | 11 | <0.001 | 0.54 |
| Pulmonary circulatory diseases | 15 | 11 | 14 | <0.001 | <0.001 |
| Hospital characteristics | |||||
| Annual ED visit volume, median (IQR) | 42,704 (29,50562,470) | 44,119 (29,89564,097) | 46,695 (31,29866,235) | 0.02 | 0.0003 |
| Annual ED volume of COPD exacerbation with respiratory failure, median (IQR) | 45 (2672) | 42 (2368) | 38 (2364) | <0.001 | <0.001 |
| Urban/rural and teaching status, % | <0.001 | <0.001 | |||
| Metropolitan nonteaching | 53 | 52 | 47 | ||
| Metropolitan teaching | 31 | 35 | 39 | ||
| Nonmetropolitan | 16 | 13 | 13 | ||
| US region, % | <0.001 | <0.001 | |||
| Northeast | 28 | 16 | 36 | ||
| Midwest | 17 | 22 | 15 | ||
| South | 41 | 45 | 32 | ||
| West | 14 | 17 | 17 | ||
The unadjusted differences in outcomes are shown in Table 2. The combined‐use group had the highest inpatient mortality, longest LOS, and highest charges, followed by the IMV and NIV groups. In general, complications were few across all 3 groups, but the rate of iatrogenic pneumothorax was notably lower in the NIV group. Table 3 details the statistically significant predictors of NIV use in the propensity score model. Similar to the unadjusted analysis, older age, high‐income neighborhoods, Medicare insurance, and some comorbidities were positively associated with NIV use (eg, pulmonary circulatory disorders and liver disease), whereas a few comorbidities were negatively associated with NIV use (eg, pneumonia, and alcohol and drug abuse). With respect to hospital characteristics, higher case volumes of COPD exacerbation/respiratory failure, Northeastern and nonmetropolitan hospitals, and more recent years were associated with NIV use.
| Outcome | NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value. A vs B | P Value, B vs C |
|---|---|---|---|---|---|
| |||||
| Inpatient mortality, n (%) | 825 (8) | 2,454 (16) | 407 (18) | <0.001 | 0.04 |
| Hospital length of stay, median (IQR), d | 5 (48) | 8 (513) | 10 (716) | <0.001 | <0.001 |
| Hospital charge per visit, median (IQR), $ | 26,002 (15,74744,638) | 53,432 (31,99892,664) | 64,585 (39,024110,336) | <0.001 | <0.001 |
| Complications* | |||||
| Ventilator‐associated pneumonia, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.09 | 1.00 |
| Facial injury, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.26 | 1.00 |
| Iatrogenic pneumothorax, n (%) | 10 (0.1) | 90 (0.6) | 14 (0.6) | <0.001 | 0.90 |
| Patient Characteristics | Adjusted OR (95% CI)* | P Value |
|---|---|---|
| ||
| Age, y | ||
| 4049 | 1.00 (Reference) | |
| 5059 | 0.96 (0.84‐1.11) | 0.61 |
| 6069 | 0.96 (0.84‐1.10) | 0.56 |
| 7079 | 1.09 (0.94‐1.25) | 0.25 |
| 80 | 1.30 (1.12‐1.52) | 0.001 |
| Quartile for median household income of patient ZIP code, $ | ||
| 138,999 | 1.00 (Reference) | |
| 39,00047,999 | 1.13 (1.05‐1.21) | 0.001 |
| 48,00062,999 | 1.21 (1.12‐1.30) | <0.001 |
| 63,000 | 1.21 (1.11‐1.32) | <0.001 |
| Insurance status | ||
| Medicare | 1.00 (Reference) | |
| Medicaid | 0.79 (0.72‐0.88) | <0.001 |
| Private | 0.88 (0.81‐0.96) | 0.004 |
| Self‐pay | 0.68 (0.56‐0.82) | <0.001 |
| Other | 0.88 (0.73‐1.07) | 0.22 |
| Season | ||
| Winter (January 1March 31) | 1.00 (Reference) | |
| Spring (April 1June 30) | 1.06 (0.99‐1.14) | 0.11 |
| Summer (July 1September 30) | 1.17 (1.08‐1.26) | <0.001 |
| Fall (October 1December 31) | 1.24 (1.15‐1.33) | <0.001 |
| Comorbidity | ||
| CHF | 0.90 (0.85‐0.95) | <0.001 |
| Pulmonary circulatory disorders | 1.40 (1.29‐1.52) | <0.001 |
| Diabetes, complicated | 1.25 (1.08‐1.44) | 0.002 |
| Liver disease | 1.79 (1.40‐2.28) | <0.001 |
| Coagulopathy | 0.54 (0.46‐0.63) | <0.001 |
| Obesity | 1.52 (1.41‐1.65) | <0.001 |
| Weight loss | 0.50 (0.44‐0.57) | <0.001 |
| Fluid and electrolyte disorders | 0.84 (0.80‐0.89) | <0.001 |
| Deficiency anemia | 0.83 (0.78‐0.90) | <0.001 |
| Alcohol abuse | 0.66 (0.58‐0.76) | <0.001 |
| Drug abuse | 0.74 (0.62‐0.88) | 0.001 |
| Psychoses | 1.22 (1.10‐1.37) | <0.001 |
| Depression | 1.45 (1.34‐1.57) | <0.001 |
| Pneumonia | 0.48 (0.45‐0.51) | <0.001 |
| Valvular heart disease | 0.87 (0.77‐0.97) | 0.01 |
| Neurological disorders | 0.89 (0.80‐0.98) | 0.02 |
| RA/collagen vascular diseases | 1.25 (1.02‐1.53) | 0.04 |
| Blood‐loss anemia | 0.72 (0.53‐0.97) | 0.03 |
| Hospital characteristics | ||
| Annual ED visit volume, per 1000‐visit increase | 0.997 (0.996‐0.998) | <0.001 |
| Annual ED volume of COPD exacerbation with respiratory failure, per 10‐visit increase | 1.03 (1.02‐1.03) | <0.001 |
| Urban/rural and teaching status | ||
| Metropolitan nonteaching | 1.00 (Reference) | |
| Metropolitan teaching | 0.91 (0.85‐0.97) | 0.006 |
| Nonmetropolitan | 1.30 (1.20‐1.42) | <0.001 |
| US region | ||
| Northeast | 1.00 (Reference) | |
| Midwest | 0.44 (0.40‐0.48) | <0.001 |
| South | 0.54 (0.50‐0.58) | <0.001 |
| West | 0.51 (0.46‐0.56) | <0.001 |
| Calendar year | ||
| 2006 | 1.00 (Reference) | |
| 2007 | 1.30 (1.22‐1.39) | <0.001 |
| 2008 | 1.65 (1.54‐1.76) | <0.001 |
In terms of propensity score distributions (see Supporting Information, Figure E1, in the online version of this article), there was sufficient overlap of the NIV and IMV groups. After matching on propensity score for the NIV and IMV groups, the differences in baseline characteristics were all balanced (see Supporting Information, Table E1, in the online version of this article), as indicated by <10% standardized differences in all covariates between the 2 groups. Finally, in the propensity scorematched cohort (see Supporting Information, Table E2, in the online version of this article), NIV use remained associated with significantly lower inpatient mortality (risk ratio: 0.54; 95% CI: 0.50‐0.59, P<0.001), a shorter hospital LOS (mean difference, 3.2 days; 95% CI: 3.4 to 2.9 days, P<0.001), and lower hospital charges (mean difference, P<$35,012; 95% CI: $36,848 to $33,176, P<0.001), compared with IMV use. Use of NIV also was associated with a lower rate of iatrogenic pneumothorax than IMV use (0.05% vs 0.5%, P<0.001).
Using hospital preference for NIV vs IMV as an instrument, the instrumental analysis confirmed the benefits of NIV use, with a 5% reduction in inpatient mortality in the NIV‐preferring hospitals (risk difference, P<5%; 95% CI: P<1.8% to P<8.3%).
In the sensitivity analysis to assess the impact of an unmeasured confounder, the confounder would have had to have a very strong impact on outcome (risk ratio: 5) and a severe exposure‐confounder imbalance (odds ratio of exposure on confounder: 5) to reduce the observed association to 1.0. In other words, an individual unmeasured confounder is unlikely to explain the observed association.
DISCUSSION
In this nationally representative sample of 67,651 ED visits for AECOPD with acute respiratory failure, we found that NIV use was increasing from 2006 to 2008. However, the utilization of NIV remained low (16% in 2008) and varied widely by patient and hospital characteristic. As with all observational studies, causality cannot be inferred definitely; however, our study suggests that, NIV usecompared with IMV usewas associated with potentially important benefits: a reduction of inpatient mortality by 46%, shortened hospital LOS by 3 days, reduced hospital charges by approximately $35,000 per visit, and modestly reduced risk of iatrogenic pneumothorax.
