User login
Disseminated Invasive Candidiasis in an Immunocompetent Host
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
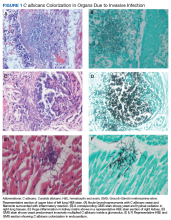
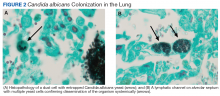
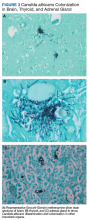
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
Candida albicans (C albicans) is a normal commensal in the human gastrointestinal (GI) tract. In addition to localized infections in healthy human beings, dissemination with fatal outcome can occur in immunocompromised individuals.1
Invasive candidiasis (IC) due to C albicans is the most common nosocomial mycosis in the world and has 2 forms, candidemia and deep-seated tissue candidiasis, which can lead to multisystem organ failure.2 The deep-seated form may originate from nonhematogenous routes, such as introduction through a peritoneal catheter or ascending infection from cystitis.2 In addition, about 50% of primary candidemia cases lead to secondary deep-seated candidiasis; however, only about 40% of these cases show positive blood cultures. Since the window of opportunity for a positive culture is narrow, active candidemia may be missed.3,4
Once developed, the prognosis for IC is grim: Mortality is 40% regardless of therapy.2 IC typically occurs in immunocompromised hosts; IC in immunocompetent persons has rarely been reported.5,6 It is challenging to diagnose IC in the immunocompetent patients as 50% to 70% of the general population is naturally colonized by this organism, and when found, it is assumed to be mostly innocuous. Neutrophil-driven cell-mediated immunity associated with IL-1 and IL-17 response prevent fungal growth and dissemination, protecting the immunocompetent host.7
We report on a patient who showed no neutropenia or leukocytopenia but developed disseminated candidiasis. This report is one of the rare cases of full-blown disseminated candidiasis with lesions related to C albicans found in almost all of the important organs.
Case Presentation
A 67-year-old male patient with a history of hypertension, peripheral vascular disease, daily heavy alcohol consumption, and a 50-pack-year history of smoking developed gangrene of the left fifth toe. He underwent vascular surgery consultation with an aortogram/left lower extremity angiography that showed occlusion of the left external iliac artery as well as the left common femoral artery. It was decided to improve inflow in the common iliac artery by placing a bare metal stent and subsequent balloon dilatation before a right to left femoral to femoral artery bypass. The patient tolerated the procedure well and was discharged home.
Two days later, the patient was admitted to a US Department of Veterans Affairs (VA) complexity level 1a hospital with weakness and worsening pain in the left lower extremities. Examination revealed chronic ischemic changes in the feet bilaterally and evidence of dry gangrene in the left fifth toe requiring femoral bypass surgery. But poor nutritional status and cardiac status prevented pursuing a permanent solution.
Following completion of a stress echocardiogram, the patient developed shock with systolic blood pressure of 60 mm Hg, and atrial fibrillation (AF) with rapid ventricular rate (RVR). He was initially treated with IV fluid supplementation, vasopressor therapy, synchronized cardioversion, and IV amiodarone/anticoagulation therapy, due to his persistent AF with RVR. The patient was transferred to a tertiary care center for persistent hypothermia and received treatment with warm saline. After initial recovery with warm saline resuscitation, he had a prolonged, complicated hospital course in which he developed progressive respiratory failure requiring intubation and critical care support. He developed a right internal jugular deep venous thrombosis, heparin-induced thrombocytopenia, lower GI bleeding requiring emergent embolization by interventional radiology, inferior vena cava filter placement, renal failure requiring dialysis, small bowel obstruction secondary to right lower quadrant phlegmon and perforation requiring small bowel resection and end ileostomy. His antibiotic regimen included therapy with vancomycin and piperacillin-tazobactam.
He eventually recovered and was extubated and subsequently transferred back to the VA hospital where cefepime was initiated because of suspicion of a urinary tract infection and septicemia (urine cultures eventually grew C albicans). Over the subsequent 3 days, the patient’s renal output and hyperkalemia worsened, he also developed increased anion gap metabolic acidosis and was intubated again and placed on full mechanical ventilatory support. His blood cultures were negative, and sputum cultures revealed normal respiratory flora and 1+ C albicans. Infectious diseases consultation recommended an abdominal ultrasound, which revealed nonspecific findings. The antibiotic regimen was changed to daptomycin and piperacillin-tazobactam. A follow-up chest X-ray revealed a developing right lower lobe pneumonia and hilar prominence suggestive of lymphadenopathy. The patient’s clinical condition deteriorated, and he subsequently developed cardiac arrest; resuscitation was not successful and he expired.
