User login
Long-Term Oxygen Therapy and Risk of Fire-Related Events
Chronic obstructive pulmonary disease (COPD) has been the third leading cause of death in the US since 2008.1 Current management of COPD includes smoking cessation, adequate nutrition, medication therapy, pulmonary rehabilitation, and vaccines.2 Outside of pharmacologic management, oxygen therapy has become a staple treatment of chronic hypoxemic respiratory failure due to COPD. Landmark trials, including the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) study, demonstrated improved survival in patients with COPD and hypoxemia, particularly if these patients received oxygen for 18 hours per day.3,4 NOTT prospectively evaluated 203 patients at 6 centers who were randomly allocated to either continuous oxygen therapy or 12-hour nocturnal oxygen therapy. The overall mortality in the nocturnal oxygen therapy group was 1.94 times that in the continuous oxygen therapy group (P = .01).3 The MRC study included 87 patients who were randomized to oxygen therapy or no oxygen; risk of death was 12% per year in the treated group vs 29% per year in the control group (P = .04).4 The effectiveness of long-term oxygen therapy (LTOT) in active smokers continues to be a source of debate; although 50% of patients in the NOTT trial were smokers, there was no subgroup analysis of whether smoking status had an impact on survival in those on continuous oxygen therapy.
Although many therapies are available for the treatment of COPD, the most effective treatment to prevent the progression of COPD is smoking cessation. Resources like smoking cessation programs, nicotine patches, and medications, such as bupropion and varenicline, are available to aid smoking cessation.5 However, many patients are unable to quit tobacco use despite their best efforts using available resources, and they continue to smoke even with progressive COPD. Long-time smokers also are likely to continue smoking while on LTOT, which increases their risk for fire-related injury.6-8
Traditional indications are being scrutinized after the LTOT trial found no benefit with respect to time to death or first hospitalization among patients with stable COPD and resting or exercise-induced moderate desaturation.9
Although oxygen accelerates combustion and is a potential fire hazard, LTOT has been prescribed even to active smokers as the 2 landmark trials did not exclude patients who were active smokers from receiving oxygen therapy.3,4 Therefore, LTOT has traditionally been prescribed to veterans who are actively smoking, despite the fire hazard. Attempts at mitigating hazards related to oxygen therapy in active smokers include counseling extensively about safety measures (which includes avoiding open flames such as candles, large fires, or sparks when on LTOT and providing Home Safety Agreements—a written contract between prescriber and patient wherein the patient agrees to abide by the terms of the US Department of Veterans Affairs (VA) to mitigate hazards related to LTOT in order to receive LTOT (eAppendix
Methods
With this practice in mind, we conducted an institutional review board approved retrospective chart review of all veterans with diagnosis of COPD within the Central Texas Veterans Health Care System (CTVHCS) who were prescribed new LTOT between October 1, 2010 and September 30, 2015. Given the retrospective nature of the chart review, patient consent was not obtained. Inclusion criteria were veterans aged > 18 years who had a confirmed diagnosis of COPD by spirometry and who met criteria for either continuous or ambulation- only oxygen therapy.
Criteria for exclusion included patients with hypoxemia not solely attributable to COPD or due to diseases other than COPD. We reviewed encounters in these patients’ charts, including follow-up in the clinic of the providers prescribing oxygen, to assess for fire-related incidents, defined as events wherein fire was visualized by the patient or by individuals living with the patient and with report provided to medical equipment company providing oxygen; the patient did not have to seek medical care to qualify for fire-related incident. Of the 158 patients who met the criteria for inclusion in the study, 152 were male.
Statistics
Bayesian logistic regression was used to model the outcome variable fire-related incident with the predictors smoking status, age, race, depression, PTSD, and type of oxygen used. Mental health disorders have significant effect on substance use disorders, such as alcohol use. Depression and PTSD were more common mental health diagnoses found in our patient population. Additionally, due to the small sample size, these psychiatric diagnoses were chosen to evaluate the impact of mental health disorders on firerelated events.
Although the sample size of events was small, weakly informative normal priors (0, 2.5) were used to shrink parameter estimates toward 0 and minimize overfitting. Weakly informative normal priors have also been suggested to deal with the problem of quasi-complete separation, where in our case, both smoking and no-PTSD perfectly predicted the 9 fire-related incidents.10 All input variables were centered and scaled as recommended. 9 The model fit well as assessed by posterior predictive checks, and Rhat was 1.00 for all parameters, indicating that all chains converged. Analysis was completed in R version 3.5.1 using the ‘brms’ package for Bayesian modeling.11
Results
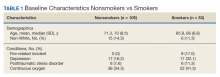
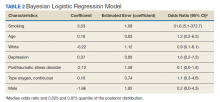
The mean age for the 158 included patients was 71.3 years in nonsmokers and 65.9 years in smokers. Fifty-three of the included patients were active smokers when LTOT was initiated. Nine veterans had fire-related incidents during the study period. All 9 patients were actively smoking (about 17%) at the time of the fire incidents. There were no deaths, and 5 patients required hospitalization due to facial burns resulting from the fire-related incidents. Our study focused on 5 baseline characteristics in our population (Table 1). After gathering data, our group inferred that these characteristics had a potential relationship to fire-related incidents compared with other variables that were studied. Future studies could look at other patient characteristics that may be linked to fire-related incidents in patients on LTOT. For example, not having PTSD also perfectly predicts fire-related incidents in our data (ie, none of the participants who had fire-related incidents had PTSD). Although this finding was not within the 95% confidence interval (CI) in the model, it does show that care must be taken when interpreting effects from small samples (Table 2). The modelestimated odds of a fire-related incident occurring in a smoker were 31.6 (5.1-372.7) times more likely than were the odds of a firerelated incident occurring in a nonsmoker, holding all other predictors at their reference level; 95% CI for the odds ratios for all other predictors in the model included a value of 1.
Discussion
This study showed evidence of increased odds of fire-related events in actively smoking patients receiving LTOT compared with patients who do not actively smoke while attempting to adjust for potential confounders. Of the 9 patients who had fire events, 5 required hospitalization for burns.