A recent analysis using the US Nationwide Inpatient Sample has shown increasing use of NIV and concomitant decreasing mortality in AECOPD over time.[28] Our analysis confirmed these favorable trends in the United States using a much larger NEDS sample (28 million visits in the NEDS vs 8 million visits in the Nationwide Inpatient Sample per year). Despite these favorable trends, NIV was still underutilized for AECOPD with respiratory failure in the United States (16% in 2008) compared with major European countries (40%).[29] Although our study lacked clinical details to arrive at the optimal rate of NIV use, the low rate of NIV use is concerning and suggests room for improvement in NIV use in appropriate patients as outlined by the current COPD guidelines.[18, 30] Why is NIV not widely adopted, given its demonstrated efficacy? Previous surveys have identified several perceived reasons for low NIV use, including lack of physician knowledge, insufficient respiratory therapist training, inadequate equipment, and time required for setting up NIV.[29, 31, 32] Our study adds to the literature by showing the actual predictors of NIV use in the real world. Our data showed that the early adopters were hospitals with higher case volumes, and hospitals in the Northeast and in nonmetropolitan areas. A higher case volume has been linked with lower mortality in AECOPD (ie, practice makes perfect),[33] and frequent NIV use could explain the lower AECOPD mortality in highcase volume centers. Alternatively, smaller hospitals tend to have moonlighters working in EDs who may not be board certified in emergency medicine. Perhaps the logical next step is to conduct a qualitative study to understand the specifics of best practices and provider characteristics in these Northeastern, highercase volume centers. Another incentive to promote NIV use in clinical practice is the cost‐effectiveness associated with this intervention, as previous studies have shown that, compared with usual care, receiving NIV was associated with a reduction in costs, mainly through reduced use of the ICU.[34, 35]
Some patient factors associated with NIV use may be well justified. For example, older AECOPD patients may have an advance directive describing their treatment wishes (eg, do‐not‐intubate order),[36] and therefore NIV was preferred to IMV. Also, our data suggested AECOPD patients with a suspected pneumonia component were less likely to be placed on NIV, which is consistent with COPD guideline recommendations.[18, 30] As outlined in the current guidelines, the major contraindications to NIV include impending respiratory arrest, excessive respiratory secretions, massive gastrointestinal bleeding, recent facial trauma, or altered mental status.[18, 30] By contrast, some factors associated with NIV use may be targeted for intervention, such as lower rates of NIV use in the uninsured, patients who live in low‐income neighborhoods, and hospitals in US regions other than the Northeast.
Current guidelines recommend using NIV in AECOPD patients with early signs of respiratory failure, such as arterial pH of 7.257.35 or pCO2 45 mm Hg.[18, 30] When NIV is considered as the modality of ventilatory support, it should probably be used as early as possible,[37] because evidence suggests that delayed use of NIV may lead to severe respiratory acidosis and increased mortality.[38] Other than in ICUs, NIV can be used on general wards and in EDs that have adequate staff training and experience, because the success rates of NIV in these settings are similar to those reported in ICU studies.[8, 36, 39] In addition, NIV is more cost‐effective when performed outside the ICU.[35] In fact, studies have found a substantial portion of patients had NIV started in the ED (one‐fourth) and on the general ward (one‐fourth).[31, 40] Given the shortage of intensivists in the United States, hospitalists begin to play an important role in provision of critical care outside the ICU.[41] Once NIV is used, it is important to ensure that it is delivered effectively and monitored closely because NIV failure has been shown to be associated with high mortality.[28, 42]
This study has some potential limitations. First, we used administrative claims that lack clinical details such as data on arterial blood gases and severity scores, and thus potential residual confounding may exist. In our study, the IMV group may be sicker than the NIV group, which could partially explain the increased mortality with IMV. However, the propensity scores overlap to a great extent between the 2 study groups, suggesting that a strong confounding bias is less likely, given the observed covariates. Furthermore, the instrumental variable and sensitivity analyses taking into account unmeasured confounders still suggested the benefits of NIV. Second, the NEDS does not contain data on the location where NIV was initiated (eg, ED, ward, or ICU) or the timing of initiating NIV or IMV. As a result, for the combined‐use group, we could not further distinguish the group switching from NIV to IMV (ie, NIV failure)[42] or from IMV to NIV (ie, NIV as a weaning strategy).[43] Accordingly, we chose to focus on the comparativeness effectiveness of NIV vs IMV. Third, although the NEDS data have undergone quality‐control procedures,[44] some misclassification may exist in identifying patient population and interventions. Finally, the analysis may not reflect the most recent trend in NIV use, as the 2010 NEDS data have just been released. In addition, although the study is the largest to date on this topic, our findings may not be generalizable to EDs that were not part of the NEDS.
In summary, in this nationally representative ED and inpatient database, NIV use is increasing for AECOPD with acute respiratory failure; however, its adoption remains low and varies widely between US hospitals. Our observational study suggests that NIV appears to be more effective and safer than IMV in the real‐world setting. There is an opportunity to increase the use of NIV as recommended in guidelines and to promote the use NIV in replacement of IMV in patients with severe AECOPD. Given the increasing mortality burden of COPD, such a strategy may help reduce COPD mortality at the population level, thereby fulfilling the objectives of Healthy People 2020.
Disclosure
Partial results from this study were presented at the 2012 Society for Academic Emergency Medicine Annual Meeting, Chicago, Illinois, May 912, 2012. This project was supported by grant number R03HS020722 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to disclose.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports, 2011. Deaths: final data for 2008. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. Accessed August 15, 2012.
- , , , . Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259.
- , , . National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2008;15:1275–1283.
- , , , , . Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16.
- US Department of Health and Human Services. Healthy People 2020. Objectives for Respiratory Diseases. Available at: http://www.healthypeople. gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=36. Accessed May 3, 2012.
- . Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;47:31s–37s.
- , , , et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935.
- , , , et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
- , , , et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
- . Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–3924.
- , , , , , . Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol. 2009;33:791–797.
- , , , et al. Noninvasive Positive‐Pressure Ventilation (NPPV) for Acute Respiratory Failure. Rockville, MD: Agency for Healthcare Research and Quality; July 2012: Report 12‐EHC089‐EF. Available at: http://effectivehealthcareahrqgov/ehc/products/273/1180/CER68_NPPV_FinalReport_20120706pdf. Accessed December 11, 2012.
- Healthcare Cost and Utilization Project (HCUP). HCUP Nationwide Emergency Department Sample (NEDS). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/nedsoverview.jsp. Accessed April 15, 2012.
- American Hospital Association. Annual survey database. Available at: http://www.ahadata.com/ahadata/html/AHASurvey.html. Accessed April 15, 2012.
- , , , . Age‐related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252e1–1258e1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). NHLBI/WHO Global Strategy for the Diagnosis, Management and Prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 15, 2012.
- .US Bureau of the Census. Census regions and divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed April 9, 2012.
- .Healthcare Cost and Utilization Project. Comorbidity software. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 15, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Healthcare Cost and Utilization Project (HCUP). HCUP Methods Series. Hierarchical Modeling Using HCUP Data. Rockville, MD: Agency for Healthcare Research and Quality; 2012: Report 2007‐01. Available at: http://www.hcup‐us.ahrq.gov/reports/methods/2007_01.pdf. Accessed April 15, 2012.
- , , . Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- , , , , , . Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042.
- . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554.
- . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
- , , , , . A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36:362–369.
- , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- , , , . Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129:1226–1233.
- , , . A survey of the use of noninvasive ventilation in academic emergency departments in the United States. Respir Care. 2009;54:1306–1312.
- , , . Emergency department case volume and patient outcomes in acute exacerbations of chronic obstructive pulmonary disease. Acad Emerg Med. 2012;19:656–663.
- , , , , . Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102.
- , , , . Cost effectiveness of ward based non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956.
- , , . Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do‐not‐intubate” patients. Crit Care Med. 2005;33:1976–1982.
- , . Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: “Don't think twice, it's alright!”. Am J Respir Crit Care Med. 2012;185:121–123.
- , , , , . Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48.
- , , , , . Use of noninvasive positive‐pressure ventilation on the regular hospital ward: experience and correlates of success. Respir Care. 2006;51:1237–1243.
- , , , et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477–1483.
- . Hospitalists and intensivists: partners in caring for the critically ill—the time has come. J Hosp Med. 2010;5:1–3.
- , , , , , . Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825.
- , , , . Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127.
- Healthcare Cost and Utilization Project (HCUP). HCUP Quality Control Procedures. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/db/quality.jsp. Accessed December 15, 2012.
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States,[1] and its rising mortality trend is unique among the top 5 causes of death.[2] Acute exacerbations of COPD (AECOPD) are important events in the natural history of COPD, accounting for 1.5 million emergency department (ED) visits and 726,000 hospitalizations each year in the United States.[3, 4] Given the significant morbidity and mortality from AECOPD, Healthy People 2020 lists reducing deaths, hospitalizations, and ED visits as the key objectives for COPD.[5]
Over the past 2 decades, noninvasive ventilation (NIV) has emerged as a potentially useful treatment modality in AECOPD patients with acute respiratory failure. Noninvasive ventilation commonly refers to positive‐pressure ventilatory support delivered through a nasal or full‐face mask, such as bilevel positive airway pressure.[6] A number of randomized controlled trials[7, 8, 9] and meta‐analyses[10] have suggested a mortality‐reduction benefit with NIV use compared with standard medical care in AECOPD. To our knowledge, however, very few small randomized controlled trials compared NIV vs invasive mechanical ventilation (IMV) head‐to‐head,[11, 12, 13] and a recent evidence review found only 5 studies (405 subjects) on this topic.[14] Collectively, the limited evidence from randomized trials showed that NIV use resulted in similar intensive care unit (ICU) and in‐hospital mortality, fewer complications (eg, ventilator‐associated pneumonia and sepsis), and shorter hospital length of stays (LOS). Given that these trials have a smaller sample size and tend to exclude older patient (age >75 years) or patients with multiple comorbidities, there is a need to better understand the adoption and effectiveness of NIV treatment for AECOPD in a much larger patient population in the real‐world setting using observational data.
To address these knowledge gaps in the literature, we analyzed data from a large, nationally representative ED and inpatient sample. The objective of the present analysis was 2‐fold: (1) to characterize the use of NIV and IMV in AECOPD patients with acute respiratory failure at a national level; and (2) to compare the effectiveness of NIV vs IMV in the real‐world setting.