Outcome and Follow-up
An autopsy disclosed the cause of death to be bilateral candida pneumonia, part of a disseminated (invasive) candidiasis, in a patient rendered vulnerable to such infection by peripheral vascular disease and renal insufficiency. Purulent inflammation was noted at the site of disarticulation of the left foot and confluent consolidation of the lower lobes of both lungs as well as focal consolidation of the middle lobe of the right lung. Examination of histologic sections, with staining both by routine method (hematoxylin and eosin) and the Grocott-Gömöri methenamine silver method for fungus, disclosed fungal forms (yeast and filamentous) in most tissues, including the lungs (Figure 1 A and B) and kidneys (Figure 1 C and D). The pulmonary sections in addition to massive inflammation showed macrophages with engulfed yeast (Figure 2 A) and a lymphatic channel, stuffed with yeast in an alveolar septum (Figure 2 B). These findings confirmed the antemortem presence of the fungus and the body’s response to it. Inflammation was noted around glomeruli overgrown by candida (Figure 1 C and D); fungi also were seen in capsular regions (not depicted). C albicans was present in the myocardium (Figure 1 E and F), brain, thyroid, and adrenal glands (Figure 3); the only organ without C albicans was the liver, either because invasion was truly absent here or because sampling had not managed to retrieve it.
Paraffin-embedded blocks of lung tissue, sent to the University of Washington Molecular Diagnosis Microbiology Laboratory for broad-range polymerase chain reaction (PCR) identification, were positive for C albicans after extraction of gDNA and conduction of PCR using internal transcribed spacer 1 and 2 specific primers.
Discussion
IC is rare among immunocompetent individuals, but C albicans can evolve into a fatal disseminated infection. We report an atypical case of IC, with profound pulmonary infection in a patient who died 1 month after hospitalization for lower extremity pain.
Cell-mediated immunity involving neutrophils and macrophages plays a major role in protection against candidiasis, while cytokines and chemokines involve regulating balanced immunity.1,2 A series of recent studies show that alcohol impairs neutrophil-mediated killing and phagocytic-mediated uptake of a pathogen in this process.8,9 As the patient chronically misused alcohol, his immune system may have experienced a subclinical immunosuppression, which would have become clinically relevant once C albicans was introduced systemically. Recent studies of bacterial pathogenesis and alcoholism strongly support this hypothesis.10,11
Most patients with the unusual diagnosis of candida pneumonia have had a background of malignancy or immunosuppressive factors (eg, administration of corticosteroids).12 In a series of 20 cases, 14 had sputum cultures positive for the organism, 6 had positive urine cultures, and 6 had positive blood cultures. Chest radiographs usually showed confluent bronchopneumonia. Five patients were diagnosed antemortem and treated with amphotericin B, but none survived.13 In the literature a positive blood culture or demonstration of yeast within pulmonary histiocytes has been considered proof of the pathogenicity of the fungus, as opposed to noninvasive colonization of the airways, a common occurrence in patients receiving mechanical ventilation.2
As previously discussed, blood cultures are often negative with invasive candidiasis, as the window of opportunity is short and may be missed. As shown in murine models, it is easy to miss a narrow window of candidemia, leading to false-negative blood cultures in clinical practice.14,15 Mouse model studies also have found that the window of candidemia is very short in disseminated candidiasis as a lethal IV dose of C albicans disappeared from blood within 48 hours of postinoculation.15 The biomarker of serum procalcitonin is a great diagnostic resource for the elimination of a likely bacterial sepsis, and conversely, the early suspicion of a fungemia, as serum procalcitonin would typically be elevated in a bacterial but not a fungal septicemia.16 The average cost per test is only about $30, and we recommend testing for serum procalcitonin as well as monitoring of serum lactate levels in cases of nonresponding septicemia.