A similar retrospective cohort study by Sharma and colleagues in 2015 demonstrated an increased risk of burn-related injury when on LTOT but reiterated that the benefit of oxygen outweighs the risk of burn-related injury in patients requiring oxygen therapy.12 Interestingly, Sharma and colleagues were unable to identify smoking status for the patients studied but further identified factors associated with burn injury to include male sex, low socioeconomic status, oxygen therapy use, and ≥ 3 comorbidities. The study’s conclusion recommended continued education by health care professionals (HCPs) to their patients on LTOT regarding potential for burn injury. In the same vein, the VA National Center for Ethics in Health Care noted that “clinicians should familiarize themselves with the risks and benefits of LTOT; should inform their patients of the risks and benefits without exaggerating the risk associated with smoking; avoid undue coercion inherent in the clinician’s ability to withdraw LTOT; reduce the risk to the greatest degree possible; and consider termination of LTOT in very extreme cases and in consultation with a multidisciplinary committee.”13
This statement is in contrast to the guidelines and policies of other countries, such as Sweden, where smoking is a direct contraindication for prescription of oxygen therapy, or in Australia and New Zealand, where the Thoracic Society of Australia and New Zealand oxygen therapy guidelines recommend against prescription of LTOT, citing “increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit.”6,14
The prevalence of oxygen therapy introduces the potential for fire-related incidents with subsequent injury requiring medical care. There are few studies regarding home oxygen fire in the US due to the lack of a uniform reporting system. One study by Wendling and Pelletier analyzed deaths in Maine, Massachusetts, New Hampshire, and Oklahoma between 2000 and 2007 and found 38 deaths directly attributable to home oxygen fires as a result of smoking.15 Further, the Consumer Product Safety Commission’s National Electronic Injury Surveillance System between 2003 and 2006 attributed 1,190 thermal burns related to home oxygen fires; the majority of which were ignited by tobacco smoking.15 The Swedish National Register of Respiratory Failure (Swedevox) published prospective population-based, consecutive cohort study that collected data over 17 years and evaluated the risk of fire-related incident in those on LTOT. Of the 12,497 patients sampled, 17 had a burn injury and 2 patients died. The low incidence of burn injury on LTOT was attributed to the strict guidelines instituted in Sweden for doctors to avoid prescribing LTOT to actively smoking patients.6 A follow-up study by Tanash and colleagues compared the risk of burn injury in each country, respectively. The results found an increased number of burn injuries in those on oxygen therapy in Denmark, a country with fewer restrictions on smoking compared with those of Sweden.7 Similarly, our results showed that the rate of fire and burn injuries was exclusively among veterans who were active smokers. All patients who were prescribed oxygen therapy at CTVHCS received counseling and signed Home Safety Agreements. Despite following the recommendations set forth by the VA on counseling, extensive harm reduction techniques, and close follow-up, we found there was still a high incidence of fires in veterans with COPD on LTOT who continue to smoke.
The findings from our study concur with those previously published regarding the risk of home oxygen fire and concomitant smoking, supporting the idea for more regulated and concrete guidelines for prescribing LTOT to those requiring it.8
Limitations
The major limitation was the small sample size of our study. Another limitation was that our study population is predominantly male as is common in veteran cohorts. In fiscal year 2016, the veteran population of Texas was 1,434,361 males and 168,967 females.16 According to Franklin and colleagues, HCPs noticed an increase use of long-term oxygen among women compared with that of men.17
Conclusions
Our study showed an increased odds of firerelated incidents of patients while on LTOT, strengthening the argument that even with extensive education, those who smoke and are on LTOT continue to put themselves at risk of a fire-related incident. This finding stresses the importance of continuing patient education on the importance of smoking cessation prior to administration of LTOT or avoiding fire hazards while on LTOT. Further research into LTOT and fire hazards could help in implementing a more structured approval process for patients who want to obtain LTOT. We propose further studies evaluating risk factors for the incidence of fire events among patients prescribed LTOT. A growing and aging population with a need for LTOT necessitates examination of oxygen safe prescribing.
1. Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States 2000-2014. https:// www.cdc.gov/nchs/data/databriefs/db256.pdf. Published September 2016. Accessed September 10, 2020.
2. Itoh M, Tsuji T, Nemoto K, Nakamura H, Aoshiba K. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316-1335. doi:10.3390/nu5041316
3. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391. doi:10.7326/0003-4819-93-3-391
4. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. doi:10.1016/S0140-6736(81)91970-X
5. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233-239. doi:10.7326/0003-4819-142-4 -200502150-00005
6. Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479-2484. doi:10.2147/COPD.S91508
7. Tanash HA, Ringbaek T, Huss F, Ekström M. Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chronic Obstruct Pulmon Dis. 2017;12:193-197. doi:10.2147/COPD.S119949
8. Kassis SA, Savetamal A, Assi R, et al. Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg. 2014;218(6):1182-1186. doi:10.1016/j.jamcollsurg.2013.12.055
9. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. doi:10.1056/NEJMoa1604344
10. Ghosh J, Li Y, Mitra R. On the use of Cauchy prior distributions for Bayesian logistic regression. Bayesian Anal. 2018;13(2):359-383. doi:10.1214/17-ba1051
11. Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80(1). doi:10.18637/jss.v080.i01
12. Sharma G, Meena R, Goodwin JS, Zhang W, Kuo Y-F, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90(4):492-499. doi:10.1016/j.mayocp.2014.12.024
13. US Department of Veterans Affairs, National Ethics Committee. Ethical considerations that arise when a home care patient on long term oxygen therapy continues to smoke. http://vaww.ethics.va.gov/docs/necrpts/NEC_Report_20100301_Smoking_while_on_LTOT.pdf. Published March 2010. [Nonpublic, source not verified.]
14. McDonald C F, Whyte K, Jenkins S, Serginson J. Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. doi:10.1111/resp.12678
15. Centers for Disease Control and Prevention (CDC). Fatal fires associated with smoking during long-term oxygen therapy--Maine, Massachusetts, New Hampshire, and Oklahoma, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(31):852-854.
16. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Population tables: the state, age/gender, 2016. https://www.va.gov/vetdata/Veteran_ Population.asp. Updated August 5, 2020. Accessed September 11, 2020.