METHODS
Study Design and Setting
We conducted a retrospective cohort study using data from the 20062008 Nationwide Emergency Department Sample (NEDS),[15] a component of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The NEDS is nationally representative of all community hospitalbased EDs in the United States, defined by the American Hospital Association as all nonfederal, short‐term, general, and other specialty hospitals.[16] Community hospitals include academic medical centers if they are nonfederal short‐term hospitals. The NEDS was constructed using administrative records from the State Emergency Department Databases and the State Inpatient Databases. The former captures information on ED visits that do not result in an admission (ie, treat‐and‐release visits or transfers to another hospital); the latter contains information on patients initially seen in the ED and then admitted to the same hospital. Taken together, the resulting NEDS represents all ED visits regardless of disposition and contains information on short‐term outcomes for patients admitted through the ED. In other words, the NEDS is the largest all‐payer ED and inpatient database in the United States. The NEDS represents an approximately 20% stratified sample of US hospital‐based EDs, containing more than 28 million records of ED visits from approximately 1000 hospitals each year. Additional details of the NEDS can be found elsewhere.[15, 17] We received a waiver for this analysis from our institutional review board.
Study Population
Patient visits were included in this analysis if they carried any COPD‐related diagnostic code (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 491.xx [chronic bronchitis], 492.xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) as their primary ED diagnosis and any acute respiratory failure code (ie, 518.81 [acute respiratory failure], 518.82 [pulmonary insufficiency not elsewhere classified, 518.84 [acute and chronic respiratory failure], or 799.1 [respiratory arrest]) as their secondary diagnosis. Patient visits with a primary diagnosis of acute respiratory failure and a secondary diagnosis of COPD were also included. Patients age <40 years were excluded, because they are much less likely to have COPD.[18]
Modes of Mechanical Ventilation
The primary exposure variable was mode of mechanical ventilation. To compare the effectiveness of different ventilatory modes, patients were divided into 3 groups according to the ventilation mode they received: (1) NIV alone, (2) IMV alone, and (3) combined modes of NIV and IMV. The use of NIV was identified by using Current Procedural Terminology (CPT) code of 94660 or ICD‐9 procedure code 93.90, whereas the use of IMV was identified by using CPT code of 31500 or ICD‐9 procedure code 96.04 or 96.7x.
Patient‐Level and Emergency DepartmentLevel Variables
The NEDS contains information on patient demographics, national quartiles for median household income based on the patient's ZIP code, payment sources, ICD‐9‐CM diagnoses and procedures, ED disposition, hospital LOS, and hospital disposition. Hospital characteristics include annual visit volume, urban‐rural status, ownership, teaching status, and US region. Geographic regions (Northeast, South, Midwest, and West) were defined according to Census Bureau boundaries.[19] To adjust for confounding by patient mix, Elixhauser comorbidity measures were derived based on the ICD‐9 codes, using the Agency for Healthcare Research and Quality's Comorbidity Software.[20] This risk‐adjustment tool has been derived and validated extensively.[21]
Outcome Measures
The outcome measures were all‐cause inpatient mortality, hospital LOS, hospital charges, and ventilator‐related complications. Three ventilator‐related complications were identified using ICD‐9 procedure codes: ventilator‐associated pneumonia (997.31), facial injury (910.x), and iatrogenic pneumothorax (512.1).
Statistical Analysis
Summary statistics are presented as proportions (with 95% confidence intervals [CI]), means (with standard deviations [SD]), or medians (with interquartile ranges). Bivariate associations were examined using Student t tests, Kruskal‐Wallis tests, and [2] tests, as appropriate. Emergency department and discharge weights were used to obtain national estimates at the ED and visit level. At all other times (eg, the propensity score and instrumental variable analyses), the unweighted cohort was analyzed, because survey weights are generally not advised for propensity score analysis using complex survey data.[22]
Propensity Score Analysis
To adjust for baseline patient and ED characteristics that may have confounded the relationship between ventilation mode and clinical outcomes, we performed propensity score and instrumental variable analyses. To compare the effectiveness of NIV vs IMV, a propensity score or predicted probability of NIV was estimated using a logistic‐regression model with all patient characteristics (age, sex, quartiles for median household income, weekend admission, insurance status, season, calendar year, and comorbid conditions) and ED characteristics (urban/rural and teaching status, US region, annual ED volume, and annual volume of AECOPD with respiratory failure) as the independent variables. We then performed 1:1 propensity score matching based on a nearest‐neighbor algorithm with caliper distance of 0.01. Although propensity score matching may result in a smaller sample, it provides a clinically relevant estimate of treatment effect because subjects in the matched sample are potential candidates for either treatment option.[23, 24] An absolute standardized difference between characteristics of <10% was considered as adequate balance.[25]
Instrumental Variable Analysis
When hospitals always or nearly always use NIV or IMV, this suggests the choice is largely independent of patient characteristics, and it is possible to use the hospital preference as a proxy for the actual treatment choice (ie, an instrument variable). The instrumental variable analysis simulates a natural randomization of patients to 2 hospital groups with high and low NIV use.
The main difference between instrumental variable and propensity score analysis is that the former could potentially adjust for unmeasured confounders.[26] We used Stata procedure IVREG to estimate the outcome differences between NIV‐preferring hospitals (NIV use in 90% of patients) and IMV‐preferring hospitals (NIV use in 10% of patients).
All odds ratios (ORs) and ‐coefficients are presented with 95% CIs. All analyses were performed using Stata 12.0 software (StataCorp, College Station, TX). All P values are 2‐sided, with P<0.05 considered statistically significant.
Sensitivity Analyses
We conducted a sensitivity analysis to determine whether it was plausible that an unmeasured confounder could completely explain the observed results. The risk ratio of a hypothetical unmeasured confounder on study outcome and the exposure‐confounder imbalance were both varied to see at what point the observed association was reduced to 1.0.[27]
RESULTS
Patient and ED Characteristics
The 20062008 NEDS sample contained 67,651 ED visits for AECOPD with acute respiratory failure from 1594 US EDs. After the weighting procedure, there were an estimated 101,000 visits annually for AECOPD with acute respiratory failure from approximately 4700 US EDs. In the weighted analysis, the mean patient age of these visits was 68 years, and 56% were made by women. Ninety‐six percent were admitted to the hospital. Of these, the mortality rate was 9% and the mean hospital LOS was 7 days. Figure 1 shows the secular trends in NIV, IMV, and the combined use over the 3‐year study period. Use of IMV decreased from 28% in 2006 to 19% in 2008 (P<0.001), whereas NIV use increased slightly from 14% in 2006 to 16% in 2008 (P=0.049); the combined use of both ventilation modalities remained stable (4%). Inpatient mortality decreased from 10% in 2006 to 7% in 2008 (P<0.001).

Figure 2 shows that the frequency of NIV use (including combined use of NIV and IMV) varied widely between hospitals, ranging from 0% to 100% with a median of 11%. In the unweighted cohort of AECOPD with acute respiratory failure, 43% received some forms of ventilatory support. Table 1 shows the patient and hospital characteristics of the patients receiving ventilatory support: 36% received NIV, 56% received IMV, and 8% received combined use. In general, patients receiving combined use of NIV and IMV tended to have more comorbidities (eg, congestive heart failure and pneumonia) compared with the NIV‐alone or IMV‐alone groups. With respect to hospital characteristics, NIV was used more often in hospitals with higher volumes of COPD exacerbation and respiratory failure, in nonmetropolitan hospitals, and in hospitals in the Northeast.

| NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value, A vs B | P Value, B vs C | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age, y, | <0.001 | 0.64 | |||
| 4049 | 5 | 5 | 5 | ||
| 5059 | 17 | 18 | 19 | ||
| 6069 | 31 | 33 | 33 | ||
| 7079 | 30 | 29 | 29 | ||
| 80 | 17 | 15 | 13 | ||
| Female sex, % | 57 | 53 | 54 | <0.001 | 0.87 |
| Quartile for median household income of patient ZIP code, $, % | <0.001 | <0.001 | |||
| 138,999 | 30 | 34 | 29 | ||
| 39,00047,999 | 28 | 28 | 28 | ||
| 48,00062,999 | 24 | 22 | 24 | ||
| 63,000 | 18 | 15 | 19 | ||
| Weekend admission, % | 27 | 28 | 28 | 0.07 | 0.80 |
| Insurance status, % | <0.001 | 0.91 | |||
| Medicare | 74 | 70 | 70 | ||
| Medicaid | 9 | 12 | 12 | ||
| Private | 12 | 13 | 13 | ||
| Self‐pay | 2 | 3 | 2 | ||
| Other | 2 | 2 | 2 | ||
| Season, % | <0.001 | 0.16 | |||
| Winter (January 1March 31) | 29 | 32 | 31 | ||
| Spring (April 1June 30) | 24 | 25 | 26 | ||
| Summer (July 1September 30) | 22 | 20 | 19 | ||
| Fall (October 1December 31) | 25 | 22 | 24 | ||
| No. of comorbidities, median (IQR) | 4 (35) | 4 (35) | 4 (36) | <0.001 | <0.001 |
| Selected comorbidities, % | |||||
| Hypertension | 56 | 55 | 55 | 0.01 | 0.65 |
| CHF | 38 | 40 | 44 | 0.001 | <0.001 |
| Fluid and electrolyte disorders | 37 | 44 | 49 | <0.001 | <0.001 |
| Diabetes, uncomplicated | 27 | 26 | 29 | 0.04 | 0.002 |
| Pneumonia | 19 | 34 | 39 | <0.001 | <0.001 |
| Deficiency anemia | 16 | 19 | 19 | <0.001 | 0.39 |
| Obesity | 18 | 12 | 17 | <0.001 | <0.001 |
| Depression | 15 | 11 | 11 | <0.001 | 0.54 |
| Pulmonary circulatory diseases | 15 | 11 | 14 | <0.001 | <0.001 |
| Hospital characteristics | |||||
| Annual ED visit volume, median (IQR) | 42,704 (29,50562,470) | 44,119 (29,89564,097) | 46,695 (31,29866,235) | 0.02 | 0.0003 |
| Annual ED volume of COPD exacerbation with respiratory failure, median (IQR) | 45 (2672) | 42 (2368) | 38 (2364) | <0.001 | <0.001 |
| Urban/rural and teaching status, % | <0.001 | <0.001 | |||
| Metropolitan nonteaching | 53 | 52 | 47 | ||
| Metropolitan teaching | 31 | 35 | 39 | ||
| Nonmetropolitan | 16 | 13 | 13 | ||
| US region, % | <0.001 | <0.001 | |||
| Northeast | 28 | 16 | 36 | ||
| Midwest | 17 | 22 | 15 | ||
| South | 41 | 45 | 32 | ||
| West | 14 | 17 | 17 | ||
The unadjusted differences in outcomes are shown in Table 2. The combined‐use group had the highest inpatient mortality, longest LOS, and highest charges, followed by the IMV and NIV groups. In general, complications were few across all 3 groups, but the rate of iatrogenic pneumothorax was notably lower in the NIV group. Table 3 details the statistically significant predictors of NIV use in the propensity score model. Similar to the unadjusted analysis, older age, high‐income neighborhoods, Medicare insurance, and some comorbidities were positively associated with NIV use (eg, pulmonary circulatory disorders and liver disease), whereas a few comorbidities were negatively associated with NIV use (eg, pneumonia, and alcohol and drug abuse). With respect to hospital characteristics, higher case volumes of COPD exacerbation/respiratory failure, Northeastern and nonmetropolitan hospitals, and more recent years were associated with NIV use.