The C albicans in this case may have been introduced hematogenously from the amputation site or through an ascending cystitis, or possibly have been derived from commensal flora in the GI tract. The iron supplementation provided to the patient may have promoted the growth and virulence of the candida; studies have shown that the kidneys assimilate increased levels of iron during disseminated candidiasis thus providing a more favorable site for colonization.17The presence of C albicans in a single collection of sputum or urine does not ordinarily indicate infection in an immunocompetent individual. Estimation of serum procalcitonin, a biomarker for bacterial infection and sepsis, might be useful if negative, for turning attention to a nonbacterial (such as, candida) source as the causative agent.18
Conclusion
C albicans can rarely cause disseminated disease in nonimmunocompromised critically ill patients. Low serum procalcitonin levels in a septic patient might indicate nonbacterial cause such as candidiasis. Even with disseminated candidiasis, blood cultures may remain negative.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
1. Navarathna DH, Stein EV, Lessey-Morillon EC, Nayak D, Martin-Manso G, Roberts DD. CD47 promotes protective innate and adaptive immunity in a mouse model of disseminated candidiasis. PLoS One. 2015;10(5):e0128220.
2. Kullberg BJ, Arendrup MC. Invasive candidiasis. N Engl J Med. 2015;373(15):1445-1456.
3. Clancy CJ, Nguyen MH. Diagnosing invasive candidiasis. J Clin Microbiol. 2018;56(5):e01909-e01917.
4. Ericson EL, Klingspor L, Ullberg M, Ozenci V. Clinical comparison of the Bactec Mycosis IC/F, BacT/Alert FA, and BacT/Alert FN blood culture vials for the detection of candidemia. Diagn Microbiol Infect Dis. 2012;73(2):153-156.
5. Baum GL. The significance of Candida albicans in human sputum. N Engl J Med. 1960;263:70-73.
6. el-Ebiary M, Torres A, Fàbregas N, et al. Significance of the isolation of Candida species from respiratory samples in critically ill, non-neutropenic patients. An immediate postmortem histologic study. Am J Respir Crit Care Med. 1997;156(2, pt 1):583-590.
7. Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS Pathog. 2016;12(9):e1005882.
8. Karavitis J, Kovacs EJ. Macrophage phagocytosis: effects of environmental pollutants, alcohol, cigarette smoke, and other external factors. J Leukoc Biol. 2011;90(6):1065-1078.
9. Chiu C-H, Wang Y-C, Yeh K-M, Lin J-C, Siu LK, Chang F-Y. Influence of ethanol concentration in the phagocytic function of neutrophils against Klebsiella pneumoniae isolates in an experimental model. J Microbiol Immunol Infect. 2018;51(1):64-69.
10. Khocht A, Schleifer S, Janal M, Keller S. Neutrophil function and periodontitis in alcohol-dependent males without medical disorders. J Int Acad Periodontol. 2013;15(3):68-74.
11. Gandhi JA, Ekhar VV, Asplund MB, et al. Alcohol enhances Acinetobacter baumannii-associated pneumonia and systemic dissemination by impairing neutrophil antimicrobial activity in a murine model of infection. PLoS One. 2014;9(4):e95707.
12. Mohsenifar Z, Chopra SK, Johnson BL, Simmons DH. Candida pneumonia: experience with 20 patients. West J Med. 1979;131(3):196-200.
13. Jones JM. Laboratory diagnosis of invasive candidiasis. Clin Microbiol Rev. 1990;3(1):32-45.
14. Clancy CJ, Nguyen MH. Finding the “missing 50%” of invasive candidiasis: how nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin Infect Dis. 2013;56(9):1284-1292.
15. Kappe R, Mu¨ ller J. Rapid clearance of Candida albicans mannan antigens by liver and spleen in contrast to prolonged circulation of Cryptococcus neoformans antigens. J Clin Microbiol. 1991;29(8):1665-1669.
16. Balk RA, Kadri SS, Cao Z, Robinson SB, Lipkin C, Bozzette SA. Effect of procalcitonin testing on health-care utilization and costs in critically ill patients in the United States. Chest. 2017;151(1):23-33.
17. Potrykus J, Stead D, Maccallum DM, et al. Fungal iron availability during deep seated candidiasis is defined by a complex interplay involving systemic and local events. PLoS Pathog. 2013;9(10):e1003676.
18. Soni NJ, Samson DJ, Galaydick JL, Vats V, Pitrak DL, Aronson N. Procalcitonin-Guided Antibiotic Therapy. Rockville, MD: Agency for Healthcare Research and Quality (US); 2012.
