Evidence summary
GAHT may improve depression and quality of life, but not anxiety
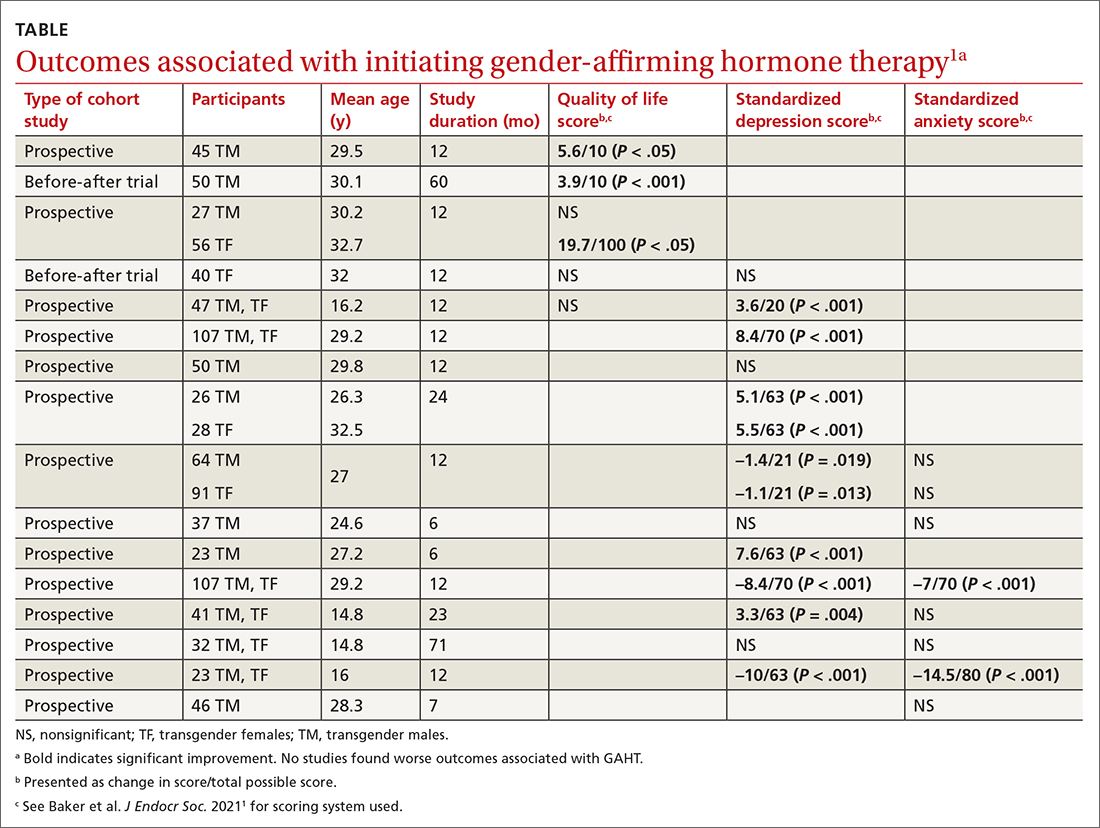
A well-done systematic review of transgender men and transgender women demonstrated that GAHT of more than a year’s duration was associated with modestly improved standardized scores for QOL, depression, and possibly anxiety.1 It was also associated with improved scores for depression in transgender adolescents.
The authors identified 15 prospective cohort studies (n = 626 transgender adults [mean age, 25-34 years]; 198 transgender adolescent girls and boys [mean age, 15-16 years]), 2 retrospective cohort studies (n = 1756 adults; mean age, 25-32 years), and 4 cross-sectional studies (n = 336 adults; mean age, 30-37 years).
Researchers recruited participants using strict eligibility criteria (psychiatric evaluation and formal diagnosis of gender dysphoria), with no prior history of GAHT, largely from gender-affirming specialty clinics at university hospitals. Most studies were conducted after the year 2000, predominantly in Europe (8 studies in Italy; 2 each in Belgium, the Netherlands, the United States, and Spain).
GAHT comprised testosterone for transgender men (14 studies used injectable testosterone cypionate, enanthate, undecanoate, or transdermal gels), estrogens (usually with an anti-androgen such as cyproterone acetate or spironolactone) for transgender women (10 studies used transdermal, oral, or injectable estradiol valerate or conjugated estrogens), and gonadotropin-releasing hormone (GnRH) therapy for transgender adolescents (3 studies).
Researchers evaluated the outcomes of QOL, depression, and anxiety with standardized scores on validated screening tools and suicide (2 studies) by medical records. GAHT in adult transgender men and transgender women was associated with modest improvements in QOL (3 of 5 studies) and depression (8 of 12 studies), and some improvement in anxiety scores (2 of 8 studies; see TABLE1). There was insufficient evidence to determine whether GAHT had any effect on suicide. In adolescent transgender girls and boys, GAHT was associated with modest improvements in depression but not QOL or anxiety scores.
The authors rated the strength of evidence from the included studies as low, based on study quality (small study sizes, uncontrolled confounding factors, and risk of bias in study designs).
Additional research supports GAHT’s association with improved outcomes
Three studies, published after the systematic review, evaluated outcomes before and after GAHT and found similar results. All studies recruited treatment-seeking participants from specialty clinics.
Continue to: An Australian propsective longitudinal..