| Outcome | NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value. A vs B | P Value, B vs C |
|---|---|---|---|---|---|
| |||||
| Inpatient mortality, n (%) | 825 (8) | 2,454 (16) | 407 (18) | <0.001 | 0.04 |
| Hospital length of stay, median (IQR), d | 5 (48) | 8 (513) | 10 (716) | <0.001 | <0.001 |
| Hospital charge per visit, median (IQR), $ | 26,002 (15,74744,638) | 53,432 (31,99892,664) | 64,585 (39,024110,336) | <0.001 | <0.001 |
| Complications* | |||||
| Ventilator‐associated pneumonia, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.09 | 1.00 |
| Facial injury, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.26 | 1.00 |
| Iatrogenic pneumothorax, n (%) | 10 (0.1) | 90 (0.6) | 14 (0.6) | <0.001 | 0.90 |
| Patient Characteristics | Adjusted OR (95% CI)* | P Value |
|---|---|---|
| ||
| Age, y | ||
| 4049 | 1.00 (Reference) | |
| 5059 | 0.96 (0.84‐1.11) | 0.61 |
| 6069 | 0.96 (0.84‐1.10) | 0.56 |
| 7079 | 1.09 (0.94‐1.25) | 0.25 |
| 80 | 1.30 (1.12‐1.52) | 0.001 |
| Quartile for median household income of patient ZIP code, $ | ||
| 138,999 | 1.00 (Reference) | |
| 39,00047,999 | 1.13 (1.05‐1.21) | 0.001 |
| 48,00062,999 | 1.21 (1.12‐1.30) | <0.001 |
| 63,000 | 1.21 (1.11‐1.32) | <0.001 |
| Insurance status | ||
| Medicare | 1.00 (Reference) | |
| Medicaid | 0.79 (0.72‐0.88) | <0.001 |
| Private | 0.88 (0.81‐0.96) | 0.004 |
| Self‐pay | 0.68 (0.56‐0.82) | <0.001 |
| Other | 0.88 (0.73‐1.07) | 0.22 |
| Season | ||
| Winter (January 1March 31) | 1.00 (Reference) | |
| Spring (April 1June 30) | 1.06 (0.99‐1.14) | 0.11 |
| Summer (July 1September 30) | 1.17 (1.08‐1.26) | <0.001 |
| Fall (October 1December 31) | 1.24 (1.15‐1.33) | <0.001 |
| Comorbidity | ||
| CHF | 0.90 (0.85‐0.95) | <0.001 |
| Pulmonary circulatory disorders | 1.40 (1.29‐1.52) | <0.001 |
| Diabetes, complicated | 1.25 (1.08‐1.44) | 0.002 |
| Liver disease | 1.79 (1.40‐2.28) | <0.001 |
| Coagulopathy | 0.54 (0.46‐0.63) | <0.001 |
| Obesity | 1.52 (1.41‐1.65) | <0.001 |
| Weight loss | 0.50 (0.44‐0.57) | <0.001 |
| Fluid and electrolyte disorders | 0.84 (0.80‐0.89) | <0.001 |
| Deficiency anemia | 0.83 (0.78‐0.90) | <0.001 |
| Alcohol abuse | 0.66 (0.58‐0.76) | <0.001 |
| Drug abuse | 0.74 (0.62‐0.88) | 0.001 |
| Psychoses | 1.22 (1.10‐1.37) | <0.001 |
| Depression | 1.45 (1.34‐1.57) | <0.001 |
| Pneumonia | 0.48 (0.45‐0.51) | <0.001 |
| Valvular heart disease | 0.87 (0.77‐0.97) | 0.01 |
| Neurological disorders | 0.89 (0.80‐0.98) | 0.02 |
| RA/collagen vascular diseases | 1.25 (1.02‐1.53) | 0.04 |
| Blood‐loss anemia | 0.72 (0.53‐0.97) | 0.03 |
| Hospital characteristics | ||
| Annual ED visit volume, per 1000‐visit increase | 0.997 (0.996‐0.998) | <0.001 |
| Annual ED volume of COPD exacerbation with respiratory failure, per 10‐visit increase | 1.03 (1.02‐1.03) | <0.001 |
| Urban/rural and teaching status | ||
| Metropolitan nonteaching | 1.00 (Reference) | |
| Metropolitan teaching | 0.91 (0.85‐0.97) | 0.006 |
| Nonmetropolitan | 1.30 (1.20‐1.42) | <0.001 |
| US region | ||
| Northeast | 1.00 (Reference) | |
| Midwest | 0.44 (0.40‐0.48) | <0.001 |
| South | 0.54 (0.50‐0.58) | <0.001 |
| West | 0.51 (0.46‐0.56) | <0.001 |
| Calendar year | ||
| 2006 | 1.00 (Reference) | |
| 2007 | 1.30 (1.22‐1.39) | <0.001 |
| 2008 | 1.65 (1.54‐1.76) | <0.001 |
In terms of propensity score distributions (see Supporting Information, Figure E1, in the online version of this article), there was sufficient overlap of the NIV and IMV groups. After matching on propensity score for the NIV and IMV groups, the differences in baseline characteristics were all balanced (see Supporting Information, Table E1, in the online version of this article), as indicated by <10% standardized differences in all covariates between the 2 groups. Finally, in the propensity scorematched cohort (see Supporting Information, Table E2, in the online version of this article), NIV use remained associated with significantly lower inpatient mortality (risk ratio: 0.54; 95% CI: 0.50‐0.59, P<0.001), a shorter hospital LOS (mean difference, 3.2 days; 95% CI: 3.4 to 2.9 days, P<0.001), and lower hospital charges (mean difference, P<$35,012; 95% CI: $36,848 to $33,176, P<0.001), compared with IMV use. Use of NIV also was associated with a lower rate of iatrogenic pneumothorax than IMV use (0.05% vs 0.5%, P<0.001).
Using hospital preference for NIV vs IMV as an instrument, the instrumental analysis confirmed the benefits of NIV use, with a 5% reduction in inpatient mortality in the NIV‐preferring hospitals (risk difference, P<5%; 95% CI: P<1.8% to P<8.3%).
In the sensitivity analysis to assess the impact of an unmeasured confounder, the confounder would have had to have a very strong impact on outcome (risk ratio: 5) and a severe exposure‐confounder imbalance (odds ratio of exposure on confounder: 5) to reduce the observed association to 1.0. In other words, an individual unmeasured confounder is unlikely to explain the observed association.
DISCUSSION
In this nationally representative sample of 67,651 ED visits for AECOPD with acute respiratory failure, we found that NIV use was increasing from 2006 to 2008. However, the utilization of NIV remained low (16% in 2008) and varied widely by patient and hospital characteristic. As with all observational studies, causality cannot be inferred definitely; however, our study suggests that, NIV usecompared with IMV usewas associated with potentially important benefits: a reduction of inpatient mortality by 46%, shortened hospital LOS by 3 days, reduced hospital charges by approximately $35,000 per visit, and modestly reduced risk of iatrogenic pneumothorax.
A recent analysis using the US Nationwide Inpatient Sample has shown increasing use of NIV and concomitant decreasing mortality in AECOPD over time.[28] Our analysis confirmed these favorable trends in the United States using a much larger NEDS sample (28 million visits in the NEDS vs 8 million visits in the Nationwide Inpatient Sample per year). Despite these favorable trends, NIV was still underutilized for AECOPD with respiratory failure in the United States (16% in 2008) compared with major European countries (40%).[29] Although our study lacked clinical details to arrive at the optimal rate of NIV use, the low rate of NIV use is concerning and suggests room for improvement in NIV use in appropriate patients as outlined by the current COPD guidelines.[18, 30] Why is NIV not widely adopted, given its demonstrated efficacy? Previous surveys have identified several perceived reasons for low NIV use, including lack of physician knowledge, insufficient respiratory therapist training, inadequate equipment, and time required for setting up NIV.[29, 31, 32] Our study adds to the literature by showing the actual predictors of NIV use in the real world. Our data showed that the early adopters were hospitals with higher case volumes, and hospitals in the Northeast and in nonmetropolitan areas. A higher case volume has been linked with lower mortality in AECOPD (ie, practice makes perfect),[33] and frequent NIV use could explain the lower AECOPD mortality in highcase volume centers. Alternatively, smaller hospitals tend to have moonlighters working in EDs who may not be board certified in emergency medicine. Perhaps the logical next step is to conduct a qualitative study to understand the specifics of best practices and provider characteristics in these Northeastern, highercase volume centers. Another incentive to promote NIV use in clinical practice is the cost‐effectiveness associated with this intervention, as previous studies have shown that, compared with usual care, receiving NIV was associated with a reduction in costs, mainly through reduced use of the ICU.[34, 35]
Some patient factors associated with NIV use may be well justified. For example, older AECOPD patients may have an advance directive describing their treatment wishes (eg, do‐not‐intubate order),[36] and therefore NIV was preferred to IMV. Also, our data suggested AECOPD patients with a suspected pneumonia component were less likely to be placed on NIV, which is consistent with COPD guideline recommendations.[18, 30] As outlined in the current guidelines, the major contraindications to NIV include impending respiratory arrest, excessive respiratory secretions, massive gastrointestinal bleeding, recent facial trauma, or altered mental status.[18, 30] By contrast, some factors associated with NIV use may be targeted for intervention, such as lower rates of NIV use in the uninsured, patients who live in low‐income neighborhoods, and hospitals in US regions other than the Northeast.