Pulmonary Mucormycosis in a Patient With Uncontrolled Diabetes
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
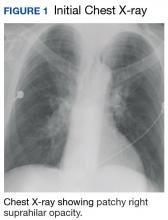
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
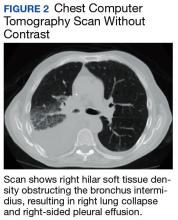
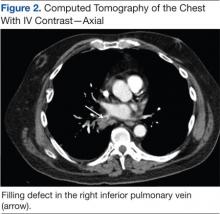
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
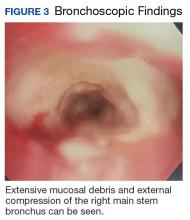
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
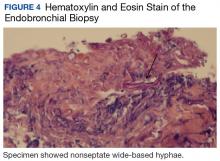
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
Mucorales fungi are ubiquitous organisms commonly inhabiting soil and can cause opportunistic infections. The majority of infections are caused by 3 genera: Rhizopus, Mucor, and Rhizomucor.1 Infection occurs by inhalation or by direct contact with damaged skin. Mucorales infections can have cutaneous, rhinocerebral, pulmonary, gastrointestinal, and central nervous system manifestations. Pulmonary mucormycosis is often rapidly progressive with angioinvasion and fulminant necrosis causing acute dyspnea, hemoptysis, and chest pain. More indolent pulmonary Mucorales infections can mimic a pulmonary mass with occasional cavitation found on imaging studies similar to other fungal infections (eg, Aspergillus).2 Risk factors include severe uncontrolled diabetes mellitus (DM), recurrent diabetic ketoacidosis (DKA), immunosuppression due to congenital or acquired causes, hematologic malignancies, and chronic renal failure.3 The authors present a case of a patient with recurrent DKA and pulmonary mucormycosis.
Case Presentation
A 62-year-old male with DM and a more than 30-pack-year smoking history presented to the emergency department with abdominal pain and chest pain ongoing for about 1 week. The patient had a history of frequent admissions with DKA and medication nonadherence.
On admission, the patient was hemodynamically stable. His vital signs were: temperature 97.4° F, heart rate 89 bpm, respirations 24 breathes per minute, blood pressure 146/86 mm Hg, and oxygen saturation 94% on ambient air. The patient appeared ill but the physical examination was otherwise unremarkable. Laboratory results revealed a white blood cell count of 24,400 with neutrophilic predominance, blood glucose 658 mg/dL, creatinine clearance 2.16 mL/min/1.73 m2, sodium level 124 mEq/L, bicarbonate 6 mEq/L, anion gap 27 mEq/L, 6.8 pH, partial pressure of CO2 11 mm Hg, and lactic acid 2.3 mmol/L.
The patient admitted for DKA management and placed on an insulin drip. Although he did not have a fever or cough productive of sputum or hemoptysis, there was concern that pneumonia might have precipitated DKA. A chest X-ray revealed a patchy, right suprahilar opacity (Figure 1).
The patient was placed on vancomycin 1,000 mg every 12 hours and cefepime 2,000 mg every 12 hours for possible hospital-acquired pneumonia because of his history of recent DKA hospitalization. Once the patient’s anion gap was closed and metabolic acidosis was resolved, the insulin drip was discontinued, and the patient was transferred to the general medical ward for further management. There, he continued to report having chest pain. A computed tomography (CT) scan without contrast of the chest (contrast was held due to recent acute kidney injury) revealed right hilar soft tissue density obstructing the bronchus intermidius, which had resulted in a right-lung collapse and right-sided pleural effusion (Figure 2). The left lung was clear, and there was no evidence of nodularity.
Given the patient’s extensive smoking history, the initial concern was for pulmonary malignancy. The decision was made to proceed with bronchoscopy with endobronchial ultrasound-guided transbronchial needle biopsy. Endobronchial brushings and biopsies of R11, 7, right bronchus intermedius, and right upper lobe were obtained. Gross inspection of the airway revealed markedly abnormal-appearing mucosa involving the take off to the right upper lobe and the entire bronchus intermedius with friable, cobblestoned, and edematous mucosa. Biopsies and immunostaining for occult carcinoma markers, including CD-56, TTF-1, Synaptophysin A, chromogranin, AE1/AE3, and CK-5/6, were negative for malignancy. Final microbiologic analysis was positive for Mucor. There was no evidence of bacterial or mycobacterial growth.