17. Franklin KA, Gustafson T, Ranstam J, Ström K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease--gender differences. Respir Med. 2007;101(7):1506-1511. doi:10.1016/j.rmed.2007.01.009
Chronic obstructive pulmonary disease (COPD) has been the third leading cause of death in the US since 2008.1 Current management of COPD includes smoking cessation, adequate nutrition, medication therapy, pulmonary rehabilitation, and vaccines.2 Outside of pharmacologic management, oxygen therapy has become a staple treatment of chronic hypoxemic respiratory failure due to COPD. Landmark trials, including the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) study, demonstrated improved survival in patients with COPD and hypoxemia, particularly if these patients received oxygen for 18 hours per day.3,4 NOTT prospectively evaluated 203 patients at 6 centers who were randomly allocated to either continuous oxygen therapy or 12-hour nocturnal oxygen therapy. The overall mortality in the nocturnal oxygen therapy group was 1.94 times that in the continuous oxygen therapy group (P = .01).3 The MRC study included 87 patients who were randomized to oxygen therapy or no oxygen; risk of death was 12% per year in the treated group vs 29% per year in the control group (P = .04).4 The effectiveness of long-term oxygen therapy (LTOT) in active smokers continues to be a source of debate; although 50% of patients in the NOTT trial were smokers, there was no subgroup analysis of whether smoking status had an impact on survival in those on continuous oxygen therapy.
Although many therapies are available for the treatment of COPD, the most effective treatment to prevent the progression of COPD is smoking cessation. Resources like smoking cessation programs, nicotine patches, and medications, such as bupropion and varenicline, are available to aid smoking cessation.5 However, many patients are unable to quit tobacco use despite their best efforts using available resources, and they continue to smoke even with progressive COPD. Long-time smokers also are likely to continue smoking while on LTOT, which increases their risk for fire-related injury.6-8
Traditional indications are being scrutinized after the LTOT trial found no benefit with respect to time to death or first hospitalization among patients with stable COPD and resting or exercise-induced moderate desaturation.9
Although oxygen accelerates combustion and is a potential fire hazard, LTOT has been prescribed even to active smokers as the 2 landmark trials did not exclude patients who were active smokers from receiving oxygen therapy.3,4 Therefore, LTOT has traditionally been prescribed to veterans who are actively smoking, despite the fire hazard. Attempts at mitigating hazards related to oxygen therapy in active smokers include counseling extensively about safety measures (which includes avoiding open flames such as candles, large fires, or sparks when on LTOT and providing Home Safety Agreements—a written contract between prescriber and patient wherein the patient agrees to abide by the terms of the US Department of Veterans Affairs (VA) to mitigate hazards related to LTOT in order to receive LTOT (eAppendix
Methods
With this practice in mind, we conducted an institutional review board approved retrospective chart review of all veterans with diagnosis of COPD within the Central Texas Veterans Health Care System (CTVHCS) who were prescribed new LTOT between October 1, 2010 and September 30, 2015. Given the retrospective nature of the chart review, patient consent was not obtained. Inclusion criteria were veterans aged > 18 years who had a confirmed diagnosis of COPD by spirometry and who met criteria for either continuous or ambulation- only oxygen therapy.
Criteria for exclusion included patients with hypoxemia not solely attributable to COPD or due to diseases other than COPD. We reviewed encounters in these patients’ charts, including follow-up in the clinic of the providers prescribing oxygen, to assess for fire-related incidents, defined as events wherein fire was visualized by the patient or by individuals living with the patient and with report provided to medical equipment company providing oxygen; the patient did not have to seek medical care to qualify for fire-related incident. Of the 158 patients who met the criteria for inclusion in the study, 152 were male.
Statistics
Bayesian logistic regression was used to model the outcome variable fire-related incident with the predictors smoking status, age, race, depression, PTSD, and type of oxygen used. Mental health disorders have significant effect on substance use disorders, such as alcohol use. Depression and PTSD were more common mental health diagnoses found in our patient population. Additionally, due to the small sample size, these psychiatric diagnoses were chosen to evaluate the impact of mental health disorders on firerelated events.
Although the sample size of events was small, weakly informative normal priors (0, 2.5) were used to shrink parameter estimates toward 0 and minimize overfitting. Weakly informative normal priors have also been suggested to deal with the problem of quasi-complete separation, where in our case, both smoking and no-PTSD perfectly predicted the 9 fire-related incidents.10 All input variables were centered and scaled as recommended. 9 The model fit well as assessed by posterior predictive checks, and Rhat was 1.00 for all parameters, indicating that all chains converged. Analysis was completed in R version 3.5.1 using the ‘brms’ package for Bayesian modeling.11
Results
The mean age for the 158 included patients was 71.3 years in nonsmokers and 65.9 years in smokers. Fifty-three of the included patients were active smokers when LTOT was initiated. Nine veterans had fire-related incidents during the study period. All 9 patients were actively smoking (about 17%) at the time of the fire incidents. There were no deaths, and 5 patients required hospitalization due to facial burns resulting from the fire-related incidents. Our study focused on 5 baseline characteristics in our population (Table 1). After gathering data, our group inferred that these characteristics had a potential relationship to fire-related incidents compared with other variables that were studied. Future studies could look at other patient characteristics that may be linked to fire-related incidents in patients on LTOT. For example, not having PTSD also perfectly predicts fire-related incidents in our data (ie, none of the participants who had fire-related incidents had PTSD). Although this finding was not within the 95% confidence interval (CI) in the model, it does show that care must be taken when interpreting effects from small samples (Table 2). The modelestimated odds of a fire-related incident occurring in a smoker were 31.6 (5.1-372.7) times more likely than were the odds of a firerelated incident occurring in a nonsmoker, holding all other predictors at their reference level; 95% CI for the odds ratios for all other predictors in the model included a value of 1.
Discussion
This study showed evidence of increased odds of fire-related events in actively smoking patients receiving LTOT compared with patients who do not actively smoke while attempting to adjust for potential confounders. Of the 9 patients who had fire events, 5 required hospitalization for burns.