Current guidelines recommend using NIV in AECOPD patients with early signs of respiratory failure, such as arterial pH of 7.257.35 or pCO2 45 mm Hg.[18, 30] When NIV is considered as the modality of ventilatory support, it should probably be used as early as possible,[37] because evidence suggests that delayed use of NIV may lead to severe respiratory acidosis and increased mortality.[38] Other than in ICUs, NIV can be used on general wards and in EDs that have adequate staff training and experience, because the success rates of NIV in these settings are similar to those reported in ICU studies.[8, 36, 39] In addition, NIV is more cost‐effective when performed outside the ICU.[35] In fact, studies have found a substantial portion of patients had NIV started in the ED (one‐fourth) and on the general ward (one‐fourth).[31, 40] Given the shortage of intensivists in the United States, hospitalists begin to play an important role in provision of critical care outside the ICU.[41] Once NIV is used, it is important to ensure that it is delivered effectively and monitored closely because NIV failure has been shown to be associated with high mortality.[28, 42]
This study has some potential limitations. First, we used administrative claims that lack clinical details such as data on arterial blood gases and severity scores, and thus potential residual confounding may exist. In our study, the IMV group may be sicker than the NIV group, which could partially explain the increased mortality with IMV. However, the propensity scores overlap to a great extent between the 2 study groups, suggesting that a strong confounding bias is less likely, given the observed covariates. Furthermore, the instrumental variable and sensitivity analyses taking into account unmeasured confounders still suggested the benefits of NIV. Second, the NEDS does not contain data on the location where NIV was initiated (eg, ED, ward, or ICU) or the timing of initiating NIV or IMV. As a result, for the combined‐use group, we could not further distinguish the group switching from NIV to IMV (ie, NIV failure)[42] or from IMV to NIV (ie, NIV as a weaning strategy).[43] Accordingly, we chose to focus on the comparativeness effectiveness of NIV vs IMV. Third, although the NEDS data have undergone quality‐control procedures,[44] some misclassification may exist in identifying patient population and interventions. Finally, the analysis may not reflect the most recent trend in NIV use, as the 2010 NEDS data have just been released. In addition, although the study is the largest to date on this topic, our findings may not be generalizable to EDs that were not part of the NEDS.
In summary, in this nationally representative ED and inpatient database, NIV use is increasing for AECOPD with acute respiratory failure; however, its adoption remains low and varies widely between US hospitals. Our observational study suggests that NIV appears to be more effective and safer than IMV in the real‐world setting. There is an opportunity to increase the use of NIV as recommended in guidelines and to promote the use NIV in replacement of IMV in patients with severe AECOPD. Given the increasing mortality burden of COPD, such a strategy may help reduce COPD mortality at the population level, thereby fulfilling the objectives of Healthy People 2020.
Disclosure
Partial results from this study were presented at the 2012 Society for Academic Emergency Medicine Annual Meeting, Chicago, Illinois, May 912, 2012. This project was supported by grant number R03HS020722 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to disclose.
Chronic obstructive pulmonary disease (COPD) is now the third leading cause of death in the United States,[1] and its rising mortality trend is unique among the top 5 causes of death.[2] Acute exacerbations of COPD (AECOPD) are important events in the natural history of COPD, accounting for 1.5 million emergency department (ED) visits and 726,000 hospitalizations each year in the United States.[3, 4] Given the significant morbidity and mortality from AECOPD, Healthy People 2020 lists reducing deaths, hospitalizations, and ED visits as the key objectives for COPD.[5]
Over the past 2 decades, noninvasive ventilation (NIV) has emerged as a potentially useful treatment modality in AECOPD patients with acute respiratory failure. Noninvasive ventilation commonly refers to positive‐pressure ventilatory support delivered through a nasal or full‐face mask, such as bilevel positive airway pressure.[6] A number of randomized controlled trials[7, 8, 9] and meta‐analyses[10] have suggested a mortality‐reduction benefit with NIV use compared with standard medical care in AECOPD. To our knowledge, however, very few small randomized controlled trials compared NIV vs invasive mechanical ventilation (IMV) head‐to‐head,[11, 12, 13] and a recent evidence review found only 5 studies (405 subjects) on this topic.[14] Collectively, the limited evidence from randomized trials showed that NIV use resulted in similar intensive care unit (ICU) and in‐hospital mortality, fewer complications (eg, ventilator‐associated pneumonia and sepsis), and shorter hospital length of stays (LOS). Given that these trials have a smaller sample size and tend to exclude older patient (age >75 years) or patients with multiple comorbidities, there is a need to better understand the adoption and effectiveness of NIV treatment for AECOPD in a much larger patient population in the real‐world setting using observational data.
To address these knowledge gaps in the literature, we analyzed data from a large, nationally representative ED and inpatient sample. The objective of the present analysis was 2‐fold: (1) to characterize the use of NIV and IMV in AECOPD patients with acute respiratory failure at a national level; and (2) to compare the effectiveness of NIV vs IMV in the real‐world setting.
METHODS
Study Design and Setting
We conducted a retrospective cohort study using data from the 20062008 Nationwide Emergency Department Sample (NEDS),[15] a component of the Healthcare Cost and Utilization Project sponsored by the Agency for Healthcare Research and Quality. The NEDS is nationally representative of all community hospitalbased EDs in the United States, defined by the American Hospital Association as all nonfederal, short‐term, general, and other specialty hospitals.[16] Community hospitals include academic medical centers if they are nonfederal short‐term hospitals. The NEDS was constructed using administrative records from the State Emergency Department Databases and the State Inpatient Databases. The former captures information on ED visits that do not result in an admission (ie, treat‐and‐release visits or transfers to another hospital); the latter contains information on patients initially seen in the ED and then admitted to the same hospital. Taken together, the resulting NEDS represents all ED visits regardless of disposition and contains information on short‐term outcomes for patients admitted through the ED. In other words, the NEDS is the largest all‐payer ED and inpatient database in the United States. The NEDS represents an approximately 20% stratified sample of US hospital‐based EDs, containing more than 28 million records of ED visits from approximately 1000 hospitals each year. Additional details of the NEDS can be found elsewhere.[15, 17] We received a waiver for this analysis from our institutional review board.
Study Population
Patient visits were included in this analysis if they carried any COPD‐related diagnostic code (ie, International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] code of 491.xx [chronic bronchitis], 492.xx [emphysema], or 496.xx [chronic airway obstruction, not elsewhere classified]) as their primary ED diagnosis and any acute respiratory failure code (ie, 518.81 [acute respiratory failure], 518.82 [pulmonary insufficiency not elsewhere classified, 518.84 [acute and chronic respiratory failure], or 799.1 [respiratory arrest]) as their secondary diagnosis. Patient visits with a primary diagnosis of acute respiratory failure and a secondary diagnosis of COPD were also included. Patients age <40 years were excluded, because they are much less likely to have COPD.[18]
Modes of Mechanical Ventilation
The primary exposure variable was mode of mechanical ventilation. To compare the effectiveness of different ventilatory modes, patients were divided into 3 groups according to the ventilation mode they received: (1) NIV alone, (2) IMV alone, and (3) combined modes of NIV and IMV. The use of NIV was identified by using Current Procedural Terminology (CPT) code of 94660 or ICD‐9 procedure code 93.90, whereas the use of IMV was identified by using CPT code of 31500 or ICD‐9 procedure code 96.04 or 96.7x.
Patient‐Level and Emergency DepartmentLevel Variables
The NEDS contains information on patient demographics, national quartiles for median household income based on the patient's ZIP code, payment sources, ICD‐9‐CM diagnoses and procedures, ED disposition, hospital LOS, and hospital disposition. Hospital characteristics include annual visit volume, urban‐rural status, ownership, teaching status, and US region. Geographic regions (Northeast, South, Midwest, and West) were defined according to Census Bureau boundaries.[19] To adjust for confounding by patient mix, Elixhauser comorbidity measures were derived based on the ICD‐9 codes, using the Agency for Healthcare Research and Quality's Comorbidity Software.[20] This risk‐adjustment tool has been derived and validated extensively.[21]
Outcome Measures
The outcome measures were all‐cause inpatient mortality, hospital LOS, hospital charges, and ventilator‐related complications. Three ventilator‐related complications were identified using ICD‐9 procedure codes: ventilator‐associated pneumonia (997.31), facial injury (910.x), and iatrogenic pneumothorax (512.1).