Due to continued suspicion for malignancy and lack of histologic yield, the patient underwent a repeat endobronchial ultrasound-guided needle biopsy. On this occasion, gross inspection revealed significant mucosal necrosis and extensive, extrinsic bronchial compression starting from the right bronchial division and notable throughout the right middle and lower lobes (Figure 3).
Bronchial washings revealed necrotic material with rare fungal hyphae present. Biopsies yielded necrotic material or lung tissue containing nonseptate hyphae with rare, right-angle branching consistent with Mucor (Figures 4 and 5). Malignancy was not present in the specimens obtained.
Based on the bronchoscopy results, thoracic surgery and infectious disease specialists were consulted. Surgical intervention was not recommended because of concerns for potential postoperative complications. The infectious disease specialists recommended initiation of liposomal amphotericin B at 10 mg/kg/d. Magnetic resonance imaging of the head showed parietal lobe enhancement with restricted diffusion most consistent with prior infarct. Paranasal sinus disease also was demonstrated. The latter findings prompted further evaluation. The patient underwent right and left endoscopic resection of concha bullosa as well as left maxillary endoscopic antrostomy. Gross examination showed thick mucosa in left concha bullosa, polypoid changes anterior to bulla ethmoidalis, and clear left maxillary sinus. The procedure had to be aborted when the patient experienced cardiac arrest secondary to ventricular fibrillation; he was successfully resuscitated.
Samples from the contents of right and left sinuses as well as left concha bullosa were submitted to pathology, showing benign respiratory mucosa with chronic inflammation and foci of bone without fungal elements. There was no other evidence of disseminated mucormycosis. The patient had a prolonged hospital course complicated by progressive hypoxemia, acute kidney injury, and toxic metabolic encephalopathy. Three months after his original diagnosis, he sustained another cardiac arrest in the hospital. Shortly after achieving return of spontaneous circulation and initiation of invasive mechanical ventilation, the family elected to withdraw care. The family declined an autopsy.
Discussion
This article describes a case of subacute pulmonary mucormycosis in a patient with recurrent DKA. Although patients with poorly controlled DM commonly present with the rhinocerebral form of mucormycosis, pulmonary involvement with a subacute course has been described. Determining the final diagnosis for the current patient was challenging due to the subtlety of his respiratory symptoms and the inconsistent initial findings on chest radiography. A pulmonary disease was finally suspected when a mass was found on the CT scan. However, the middle mediastinal mass was more suspicious for malignancy, particularly given the patient’s smoking history and persistent hyponatremia. In fact, the lack of any neoplastic findings on the initial endobronchial biopsy prompted the health care team to pursue a second biopsy that was consistent with mucormycosis.
This case demonstrates the challenges of prompt diagnosis and treatment of this potentially fatal infection. Furthermore, the extent of the disease at diagnosis precluded this patient from having a surgical intervention, which has been associated with better outcomes than those of medical management alone. Finally, it remains unknown whether the patient had an underlying malignancy, which could have increased the likelihood of pulmonary mucormycosis; the biopsy yield may have been confounded by repeated sampling of necrotic material caused by mucormycosis. Further investigation of any potential pulmonary neoplasm was limited by the patient’s clinical condition and the poor prognosis due to the extent of infection.
Mucorales is an order of fungi comprised of 6 main families that have potential to cause a variety of infections. The genera Mucor, Rhizopus, and Rhizomucor cause the majority of infections.1 Mucormycosis (infection with Mucorales) is generally a rare fungal infection with an incidence of about 500 cases per year in the U.S. However, the incidence is increasing with an aging population, higher prevalence of DM and chronic kidney disease, and a growing population of immunocompromised patients due to advances in cancer therapy and transplantation. Risk factors for pulmonary mucormycosis include conditions associated with congenital and acquired immunodeficiency: hematologic malignancies, uncontrolled DM, solid tumors, and organ transplantation.2
Presentation
Notably, there seems to be an association between specific organ system involvement and predisposing conditions. Pulmonary mucormycosis occurs much less frequently than does the rhinocerebral form in patients with DKA but occurs more commonly in patients with neutropenia that is due to chemotherapy or hematopoietic stem cell transplantation (HSCT) for the treatment of hematologic malignancies.2
The mechanisms for preferential site infection are not well understood with current knowledge of mucormycosis pathogenesis. Current research demonstrates monocytes and neutrophils may play a vital role in the body’s defense against Mucor by both phagocytosis and oxidative damage. Chemotaxis and oxidative cell lysis seem to be compromised in states of hyperglycemia and acidosis. Iron metabolism repeatedly has been shown to play a role in the pathogenesis of mucormycosis. Specifically, patients receiving deferoxamine seem to have a predisposition to Mucorales infections, presumably due to the increased iron supply to the fungus.4 Notably, systemic acidosis also facilitates higher concentrations of available serum iron.