A similar retrospective cohort study by Sharma and colleagues in 2015 demonstrated an increased risk of burn-related injury when on LTOT but reiterated that the benefit of oxygen outweighs the risk of burn-related injury in patients requiring oxygen therapy.12 Interestingly, Sharma and colleagues were unable to identify smoking status for the patients studied but further identified factors associated with burn injury to include male sex, low socioeconomic status, oxygen therapy use, and ≥ 3 comorbidities. The study’s conclusion recommended continued education by health care professionals (HCPs) to their patients on LTOT regarding potential for burn injury. In the same vein, the VA National Center for Ethics in Health Care noted that “clinicians should familiarize themselves with the risks and benefits of LTOT; should inform their patients of the risks and benefits without exaggerating the risk associated with smoking; avoid undue coercion inherent in the clinician’s ability to withdraw LTOT; reduce the risk to the greatest degree possible; and consider termination of LTOT in very extreme cases and in consultation with a multidisciplinary committee.”13
This statement is in contrast to the guidelines and policies of other countries, such as Sweden, where smoking is a direct contraindication for prescription of oxygen therapy, or in Australia and New Zealand, where the Thoracic Society of Australia and New Zealand oxygen therapy guidelines recommend against prescription of LTOT, citing “increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit.”6,14
The prevalence of oxygen therapy introduces the potential for fire-related incidents with subsequent injury requiring medical care. There are few studies regarding home oxygen fire in the US due to the lack of a uniform reporting system. One study by Wendling and Pelletier analyzed deaths in Maine, Massachusetts, New Hampshire, and Oklahoma between 2000 and 2007 and found 38 deaths directly attributable to home oxygen fires as a result of smoking.15 Further, the Consumer Product Safety Commission’s National Electronic Injury Surveillance System between 2003 and 2006 attributed 1,190 thermal burns related to home oxygen fires; the majority of which were ignited by tobacco smoking.15 The Swedish National Register of Respiratory Failure (Swedevox) published prospective population-based, consecutive cohort study that collected data over 17 years and evaluated the risk of fire-related incident in those on LTOT. Of the 12,497 patients sampled, 17 had a burn injury and 2 patients died. The low incidence of burn injury on LTOT was attributed to the strict guidelines instituted in Sweden for doctors to avoid prescribing LTOT to actively smoking patients.6 A follow-up study by Tanash and colleagues compared the risk of burn injury in each country, respectively. The results found an increased number of burn injuries in those on oxygen therapy in Denmark, a country with fewer restrictions on smoking compared with those of Sweden.7 Similarly, our results showed that the rate of fire and burn injuries was exclusively among veterans who were active smokers. All patients who were prescribed oxygen therapy at CTVHCS received counseling and signed Home Safety Agreements. Despite following the recommendations set forth by the VA on counseling, extensive harm reduction techniques, and close follow-up, we found there was still a high incidence of fires in veterans with COPD on LTOT who continue to smoke.
The findings from our study concur with those previously published regarding the risk of home oxygen fire and concomitant smoking, supporting the idea for more regulated and concrete guidelines for prescribing LTOT to those requiring it.8
Limitations
The major limitation was the small sample size of our study. Another limitation was that our study population is predominantly male as is common in veteran cohorts. In fiscal year 2016, the veteran population of Texas was 1,434,361 males and 168,967 females.16 According to Franklin and colleagues, HCPs noticed an increase use of long-term oxygen among women compared with that of men.17
Conclusions
Our study showed an increased odds of firerelated incidents of patients while on LTOT, strengthening the argument that even with extensive education, those who smoke and are on LTOT continue to put themselves at risk of a fire-related incident. This finding stresses the importance of continuing patient education on the importance of smoking cessation prior to administration of LTOT or avoiding fire hazards while on LTOT. Further research into LTOT and fire hazards could help in implementing a more structured approval process for patients who want to obtain LTOT. We propose further studies evaluating risk factors for the incidence of fire events among patients prescribed LTOT. A growing and aging population with a need for LTOT necessitates examination of oxygen safe prescribing.
Chronic obstructive pulmonary disease (COPD) has been the third leading cause of death in the US since 2008.1 Current management of COPD includes smoking cessation, adequate nutrition, medication therapy, pulmonary rehabilitation, and vaccines.2 Outside of pharmacologic management, oxygen therapy has become a staple treatment of chronic hypoxemic respiratory failure due to COPD. Landmark trials, including the Nocturnal Oxygen Therapy Trial (NOTT) and Medical Research Council (MRC) study, demonstrated improved survival in patients with COPD and hypoxemia, particularly if these patients received oxygen for 18 hours per day.3,4 NOTT prospectively evaluated 203 patients at 6 centers who were randomly allocated to either continuous oxygen therapy or 12-hour nocturnal oxygen therapy. The overall mortality in the nocturnal oxygen therapy group was 1.94 times that in the continuous oxygen therapy group (P = .01).3 The MRC study included 87 patients who were randomized to oxygen therapy or no oxygen; risk of death was 12% per year in the treated group vs 29% per year in the control group (P = .04).4 The effectiveness of long-term oxygen therapy (LTOT) in active smokers continues to be a source of debate; although 50% of patients in the NOTT trial were smokers, there was no subgroup analysis of whether smoking status had an impact on survival in those on continuous oxygen therapy.
Although many therapies are available for the treatment of COPD, the most effective treatment to prevent the progression of COPD is smoking cessation. Resources like smoking cessation programs, nicotine patches, and medications, such as bupropion and varenicline, are available to aid smoking cessation.5 However, many patients are unable to quit tobacco use despite their best efforts using available resources, and they continue to smoke even with progressive COPD. Long-time smokers also are likely to continue smoking while on LTOT, which increases their risk for fire-related injury.6-8
Traditional indications are being scrutinized after the LTOT trial found no benefit with respect to time to death or first hospitalization among patients with stable COPD and resting or exercise-induced moderate desaturation.9
Although oxygen accelerates combustion and is a potential fire hazard, LTOT has been prescribed even to active smokers as the 2 landmark trials did not exclude patients who were active smokers from receiving oxygen therapy.3,4 Therefore, LTOT has traditionally been prescribed to veterans who are actively smoking, despite the fire hazard. Attempts at mitigating hazards related to oxygen therapy in active smokers include counseling extensively about safety measures (which includes avoiding open flames such as candles, large fires, or sparks when on LTOT and providing Home Safety Agreements—a written contract between prescriber and patient wherein the patient agrees to abide by the terms of the US Department of Veterans Affairs (VA) to mitigate hazards related to LTOT in order to receive LTOT (eAppendix
Methods
With this practice in mind, we conducted an institutional review board approved retrospective chart review of all veterans with diagnosis of COPD within the Central Texas Veterans Health Care System (CTVHCS) who were prescribed new LTOT between October 1, 2010 and September 30, 2015. Given the retrospective nature of the chart review, patient consent was not obtained. Inclusion criteria were veterans aged > 18 years who had a confirmed diagnosis of COPD by spirometry and who met criteria for either continuous or ambulation- only oxygen therapy.