Statistical Analysis
Summary statistics are presented as proportions (with 95% confidence intervals [CI]), means (with standard deviations [SD]), or medians (with interquartile ranges). Bivariate associations were examined using Student t tests, Kruskal‐Wallis tests, and [2] tests, as appropriate. Emergency department and discharge weights were used to obtain national estimates at the ED and visit level. At all other times (eg, the propensity score and instrumental variable analyses), the unweighted cohort was analyzed, because survey weights are generally not advised for propensity score analysis using complex survey data.[22]
Propensity Score Analysis
To adjust for baseline patient and ED characteristics that may have confounded the relationship between ventilation mode and clinical outcomes, we performed propensity score and instrumental variable analyses. To compare the effectiveness of NIV vs IMV, a propensity score or predicted probability of NIV was estimated using a logistic‐regression model with all patient characteristics (age, sex, quartiles for median household income, weekend admission, insurance status, season, calendar year, and comorbid conditions) and ED characteristics (urban/rural and teaching status, US region, annual ED volume, and annual volume of AECOPD with respiratory failure) as the independent variables. We then performed 1:1 propensity score matching based on a nearest‐neighbor algorithm with caliper distance of 0.01. Although propensity score matching may result in a smaller sample, it provides a clinically relevant estimate of treatment effect because subjects in the matched sample are potential candidates for either treatment option.[23, 24] An absolute standardized difference between characteristics of <10% was considered as adequate balance.[25]
Instrumental Variable Analysis
When hospitals always or nearly always use NIV or IMV, this suggests the choice is largely independent of patient characteristics, and it is possible to use the hospital preference as a proxy for the actual treatment choice (ie, an instrument variable). The instrumental variable analysis simulates a natural randomization of patients to 2 hospital groups with high and low NIV use.
The main difference between instrumental variable and propensity score analysis is that the former could potentially adjust for unmeasured confounders.[26] We used Stata procedure IVREG to estimate the outcome differences between NIV‐preferring hospitals (NIV use in 90% of patients) and IMV‐preferring hospitals (NIV use in 10% of patients).
All odds ratios (ORs) and ‐coefficients are presented with 95% CIs. All analyses were performed using Stata 12.0 software (StataCorp, College Station, TX). All P values are 2‐sided, with P<0.05 considered statistically significant.
Sensitivity Analyses
We conducted a sensitivity analysis to determine whether it was plausible that an unmeasured confounder could completely explain the observed results. The risk ratio of a hypothetical unmeasured confounder on study outcome and the exposure‐confounder imbalance were both varied to see at what point the observed association was reduced to 1.0.[27]
RESULTS
Patient and ED Characteristics
The 20062008 NEDS sample contained 67,651 ED visits for AECOPD with acute respiratory failure from 1594 US EDs. After the weighting procedure, there were an estimated 101,000 visits annually for AECOPD with acute respiratory failure from approximately 4700 US EDs. In the weighted analysis, the mean patient age of these visits was 68 years, and 56% were made by women. Ninety‐six percent were admitted to the hospital. Of these, the mortality rate was 9% and the mean hospital LOS was 7 days. Figure 1 shows the secular trends in NIV, IMV, and the combined use over the 3‐year study period. Use of IMV decreased from 28% in 2006 to 19% in 2008 (P<0.001), whereas NIV use increased slightly from 14% in 2006 to 16% in 2008 (P=0.049); the combined use of both ventilation modalities remained stable (4%). Inpatient mortality decreased from 10% in 2006 to 7% in 2008 (P<0.001).

Figure 2 shows that the frequency of NIV use (including combined use of NIV and IMV) varied widely between hospitals, ranging from 0% to 100% with a median of 11%. In the unweighted cohort of AECOPD with acute respiratory failure, 43% received some forms of ventilatory support. Table 1 shows the patient and hospital characteristics of the patients receiving ventilatory support: 36% received NIV, 56% received IMV, and 8% received combined use. In general, patients receiving combined use of NIV and IMV tended to have more comorbidities (eg, congestive heart failure and pneumonia) compared with the NIV‐alone or IMV‐alone groups. With respect to hospital characteristics, NIV was used more often in hospitals with higher volumes of COPD exacerbation and respiratory failure, in nonmetropolitan hospitals, and in hospitals in the Northeast.

| NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value, A vs B | P Value, B vs C | |
|---|---|---|---|---|---|
| |||||
| Patient characteristics | |||||
| Age, y, | <0.001 | 0.64 | |||
| 4049 | 5 | 5 | 5 | ||
| 5059 | 17 | 18 | 19 | ||
| 6069 | 31 | 33 | 33 | ||
| 7079 | 30 | 29 | 29 | ||
| 80 | 17 | 15 | 13 | ||
| Female sex, % | 57 | 53 | 54 | <0.001 | 0.87 |
| Quartile for median household income of patient ZIP code, $, % | <0.001 | <0.001 | |||
| 138,999 | 30 | 34 | 29 | ||
| 39,00047,999 | 28 | 28 | 28 | ||
| 48,00062,999 | 24 | 22 | 24 | ||
| 63,000 | 18 | 15 | 19 | ||
| Weekend admission, % | 27 | 28 | 28 | 0.07 | 0.80 |
| Insurance status, % | <0.001 | 0.91 | |||
| Medicare | 74 | 70 | 70 | ||
| Medicaid | 9 | 12 | 12 | ||
| Private | 12 | 13 | 13 | ||
| Self‐pay | 2 | 3 | 2 | ||
| Other | 2 | 2 | 2 | ||
| Season, % | <0.001 | 0.16 | |||
| Winter (January 1March 31) | 29 | 32 | 31 | ||
| Spring (April 1June 30) | 24 | 25 | 26 | ||
| Summer (July 1September 30) | 22 | 20 | 19 | ||
| Fall (October 1December 31) | 25 | 22 | 24 | ||
| No. of comorbidities, median (IQR) | 4 (35) | 4 (35) | 4 (36) | <0.001 | <0.001 |
| Selected comorbidities, % | |||||
| Hypertension | 56 | 55 | 55 | 0.01 | 0.65 |
| CHF | 38 | 40 | 44 | 0.001 | <0.001 |
| Fluid and electrolyte disorders | 37 | 44 | 49 | <0.001 | <0.001 |
| Diabetes, uncomplicated | 27 | 26 | 29 | 0.04 | 0.002 |
| Pneumonia | 19 | 34 | 39 | <0.001 | <0.001 |
| Deficiency anemia | 16 | 19 | 19 | <0.001 | 0.39 |
| Obesity | 18 | 12 | 17 | <0.001 | <0.001 |
| Depression | 15 | 11 | 11 | <0.001 | 0.54 |
| Pulmonary circulatory diseases | 15 | 11 | 14 | <0.001 | <0.001 |
| Hospital characteristics | |||||
| Annual ED visit volume, median (IQR) | 42,704 (29,50562,470) | 44,119 (29,89564,097) | 46,695 (31,29866,235) | 0.02 | 0.0003 |
| Annual ED volume of COPD exacerbation with respiratory failure, median (IQR) | 45 (2672) | 42 (2368) | 38 (2364) | <0.001 | <0.001 |
| Urban/rural and teaching status, % | <0.001 | <0.001 | |||
| Metropolitan nonteaching | 53 | 52 | 47 | ||
| Metropolitan teaching | 31 | 35 | 39 | ||
| Nonmetropolitan | 16 | 13 | 13 | ||
| US region, % | <0.001 | <0.001 | |||
| Northeast | 28 | 16 | 36 | ||
| Midwest | 17 | 22 | 15 | ||
| South | 41 | 45 | 32 | ||
| West | 14 | 17 | 17 | ||
The unadjusted differences in outcomes are shown in Table 2. The combined‐use group had the highest inpatient mortality, longest LOS, and highest charges, followed by the IMV and NIV groups. In general, complications were few across all 3 groups, but the rate of iatrogenic pneumothorax was notably lower in the NIV group. Table 3 details the statistically significant predictors of NIV use in the propensity score model. Similar to the unadjusted analysis, older age, high‐income neighborhoods, Medicare insurance, and some comorbidities were positively associated with NIV use (eg, pulmonary circulatory disorders and liver disease), whereas a few comorbidities were negatively associated with NIV use (eg, pneumonia, and alcohol and drug abuse). With respect to hospital characteristics, higher case volumes of COPD exacerbation/respiratory failure, Northeastern and nonmetropolitan hospitals, and more recent years were associated with NIV use.
