User login
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
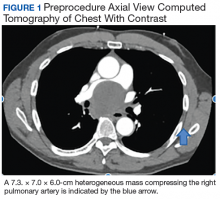
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
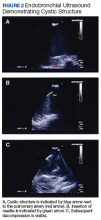
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
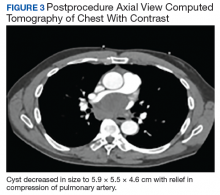
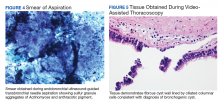
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.