Criteria for exclusion included patients with hypoxemia not solely attributable to COPD or due to diseases other than COPD. We reviewed encounters in these patients’ charts, including follow-up in the clinic of the providers prescribing oxygen, to assess for fire-related incidents, defined as events wherein fire was visualized by the patient or by individuals living with the patient and with report provided to medical equipment company providing oxygen; the patient did not have to seek medical care to qualify for fire-related incident. Of the 158 patients who met the criteria for inclusion in the study, 152 were male.
Statistics
Bayesian logistic regression was used to model the outcome variable fire-related incident with the predictors smoking status, age, race, depression, PTSD, and type of oxygen used. Mental health disorders have significant effect on substance use disorders, such as alcohol use. Depression and PTSD were more common mental health diagnoses found in our patient population. Additionally, due to the small sample size, these psychiatric diagnoses were chosen to evaluate the impact of mental health disorders on firerelated events.
Although the sample size of events was small, weakly informative normal priors (0, 2.5) were used to shrink parameter estimates toward 0 and minimize overfitting. Weakly informative normal priors have also been suggested to deal with the problem of quasi-complete separation, where in our case, both smoking and no-PTSD perfectly predicted the 9 fire-related incidents.10 All input variables were centered and scaled as recommended. 9 The model fit well as assessed by posterior predictive checks, and Rhat was 1.00 for all parameters, indicating that all chains converged. Analysis was completed in R version 3.5.1 using the ‘brms’ package for Bayesian modeling.11
Results
The mean age for the 158 included patients was 71.3 years in nonsmokers and 65.9 years in smokers. Fifty-three of the included patients were active smokers when LTOT was initiated. Nine veterans had fire-related incidents during the study period. All 9 patients were actively smoking (about 17%) at the time of the fire incidents. There were no deaths, and 5 patients required hospitalization due to facial burns resulting from the fire-related incidents. Our study focused on 5 baseline characteristics in our population (Table 1). After gathering data, our group inferred that these characteristics had a potential relationship to fire-related incidents compared with other variables that were studied. Future studies could look at other patient characteristics that may be linked to fire-related incidents in patients on LTOT. For example, not having PTSD also perfectly predicts fire-related incidents in our data (ie, none of the participants who had fire-related incidents had PTSD). Although this finding was not within the 95% confidence interval (CI) in the model, it does show that care must be taken when interpreting effects from small samples (Table 2). The modelestimated odds of a fire-related incident occurring in a smoker were 31.6 (5.1-372.7) times more likely than were the odds of a firerelated incident occurring in a nonsmoker, holding all other predictors at their reference level; 95% CI for the odds ratios for all other predictors in the model included a value of 1.
Discussion
This study showed evidence of increased odds of fire-related events in actively smoking patients receiving LTOT compared with patients who do not actively smoke while attempting to adjust for potential confounders. Of the 9 patients who had fire events, 5 required hospitalization for burns.
A similar retrospective cohort study by Sharma and colleagues in 2015 demonstrated an increased risk of burn-related injury when on LTOT but reiterated that the benefit of oxygen outweighs the risk of burn-related injury in patients requiring oxygen therapy.12 Interestingly, Sharma and colleagues were unable to identify smoking status for the patients studied but further identified factors associated with burn injury to include male sex, low socioeconomic status, oxygen therapy use, and ≥ 3 comorbidities. The study’s conclusion recommended continued education by health care professionals (HCPs) to their patients on LTOT regarding potential for burn injury. In the same vein, the VA National Center for Ethics in Health Care noted that “clinicians should familiarize themselves with the risks and benefits of LTOT; should inform their patients of the risks and benefits without exaggerating the risk associated with smoking; avoid undue coercion inherent in the clinician’s ability to withdraw LTOT; reduce the risk to the greatest degree possible; and consider termination of LTOT in very extreme cases and in consultation with a multidisciplinary committee.”13
This statement is in contrast to the guidelines and policies of other countries, such as Sweden, where smoking is a direct contraindication for prescription of oxygen therapy, or in Australia and New Zealand, where the Thoracic Society of Australia and New Zealand oxygen therapy guidelines recommend against prescription of LTOT, citing “increased fire risk and the probability that the poorer prognosis conferred by smoking will offset treatment benefit.”6,14
The prevalence of oxygen therapy introduces the potential for fire-related incidents with subsequent injury requiring medical care. There are few studies regarding home oxygen fire in the US due to the lack of a uniform reporting system. One study by Wendling and Pelletier analyzed deaths in Maine, Massachusetts, New Hampshire, and Oklahoma between 2000 and 2007 and found 38 deaths directly attributable to home oxygen fires as a result of smoking.15 Further, the Consumer Product Safety Commission’s National Electronic Injury Surveillance System between 2003 and 2006 attributed 1,190 thermal burns related to home oxygen fires; the majority of which were ignited by tobacco smoking.15 The Swedish National Register of Respiratory Failure (Swedevox) published prospective population-based, consecutive cohort study that collected data over 17 years and evaluated the risk of fire-related incident in those on LTOT. Of the 12,497 patients sampled, 17 had a burn injury and 2 patients died. The low incidence of burn injury on LTOT was attributed to the strict guidelines instituted in Sweden for doctors to avoid prescribing LTOT to actively smoking patients.6 A follow-up study by Tanash and colleagues compared the risk of burn injury in each country, respectively. The results found an increased number of burn injuries in those on oxygen therapy in Denmark, a country with fewer restrictions on smoking compared with those of Sweden.7 Similarly, our results showed that the rate of fire and burn injuries was exclusively among veterans who were active smokers. All patients who were prescribed oxygen therapy at CTVHCS received counseling and signed Home Safety Agreements. Despite following the recommendations set forth by the VA on counseling, extensive harm reduction techniques, and close follow-up, we found there was still a high incidence of fires in veterans with COPD on LTOT who continue to smoke.
The findings from our study concur with those previously published regarding the risk of home oxygen fire and concomitant smoking, supporting the idea for more regulated and concrete guidelines for prescribing LTOT to those requiring it.8
Limitations
The major limitation was the small sample size of our study. Another limitation was that our study population is predominantly male as is common in veteran cohorts. In fiscal year 2016, the veteran population of Texas was 1,434,361 males and 168,967 females.16 According to Franklin and colleagues, HCPs noticed an increase use of long-term oxygen among women compared with that of men.17
Conclusions
Our study showed an increased odds of firerelated incidents of patients while on LTOT, strengthening the argument that even with extensive education, those who smoke and are on LTOT continue to put themselves at risk of a fire-related incident. This finding stresses the importance of continuing patient education on the importance of smoking cessation prior to administration of LTOT or avoiding fire hazards while on LTOT. Further research into LTOT and fire hazards could help in implementing a more structured approval process for patients who want to obtain LTOT. We propose further studies evaluating risk factors for the incidence of fire events among patients prescribed LTOT. A growing and aging population with a need for LTOT necessitates examination of oxygen safe prescribing.
1. Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States 2000-2014. https:// www.cdc.gov/nchs/data/databriefs/db256.pdf. Published September 2016. Accessed September 10, 2020.
2. Itoh M, Tsuji T, Nemoto K, Nakamura H, Aoshiba K. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316-1335. doi:10.3390/nu5041316
3. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391. doi:10.7326/0003-4819-93-3-391
4. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. doi:10.1016/S0140-6736(81)91970-X
5. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233-239. doi:10.7326/0003-4819-142-4 -200502150-00005
6. Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479-2484. doi:10.2147/COPD.S91508
7. Tanash HA, Ringbaek T, Huss F, Ekström M. Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chronic Obstruct Pulmon Dis. 2017;12:193-197. doi:10.2147/COPD.S119949
8. Kassis SA, Savetamal A, Assi R, et al. Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg. 2014;218(6):1182-1186. doi:10.1016/j.jamcollsurg.2013.12.055
9. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. doi:10.1056/NEJMoa1604344
10. Ghosh J, Li Y, Mitra R. On the use of Cauchy prior distributions for Bayesian logistic regression. Bayesian Anal. 2018;13(2):359-383. doi:10.1214/17-ba1051
11. Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80(1). doi:10.18637/jss.v080.i01
12. Sharma G, Meena R, Goodwin JS, Zhang W, Kuo Y-F, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90(4):492-499. doi:10.1016/j.mayocp.2014.12.024
13. US Department of Veterans Affairs, National Ethics Committee. Ethical considerations that arise when a home care patient on long term oxygen therapy continues to smoke. http://vaww.ethics.va.gov/docs/necrpts/NEC_Report_20100301_Smoking_while_on_LTOT.pdf. Published March 2010. [Nonpublic, source not verified.]
14. McDonald C F, Whyte K, Jenkins S, Serginson J. Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. doi:10.1111/resp.12678
15. Centers for Disease Control and Prevention (CDC). Fatal fires associated with smoking during long-term oxygen therapy--Maine, Massachusetts, New Hampshire, and Oklahoma, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(31):852-854.
16. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Population tables: the state, age/gender, 2016. https://www.va.gov/vetdata/Veteran_ Population.asp. Updated August 5, 2020. Accessed September 11, 2020.
17. Franklin KA, Gustafson T, Ranstam J, Ström K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease--gender differences. Respir Med. 2007;101(7):1506-1511. doi:10.1016/j.rmed.2007.01.009
1. Ni H, Xu J. COPD-related mortality by sex and race among adults aged 25 and over: United States 2000-2014. https:// www.cdc.gov/nchs/data/databriefs/db256.pdf. Published September 2016. Accessed September 10, 2020.
2. Itoh M, Tsuji T, Nemoto K, Nakamura H, Aoshiba K. Undernutrition in patients with COPD and its treatment. Nutrients. 2013;5(4):1316-1335. doi:10.3390/nu5041316
3. Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease: a clinical trial. Nocturnal Oxygen Therapy Trial Group. Ann Intern Med. 1980;93(3):391. doi:10.7326/0003-4819-93-3-391
4. Long term domiciliary oxygen therapy in chronic hypoxic cor pulmonale complicating chronic bronchitis and emphysema. Report of the Medical Research Council Working Party. Lancet. 1981;1(8222):681-686. doi:10.1016/S0140-6736(81)91970-X
5. Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE. The effects of a smoking cessation intervention on 14.5-year mortality. Ann Intern Med. 2005;142(4):233-239. doi:10.7326/0003-4819-142-4 -200502150-00005
6. Tanash HA, Huss F, Ekström M. The risk of burn injury during long-term oxygen therapy: a 17-year longitudinal national study in Sweden. Int J Chron Obstruct Pulmon Dis. 2015;10:2479-2484. doi:10.2147/COPD.S91508
7. Tanash HA, Ringbaek T, Huss F, Ekström M. Burn injury during long-term oxygen therapy in Denmark and Sweden: the potential role of smoking. Int J Chronic Obstruct Pulmon Dis. 2017;12:193-197. doi:10.2147/COPD.S119949
8. Kassis SA, Savetamal A, Assi R, et al. Characteristics of patients with injury secondary to smoking on home oxygen therapy transferred intubated to a burn center. J Am Coll Surg. 2014;218(6):1182-1186. doi:10.1016/j.jamcollsurg.2013.12.055
9. Long-Term Oxygen Treatment Trial Research Group, Albert RK, Au DH, et al. A Randomized Trial of Long-Term Oxygen for COPD with Moderate Desaturation. N Engl J Med. 2016;375(17):1617-1627. doi:10.1056/NEJMoa1604344
10. Ghosh J, Li Y, Mitra R. On the use of Cauchy prior distributions for Bayesian logistic regression. Bayesian Anal. 2018;13(2):359-383. doi:10.1214/17-ba1051
11. Bürkner P-C. brms: An R package for Bayesian multilevel models using Stan. J Stat Software. 2017;80(1). doi:10.18637/jss.v080.i01
12. Sharma G, Meena R, Goodwin JS, Zhang W, Kuo Y-F, Duarte AG. Burn injury associated with home oxygen use in patients with chronic obstructive pulmonary disease. Mayo Clin Proc. 2015;90(4):492-499. doi:10.1016/j.mayocp.2014.12.024
13. US Department of Veterans Affairs, National Ethics Committee. Ethical considerations that arise when a home care patient on long term oxygen therapy continues to smoke. http://vaww.ethics.va.gov/docs/necrpts/NEC_Report_20100301_Smoking_while_on_LTOT.pdf. Published March 2010. [Nonpublic, source not verified.]
14. McDonald C F, Whyte K, Jenkins S, Serginson J. Frith P. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology. 2016;21(1):76-78. doi:10.1111/resp.12678
15. Centers for Disease Control and Prevention (CDC). Fatal fires associated with smoking during long-term oxygen therapy--Maine, Massachusetts, New Hampshire, and Oklahoma, 2000-2007. MMWR Morb Mortal Wkly Rep. 2008;57(31):852-854.