| Outcome | NIV Alone (A) (n=10,032) | IMV Alone (B) (n=15,427) | Combined Use (C) (n=2311) | P Value. A vs B | P Value, B vs C |
|---|---|---|---|---|---|
| |||||
| Inpatient mortality, n (%) | 825 (8) | 2,454 (16) | 407 (18) | <0.001 | 0.04 |
| Hospital length of stay, median (IQR), d | 5 (48) | 8 (513) | 10 (716) | <0.001 | <0.001 |
| Hospital charge per visit, median (IQR), $ | 26,002 (15,74744,638) | 53,432 (31,99892,664) | 64,585 (39,024110,336) | <0.001 | <0.001 |
| Complications* | |||||
| Ventilator‐associated pneumonia, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.09 | 1.00 |
| Facial injury, n (%) | 10 (0.1) | 10 (0.1) | 10 (0.5) | 0.26 | 1.00 |
| Iatrogenic pneumothorax, n (%) | 10 (0.1) | 90 (0.6) | 14 (0.6) | <0.001 | 0.90 |
| Patient Characteristics | Adjusted OR (95% CI)* | P Value |
|---|---|---|
| ||
| Age, y | ||
| 4049 | 1.00 (Reference) | |
| 5059 | 0.96 (0.84‐1.11) | 0.61 |
| 6069 | 0.96 (0.84‐1.10) | 0.56 |
| 7079 | 1.09 (0.94‐1.25) | 0.25 |
| 80 | 1.30 (1.12‐1.52) | 0.001 |
| Quartile for median household income of patient ZIP code, $ | ||
| 138,999 | 1.00 (Reference) | |
| 39,00047,999 | 1.13 (1.05‐1.21) | 0.001 |
| 48,00062,999 | 1.21 (1.12‐1.30) | <0.001 |
| 63,000 | 1.21 (1.11‐1.32) | <0.001 |
| Insurance status | ||
| Medicare | 1.00 (Reference) | |
| Medicaid | 0.79 (0.72‐0.88) | <0.001 |
| Private | 0.88 (0.81‐0.96) | 0.004 |
| Self‐pay | 0.68 (0.56‐0.82) | <0.001 |
| Other | 0.88 (0.73‐1.07) | 0.22 |
| Season | ||
| Winter (January 1March 31) | 1.00 (Reference) | |
| Spring (April 1June 30) | 1.06 (0.99‐1.14) | 0.11 |
| Summer (July 1September 30) | 1.17 (1.08‐1.26) | <0.001 |
| Fall (October 1December 31) | 1.24 (1.15‐1.33) | <0.001 |
| Comorbidity | ||
| CHF | 0.90 (0.85‐0.95) | <0.001 |
| Pulmonary circulatory disorders | 1.40 (1.29‐1.52) | <0.001 |
| Diabetes, complicated | 1.25 (1.08‐1.44) | 0.002 |
| Liver disease | 1.79 (1.40‐2.28) | <0.001 |
| Coagulopathy | 0.54 (0.46‐0.63) | <0.001 |
| Obesity | 1.52 (1.41‐1.65) | <0.001 |
| Weight loss | 0.50 (0.44‐0.57) | <0.001 |
| Fluid and electrolyte disorders | 0.84 (0.80‐0.89) | <0.001 |
| Deficiency anemia | 0.83 (0.78‐0.90) | <0.001 |
| Alcohol abuse | 0.66 (0.58‐0.76) | <0.001 |
| Drug abuse | 0.74 (0.62‐0.88) | 0.001 |
| Psychoses | 1.22 (1.10‐1.37) | <0.001 |
| Depression | 1.45 (1.34‐1.57) | <0.001 |
| Pneumonia | 0.48 (0.45‐0.51) | <0.001 |
| Valvular heart disease | 0.87 (0.77‐0.97) | 0.01 |
| Neurological disorders | 0.89 (0.80‐0.98) | 0.02 |
| RA/collagen vascular diseases | 1.25 (1.02‐1.53) | 0.04 |
| Blood‐loss anemia | 0.72 (0.53‐0.97) | 0.03 |
| Hospital characteristics | ||
| Annual ED visit volume, per 1000‐visit increase | 0.997 (0.996‐0.998) | <0.001 |
| Annual ED volume of COPD exacerbation with respiratory failure, per 10‐visit increase | 1.03 (1.02‐1.03) | <0.001 |
| Urban/rural and teaching status | ||
| Metropolitan nonteaching | 1.00 (Reference) | |
| Metropolitan teaching | 0.91 (0.85‐0.97) | 0.006 |
| Nonmetropolitan | 1.30 (1.20‐1.42) | <0.001 |
| US region | ||
| Northeast | 1.00 (Reference) | |
| Midwest | 0.44 (0.40‐0.48) | <0.001 |
| South | 0.54 (0.50‐0.58) | <0.001 |
| West | 0.51 (0.46‐0.56) | <0.001 |
| Calendar year | ||
| 2006 | 1.00 (Reference) | |
| 2007 | 1.30 (1.22‐1.39) | <0.001 |
| 2008 | 1.65 (1.54‐1.76) | <0.001 |
In terms of propensity score distributions (see Supporting Information, Figure E1, in the online version of this article), there was sufficient overlap of the NIV and IMV groups. After matching on propensity score for the NIV and IMV groups, the differences in baseline characteristics were all balanced (see Supporting Information, Table E1, in the online version of this article), as indicated by <10% standardized differences in all covariates between the 2 groups. Finally, in the propensity scorematched cohort (see Supporting Information, Table E2, in the online version of this article), NIV use remained associated with significantly lower inpatient mortality (risk ratio: 0.54; 95% CI: 0.50‐0.59, P<0.001), a shorter hospital LOS (mean difference, 3.2 days; 95% CI: 3.4 to 2.9 days, P<0.001), and lower hospital charges (mean difference, P<$35,012; 95% CI: $36,848 to $33,176, P<0.001), compared with IMV use. Use of NIV also was associated with a lower rate of iatrogenic pneumothorax than IMV use (0.05% vs 0.5%, P<0.001).
Using hospital preference for NIV vs IMV as an instrument, the instrumental analysis confirmed the benefits of NIV use, with a 5% reduction in inpatient mortality in the NIV‐preferring hospitals (risk difference, P<5%; 95% CI: P<1.8% to P<8.3%).
In the sensitivity analysis to assess the impact of an unmeasured confounder, the confounder would have had to have a very strong impact on outcome (risk ratio: 5) and a severe exposure‐confounder imbalance (odds ratio of exposure on confounder: 5) to reduce the observed association to 1.0. In other words, an individual unmeasured confounder is unlikely to explain the observed association.
DISCUSSION
In this nationally representative sample of 67,651 ED visits for AECOPD with acute respiratory failure, we found that NIV use was increasing from 2006 to 2008. However, the utilization of NIV remained low (16% in 2008) and varied widely by patient and hospital characteristic. As with all observational studies, causality cannot be inferred definitely; however, our study suggests that, NIV usecompared with IMV usewas associated with potentially important benefits: a reduction of inpatient mortality by 46%, shortened hospital LOS by 3 days, reduced hospital charges by approximately $35,000 per visit, and modestly reduced risk of iatrogenic pneumothorax.
A recent analysis using the US Nationwide Inpatient Sample has shown increasing use of NIV and concomitant decreasing mortality in AECOPD over time.[28] Our analysis confirmed these favorable trends in the United States using a much larger NEDS sample (28 million visits in the NEDS vs 8 million visits in the Nationwide Inpatient Sample per year). Despite these favorable trends, NIV was still underutilized for AECOPD with respiratory failure in the United States (16% in 2008) compared with major European countries (40%).[29] Although our study lacked clinical details to arrive at the optimal rate of NIV use, the low rate of NIV use is concerning and suggests room for improvement in NIV use in appropriate patients as outlined by the current COPD guidelines.[18, 30] Why is NIV not widely adopted, given its demonstrated efficacy? Previous surveys have identified several perceived reasons for low NIV use, including lack of physician knowledge, insufficient respiratory therapist training, inadequate equipment, and time required for setting up NIV.[29, 31, 32] Our study adds to the literature by showing the actual predictors of NIV use in the real world. Our data showed that the early adopters were hospitals with higher case volumes, and hospitals in the Northeast and in nonmetropolitan areas. A higher case volume has been linked with lower mortality in AECOPD (ie, practice makes perfect),[33] and frequent NIV use could explain the lower AECOPD mortality in highcase volume centers. Alternatively, smaller hospitals tend to have moonlighters working in EDs who may not be board certified in emergency medicine. Perhaps the logical next step is to conduct a qualitative study to understand the specifics of best practices and provider characteristics in these Northeastern, highercase volume centers. Another incentive to promote NIV use in clinical practice is the cost‐effectiveness associated with this intervention, as previous studies have shown that, compared with usual care, receiving NIV was associated with a reduction in costs, mainly through reduced use of the ICU.[34, 35]
Some patient factors associated with NIV use may be well justified. For example, older AECOPD patients may have an advance directive describing their treatment wishes (eg, do‐not‐intubate order),[36] and therefore NIV was preferred to IMV. Also, our data suggested AECOPD patients with a suspected pneumonia component were less likely to be placed on NIV, which is consistent with COPD guideline recommendations.[18, 30] As outlined in the current guidelines, the major contraindications to NIV include impending respiratory arrest, excessive respiratory secretions, massive gastrointestinal bleeding, recent facial trauma, or altered mental status.[18, 30] By contrast, some factors associated with NIV use may be targeted for intervention, such as lower rates of NIV use in the uninsured, patients who live in low‐income neighborhoods, and hospitals in US regions other than the Northeast.
Current guidelines recommend using NIV in AECOPD patients with early signs of respiratory failure, such as arterial pH of 7.257.35 or pCO2 45 mm Hg.[18, 30] When NIV is considered as the modality of ventilatory support, it should probably be used as early as possible,[37] because evidence suggests that delayed use of NIV may lead to severe respiratory acidosis and increased mortality.[38] Other than in ICUs, NIV can be used on general wards and in EDs that have adequate staff training and experience, because the success rates of NIV in these settings are similar to those reported in ICU studies.[8, 36, 39] In addition, NIV is more cost‐effective when performed outside the ICU.[35] In fact, studies have found a substantial portion of patients had NIV started in the ED (one‐fourth) and on the general ward (one‐fourth).[31, 40] Given the shortage of intensivists in the United States, hospitalists begin to play an important role in provision of critical care outside the ICU.[41] Once NIV is used, it is important to ensure that it is delivered effectively and monitored closely because NIV failure has been shown to be associated with high mortality.[28, 42]
This study has some potential limitations. First, we used administrative claims that lack clinical details such as data on arterial blood gases and severity scores, and thus potential residual confounding may exist. In our study, the IMV group may be sicker than the NIV group, which could partially explain the increased mortality with IMV. However, the propensity scores overlap to a great extent between the 2 study groups, suggesting that a strong confounding bias is less likely, given the observed covariates. Furthermore, the instrumental variable and sensitivity analyses taking into account unmeasured confounders still suggested the benefits of NIV. Second, the NEDS does not contain data on the location where NIV was initiated (eg, ED, ward, or ICU) or the timing of initiating NIV or IMV. As a result, for the combined‐use group, we could not further distinguish the group switching from NIV to IMV (ie, NIV failure)[42] or from IMV to NIV (ie, NIV as a weaning strategy).[43] Accordingly, we chose to focus on the comparativeness effectiveness of NIV vs IMV. Third, although the NEDS data have undergone quality‐control procedures,[44] some misclassification may exist in identifying patient population and interventions. Finally, the analysis may not reflect the most recent trend in NIV use, as the 2010 NEDS data have just been released. In addition, although the study is the largest to date on this topic, our findings may not be generalizable to EDs that were not part of the NEDS.
In summary, in this nationally representative ED and inpatient database, NIV use is increasing for AECOPD with acute respiratory failure; however, its adoption remains low and varies widely between US hospitals. Our observational study suggests that NIV appears to be more effective and safer than IMV in the real‐world setting. There is an opportunity to increase the use of NIV as recommended in guidelines and to promote the use NIV in replacement of IMV in patients with severe AECOPD. Given the increasing mortality burden of COPD, such a strategy may help reduce COPD mortality at the population level, thereby fulfilling the objectives of Healthy People 2020.
