User login
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
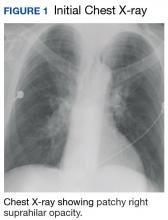
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
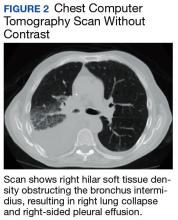
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
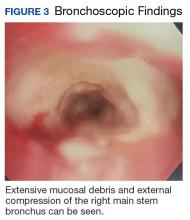
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
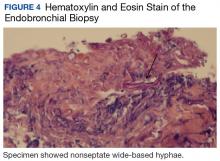
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.