16. US Department of Veteran Affairs. National Center for Veterans Analysis and Statistics. Population tables: the state, age/gender, 2016. https://www.va.gov/vetdata/Veteran_ Population.asp. Updated August 5, 2020. Accessed September 11, 2020.
17. Franklin KA, Gustafson T, Ranstam J, Ström K. Survival and future need of long-term oxygen therapy for chronic obstructive pulmonary disease--gender differences. Respir Med. 2007;101(7):1506-1511. doi:10.1016/j.rmed.2007.01.009
Infected Bronchogenic Cyst With Left Atrial, Pulmonary Artery, and Esophageal Compression
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
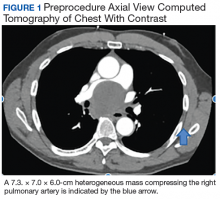
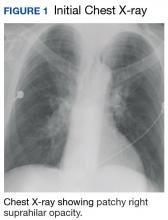
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
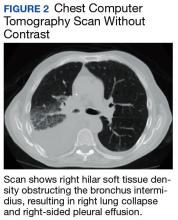
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
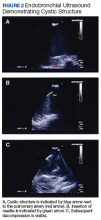
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
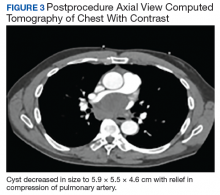
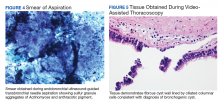
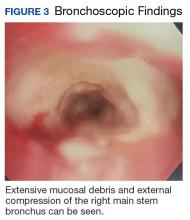
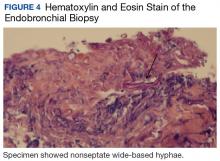
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
Bronchogenic cyst is a rare foregut malformation that typically presents during the second decade of life that arises due to aberrant development from the tracheobronchial tree.1 Mediastinal bronchogenic cyst is the most common primary cystic lesion of the mediastinum, and bronchogenic cysts of the mediastinum represent 18% of all primary mediastinal malformations.2 Patients with mediastinal bronchogenic cysts may present with symptoms of cough, dyspnea, or wheezing if there is encroachment on surrounding structures.
Rarely, bronchogenic cysts can become infected. Definitive treatment of bronchogenic cysts is surgical excision; however, endobronchial ultrasound (EBUS)-guided drainage also can be employed. EBUS-guided drainage may be used when the cyst cannot be distinguished from solid mass on computed tomography (CT) images, to relieve symptomatic compression of surrounding structures, or to provide a histologic or microbial diagnosis in cases where surgical excision is not immediately available. We present the first-ever described case of bronchogenic cyst infected with Actinomyces, diagnosed by EBUS-guided drainage as well as a review of the literature regarding infected bronchogenic cysts and management of cysts affecting mediastinal structures.
Case Presentation
A 57-year-old African American male presented with a 4-day history of continuous, sharp, substernal chest pain accompanied by dyspnea. Additionally, the patient reported progressive dysphagia to solids. The posteroanterior view of a chest X-ray showed a widened mediastinum with splaying of the carina. A contrast-enhanced CT of the chest showed a large, middle mediastinal mass of heterogenous density measuring 7.3. × 7.0 × 6.0 cm with compression of the right pulmonary artery, left atria, superior vena cava and esophagus (Figure 1).
The mass demonstrated neither clear fluid-fluid level nor rounded structure with a distinct wall and uniform attenuation consistent with pure cystic structure and, in fact, was concerning for malignant process, such as lymphoma. Due to the malignancy concern and the findings of significant compression of surrounding mediastinal structures, the decision was made to proceed with bronchoscopy and EBUS-guided transbronchial needle aspiration (EBUS-TBNA) to assist in diagnosis and potentially provide symptomatic relief.
Under general anesthesia a P160 Olympus bronchoscope was advanced into the tracheobronchial tree; bronchoscopy with airway inspection revealed splayed carina with obtuse angle but was otherwise unremarkable. Next, an EBUS P160 fiber optic Olympus bronchoscope was advanced; ultrasound demonstrated a cystic structure. The EBUS-TBNA of cystic structure yielded 20 mL of brown, purulent fluid with decompression bringing pulmonary artery in ultrasound field (Figure 2). Rapid on-site cytology was performed with no preliminary findings of malignancy. The fluid was then sent for cytology and microbiologic evaluation.
Following EBUS-guided aspiration, the patient reported significant improvement in chest pain, dyspnea, and dysphagia. A repeat chest CT demonstrated decrease in mass size to 5.9 × 5.5 × 4.6 cm with relief of the compression of the right pulmonary artery and decreased mass effect on the carina (Figure 3). Pathology ultimately demonstrated no evidence of malignancy but did demonstrate filamentous material with sulfur granules and anthracotic pigment suggestive of Actinomyces infection (Figure 4).
The patient was placed on amoxicillin/clavulanate 875 mg to 125 mg twice daily for 4 weeks based on antibiotic susceptibility testing to prevent progression to mediastinitis related to Actinomyces infection. The duration of therapy was extrapolated from treatment regimens described in case series of cervicofacial and abdominal Actinomyces infections.3 Thoracic surgery evaluation for definitive excision of cyst was recommended after the patient completed his course of antibiotics.
The patient underwent dental evaluation to identify the source of Actinomyces infection but there appeared to be no odontogenic source. The patient also had extensive skin survey with no findings of overt source of Actinomyces and CT abdomen/pelvis also identified no abscess that could be a potential source. He subsequently underwent thoracoscopic resection with pathology demonstrating a fibrous cyst wall lined with ciliated columnar epithelium consistent with diagnosis of bronchogenic cyst (Figure 5).
Discussion
Bronchogenic cysts can present at birth or later in life; patients may be asymptomatic for decades prior to discovery.4 Cysts located in the mediastinum can cause compression of the trachea and esophagus and cause cough, dyspnea, chest pain, and dysphagia.5 More life-threatening complications include infection, tracheal compression, malignant transformation, superior vena cava syndrome, or spontaneous rupture into the airway.6,7
Infection can occasionally occur, and various bacterial etiologies have been described. Hernandez-Solis and colleagues describe 12 cases of superinfected bronchogenic cysts with Staphylococcus aureus and Pseudomonas aeroginosa, the most commonly described organisms.8 Casal and colleagues describe a case of α-hemolytic Streptococci treated with amoxicillin.9 Liman and colleagues describe 2 cases of bronchogenic cyst infected with Mycobacterium and cite an additional case report by Lin and colleagues similarly infected by Mycobacterium.10,11 Only 1 case was identified to have direct bronchial communication as a potential source of introduction of infection into bronchogenic cyst. In other cases, potential sources of infection were not identified, though it was postulated that direct ventilation could be a potential source of inoculation.