Disclosure
Partial results from this study were presented at the 2012 Society for Academic Emergency Medicine Annual Meeting, Chicago, Illinois, May 912, 2012. This project was supported by grant number R03HS020722 from the Agency for Healthcare Research and Quality. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality. The authors have no conflicts of interest to disclose.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports, 2011. Deaths: final data for 2008. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. Accessed August 15, 2012.
- , , , . Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259.
- , , . National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2008;15:1275–1283.
- , , , , . Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16.
- US Department of Health and Human Services. Healthy People 2020. Objectives for Respiratory Diseases. Available at: http://www.healthypeople. gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=36. Accessed May 3, 2012.
- . Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;47:31s–37s.
- , , , et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935.
- , , , et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
- , , , et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
- . Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–3924.
- , , , , , . Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol. 2009;33:791–797.
- , , , et al. Noninvasive Positive‐Pressure Ventilation (NPPV) for Acute Respiratory Failure. Rockville, MD: Agency for Healthcare Research and Quality; July 2012: Report 12‐EHC089‐EF. Available at: http://effectivehealthcareahrqgov/ehc/products/273/1180/CER68_NPPV_FinalReport_20120706pdf. Accessed December 11, 2012.
- Healthcare Cost and Utilization Project (HCUP). HCUP Nationwide Emergency Department Sample (NEDS). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/nedsoverview.jsp. Accessed April 15, 2012.
- American Hospital Association. Annual survey database. Available at: http://www.ahadata.com/ahadata/html/AHASurvey.html. Accessed April 15, 2012.
- , , , . Age‐related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252e1–1258e1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). NHLBI/WHO Global Strategy for the Diagnosis, Management and Prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 15, 2012.
- .US Bureau of the Census. Census regions and divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed April 9, 2012.
- .Healthcare Cost and Utilization Project. Comorbidity software. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 15, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Healthcare Cost and Utilization Project (HCUP). HCUP Methods Series. Hierarchical Modeling Using HCUP Data. Rockville, MD: Agency for Healthcare Research and Quality; 2012: Report 2007‐01. Available at: http://www.hcup‐us.ahrq.gov/reports/methods/2007_01.pdf. Accessed April 15, 2012.
- , , . Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- , , , , , . Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042.
- . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554.
- . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
- , , , , . A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36:362–369.
- , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- , , , . Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129:1226–1233.
- , , . A survey of the use of noninvasive ventilation in academic emergency departments in the United States. Respir Care. 2009;54:1306–1312.
- , , . Emergency department case volume and patient outcomes in acute exacerbations of chronic obstructive pulmonary disease. Acad Emerg Med. 2012;19:656–663.
- , , , , . Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102.
- , , , . Cost effectiveness of ward based non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956.
- , , . Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do‐not‐intubate” patients. Crit Care Med. 2005;33:1976–1982.
- , . Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: “Don't think twice, it's alright!”. Am J Respir Crit Care Med. 2012;185:121–123.
- , , , , . Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48.
- , , , , . Use of noninvasive positive‐pressure ventilation on the regular hospital ward: experience and correlates of success. Respir Care. 2006;51:1237–1243.
- , , , et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477–1483.
- . Hospitalists and intensivists: partners in caring for the critically ill—the time has come. J Hosp Med. 2010;5:1–3.
- , , , , , . Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825.
- , , , . Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127.
- Healthcare Cost and Utilization Project (HCUP). HCUP Quality Control Procedures. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/db/quality.jsp. Accessed December 15, 2012.
- Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics Reports, 2011. Deaths: final data for 2008. Available at: http://www.cdc.gov/nchs/data/nvsr/nvsr59/nvsr59_10.pdf. Accessed August 15, 2012.
- , , , . Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294:1255–1259.
- , , . National study of emergency department visits for acute exacerbation of chronic obstructive pulmonary disease. Acad Emerg Med. 2008;15:1275–1283.
- , , , , . Chronic obstructive pulmonary disease surveillance—United States, 1971–2000. MMWR Surveill Summ. 2002;51:1–16.
- US Department of Health and Human Services. Healthy People 2020. Objectives for Respiratory Diseases. Available at: http://www.healthypeople. gov/2020/topicsobjectives2020/objectiveslist.aspx?topicId=36. Accessed May 3, 2012.
- . Mechanical ventilation: invasive versus noninvasive. Eur Respir J Suppl. 2003;47:31s–37s.
- , , , et al. Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease. N Engl J Med. 1995;333:817–822.
- , , . Early use of non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease on general respiratory wards: a multicentre randomised controlled trial. Lancet. 2000;355:1931–1935.
- , , , et al. Randomised controlled trial of nasal ventilation in acute ventilatory failure due to chronic obstructive airways disease. Lancet. 1993;341:1555–1557.
- , , , . Non‐invasive positive pressure ventilation for treatment of respiratory failure due to exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2004;(3):CD004104.
- , , , et al. Noninvasive vs. conventional mechanical ventilation in patients with chronic obstructive pulmonary disease after failure of medical treatment in the ward: a randomized trial. Intensive Care Med. 2002;28:1701–1707.
- . Noninvasive vs conventional mechanical ventilation in acute respiratory failure: a multicenter, randomized controlled trial. Chest. 2005;128:3916–3924.
- , , , , , . Mechanical ventilation in chronic obstructive pulmonary disease patients, noninvasive vs. invasive method (randomized prospective study). Coll Antropol. 2009;33:791–797.
- , , , et al. Noninvasive Positive‐Pressure Ventilation (NPPV) for Acute Respiratory Failure. Rockville, MD: Agency for Healthcare Research and Quality; July 2012: Report 12‐EHC089‐EF. Available at: http://effectivehealthcareahrqgov/ehc/products/273/1180/CER68_NPPV_FinalReport_20120706pdf. Accessed December 11, 2012.
- Healthcare Cost and Utilization Project (HCUP). HCUP Nationwide Emergency Department Sample (NEDS). Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/nedsoverview.jsp. Accessed April 15, 2012.
- American Hospital Association. Annual survey database. Available at: http://www.ahadata.com/ahadata/html/AHASurvey.html. Accessed April 15, 2012.
- , , , . Age‐related differences in clinical outcomes for acute asthma in the United States, 2006–2008. J Allergy Clin Immunol. 2012;129:1252e1–1258e1.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). NHLBI/WHO Global Strategy for the Diagnosis, Management and Prevention of COPD. Available at: http://www.goldcopd.org. Accessed April 15, 2012.
- .US Bureau of the Census. Census regions and divisions of the United States. Available at: http://www.census.gov/geo/www/us_regdiv.pdf. Accessed April 9, 2012.
- .Healthcare Cost and Utilization Project. Comorbidity software. Available at: http://www.hcup‐us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed April 15, 2012.
- , , , . Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27.
- Healthcare Cost and Utilization Project (HCUP). HCUP Methods Series. Hierarchical Modeling Using HCUP Data. Rockville, MD: Agency for Healthcare Research and Quality; 2012: Report 2007‐01. Available at: http://www.hcup‐us.ahrq.gov/reports/methods/2007_01.pdf. Accessed April 15, 2012.
- , , . Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98:253–259.
- , , , , , . Antibiotic therapy and treatment failure in patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. JAMA. 2010;303:2035–2042.
- . An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424.
- , , . Instrumental variable methods in comparative safety and effectiveness research. Pharmacoepidemiol Drug Saf. 2010;19:537–554.
- . Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf. 2006;15:291–303.
- , , , et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998–2008. Am J Respir Crit Care Med. 2012;185:152–159.
- , , , , . A European survey of noninvasive ventilation practices. Eur Respir J. 2010;36:362–369.
- , . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946.
- , , , . Utilization of noninvasive ventilation in acute care hospitals: a regional survey. Chest. 2006;129:1226–1233.
- , , . A survey of the use of noninvasive ventilation in academic emergency departments in the United States. Respir Care. 2009;54:1306–1312.
- , , . Emergency department case volume and patient outcomes in acute exacerbations of chronic obstructive pulmonary disease. Acad Emerg Med. 2012;19:656–663.
- , , , , . Noninvasive positive pressure ventilation in the setting of severe, acute exacerbations of chronic obstructive pulmonary disease: more effective and less expensive. Crit Care Med. 2000;28:2094–2102.
- , , , . Cost effectiveness of ward based non‐invasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: economic analysis of randomised controlled trial. BMJ. 2003;326:956.
- , , . Noninvasive positive pressure ventilation reverses acute respiratory failure in select “do‐not‐intubate” patients. Crit Care Med. 2005;33:1976–1982.
- , . Noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease: “Don't think twice, it's alright!”. Am J Respir Crit Care Med. 2012;185:121–123.
- , , , , . Acidosis, non‐invasive ventilation and mortality in hospitalised COPD exacerbations. Thorax. 2011;66:43–48.
- , , , , . Use of noninvasive positive‐pressure ventilation on the regular hospital ward: experience and correlates of success. Respir Care. 2006;51:1237–1243.
- , , , et al. Bilevel noninvasive positive pressure ventilation for acute respiratory failure: survey of Ontario practice. Crit Care Med. 2005;33:1477–1483.
- . Hospitalists and intensivists: partners in caring for the critically ill—the time has come. J Hosp Med. 2010;5:1–3.
- , , , , , . Incidence and causes of non‐invasive mechanical ventilation failure after initial success. Thorax. 2000;55:819–825.
- , , , . Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure. Cochrane Database Syst Rev. 2010:CD004127.
- Healthcare Cost and Utilization Project (HCUP). HCUP Quality Control Procedures. Rockville, MD: Agency for Healthcare Research and Quality; 2012. Available at: http://www.hcup‐us.ahrq.gov/db/quality.jsp. Accessed December 15, 2012.
Copyright © 2013 Society of Hospital Medicine