Surgical resection of mediastinal bronchogenic cysts has traditionally been considered the definitive treatment of choice.12,13 However, bronchogenic cysts may sometimes be difficult to differentiate from soft tissue tumors by chest CT, especially in cases of cysts with nonserous fluid. In particular, cysts that are infected are likely to have increased density and high attenuation on imaging; therefore, surgical excision may be delayed until diagnosis is made.14 Due to low complication rates, EBUS is increasingly used in the diagnosis and therapeutic management of bronchogenic cysts as an alternative to surgery, particularly for those who are symptomatic.15,16 Ultrasound guidance can allow for complete aspiration of the cyst, causing complete collapse of the cystic space and can facilitate adhesion between the mucosal surfaces lining the cavity and reduce recurrence.17 Nonetheless, bronchogenic cysts that are found to be infected, recur, or have a malignant component should be resected for definitive treatment.18
The mass discovered on our patient’s imaging appeared to have heterogenous attenuation consistent with malignancy rather than homogenous attenuation surrounded by a clearly demarcated wall consistent with a cystic structure; therefore, EBUS-TBNA was initially pursued and yielded an expedited diagnosis of the first-ever described bronchogenic cyst with Actinomyces superinfection as well as dramatic symptomatic relief of compression of surrounding mediastinal structures, particularly of the right pulmonary artery. As this is a congenital malformation, the patient was likely asymptomatic until the cyst became infected, after which he likely experience cyst growth with subsequent encroachment of surrounding mediastinal structures. Additionally, identification of pathogen by TBNA allowed for treatment before surgical excision, possibly avoiding accidental spread of pathogen intraoperatively.
Conclusions
Our case adds to the literature on the use of EBUS-TBNA as a diagnostic and therapeutic modality for bronchogenic cyst. While cases of mediastinitis and pleural effusion following EBUS-guided aspiration of bronchogenic cysts have been reported, complications are extremely rare.19 EBUS is increasingly favored as a means of immediate diagnosis and treatment in cases where CT imaging may not overtly suggest cystic structure and in patients experiencing compression of critical mediastinal structures.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
1. Weber T, Roth TC, Beshay M, Herrmann P, Stein R, Schmid RA. Video-assisted thoracoscopic surgery of mediastinal bronchogenic cysts in adults: a single-center experience. Ann Thorac Surg. 2004;78(3):987-991.
2. Martinod E, Pons F, Azorin J, et al. Thoracoscopic excision of mediastinal bronchogenic cysts: results in 20 cases. Ann Thorac Surg. 2000;69(5):1525-1528.
3. Könönen E, Wade WG. Actinomyces and related organisms in human infections. Clin Microbiol Rev. 2015;28(2):419-442.
4. Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61(6):1636-1640.
5. Guillem P, Porte H, Marquette CH, Wurtz A. Progressive dysphonia and acute respiratory failure: revealing a bronchogenic cyst. Eur J Cardiothorac Surg. 1997;12(6):925-927.
6. McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, Matsumoto S. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology. 2000;217(2):441-446.
7. Rammohan G, Berger HW, Lajam F, Buhain WJ. Superior vena cava syndrome caused by bronchogenic cyst. Chest. 1975;68(4):599-601.
8. Hernández-Solís A, Cruz-Ortiz H, Gutiérrez-Díaz Ceballos ME, Cicero-Sabido R. Quistes broncogénicos. Importancia de la infección en adultos. Estudio de 12 casos [Bronchogenic cysts. Importance of infection in adults. Study of 12 cases]. Cir Cir. 2015;83(2):112-116.
9. Casal RF, Jimenez CA, Mehran RJ, et al. Infected mediastinal bronchogenic cyst successfully treated by endobronchial ultrasound-guided fine-needle aspiration. Ann Thorac Surg. 2010;90(4):e52-e53.
10. Liman ST, Dogan Y, Topcu S, Karabulut N, Demirkan N, Keser Z. Mycobacterial infection of intraparenchymal bronchogenic cysts. Respir Med. 2006;100(11):2060-2062.
11. Lin SH, Lee LN, Chang YL, Lee YC, Ding LW, Hsueh PR. Infected bronchogenic cyst due to Mycobacterium avium in an immunocompetent patient. J Infect. 2005;51(3):e131-e133.
12. Gharagozloo F, Dausmann MJ, McReynolds SD, Sanderson DR, Helmers RA. Recurrent bronchogenic pseudocyst 24 years after incomplete excision. Report of a case. Chest. 1995;108(3):880-883.
13. Bolton JW, Shahian DM. Asymptomatic bronchogenic cysts: what is the best management? Ann Thorac Surg. 1992;53(6):1134-1137.
14. Sarper A, Ayten A, Golbasi I, Demircan A, Isin E. Bronchogenic cyst. Tex Heart Inst J. 2003;30(2):105-108.
15. Varela-Lema L, Fernández-Villar A, Ruano-Ravina A. Effectiveness and safety of endobronchial ultrasound-transbronchial needle aspiration: a systematic review. Eur Respir J. 2009;33(5):1156-1164.
16. Maturu VN, Dhooria S, Agarwal R. Efficacy and safety of transbronchial needle aspiration in diagnosis and treatment of mediastinal bronchogenic cysts: systematic review of case reports. J Bronchology Interv Pulmonol. 2015;22(3):195-203.
17. Galluccio G, Lucantoni G. Mediastinal bronchogenic cyst’s recurrence treated with EBUS-FNA with a long-term follow-up. Eur J Cardiothorac Surg. 2006;29(4):627-629.
18. Lee DH, Park CK, Kum DY, Kim JB, Hwang I. Clinical characteristics and management of intrathoracic bronchogenic cysts: a single center experience. Korean J Thorac Cardiovasc Surg. 2011;44(4):279-284.
19. Onuki T, Kuramochi M, Inagaki M. Mediastinitis of bronchogenic cyst caused by endobronchial ultrasound-guided transbronchial needle aspiration. Respirol Case Rep. 2014;2(2):73-75.
Pulmonary Mucormycosis in a Patient With Uncontrolled Diabetes
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.