One of the main characteristics of mucormycosis is its ability to aggressively invade blood vessels, causing thrombosis and necrosis and subsequently disseminate hematogenously or through the lymphatic system. This property, at least in large part, depends on endothelial cell damage following phagocytosis of fungus by these cells.
Of note, some of the azole class of drugs (eg, voriconazole), which may be used for antifungal prophylaxis in patients with hematologic malignancies accompanied by neutropenia, have been implicated in predisposition to mucormycosis.2 It also is commonly seen in patients undergoing HSCT. Patients with DM and DKA also can present with pulmonary mucormycosis but generally have a more indolent course unless they develop pulmonary hemorrhage.3 Infection usually occurs by inhalation.
Patients may report dyspnea, cough, and chest pain, which is sometimes accompanied by a fever. Presentation is generally indistinguishable from other causes of pneumonia, and the routinely obtained sputum cultures are usually not diagnostically significant.
Radiographic findings are variable and may include pulmonary nodules, consolidations, masses, and cavitary lesions.1 Due to tissue invasion, a CT scan of the chest might demonstrate a mass crossing mediastinal tissue planes. Definitive diagnosis requires a biopsy with a demonstration of characteristic broad-based nonseptate hyphae with tissue invasion as well as a positive culture (Figures 4 and 5).5 Due to nonspecific symptoms as well as laboratory and imaging findings, a biopsy and, therefore, definitive diagnosis are often delayed. However, postponing medical and surgical therapy for mucormycosis has been associated with worse outcomes.6 With the absence of easily available serologic tests and unspecific symptoms in early disease, many mucormycosis cases are diagnosed postmortem.
Treatments
Recently described therapy advancements have indicated improved outcomes.7 Nevertheless, prognosis remains universally poor with 65% to 70% mortality for patients with cases of isolated pulmonary mucormycosis.8 Many of these patients succumb to sepsis, respiratory failure, and hemoptysis. Patients with pulmonary mucormycosis usually die of dissemination rather than of the sequelae of the pulmonary disease. In fact, pulmonary infection seems to have the highest incidence of dissemination in patients with neutropenia. Surgical therapy seems to have more favorable outcomes than treatment with antifungals alone, especially when considering infection primarily affecting 1 lung.8
Amphotericin B remains the first-line agent for treatment of pulmonary mucormycosis. Retrospective studies show that this agent remains one of the few with activity against Mucor with reported successful outcomes. Specifically, the liposomal formulation seems to have greater efficacy.9 Strong prospective data are lacking. An increasing body of evidence supports a potential benefit from adding echinocandins.10 Although these agents have minimal activity against mucormycosis in vitro, adjunctive therapy to amphotericin resulted in better survival. Alternative regimens include the combination of amphotericin with posaconazole or itraconazole. Both these agents seem to have in vitro activity against mucormycosis pathogens, although poor absorption of these agents puts the potential benefit of such combinations in question.
In patients unable to tolerate polyenes due to adverse effects (AEs), the use of posaconazole as monotherapy has been reported with positive results. One retrospective study reported treatment success in up to 60% and stable disease in 21% of patients at 12 weeks. This study included 24 out of 36 patients with pulmonary mucormycosis.11 Significantly fewer AEs and oral administration makes posaconazole an attractive alternative treatment for mucormycosis and needs further prospective evaluation.
Novel therapies have been attempted, though without success thus far. One randomized clinical trial conducted on patients with mucormycosis attempted to determine whether capitalizing on iron metabolism by Mucor by providing adjunctive deferasirox, an iron chelator, would lead to an initial improvement in mortality. However, outcomes did not improve and resulted in higher mortality rates at 90 days in the intervention group.12
Reversal of underlying conditions remains the cornerstone of successful therapy. If possible, it is important to cease immunosuppression by avoiding corticosteroids, correcting acidosis and hyperglycemia, and discontinuing aluminum and iron chelators.13 This approach becomes problematic in patients with DM with poor glucose control due to nonadherence or lack of resources and in situations where the underlying condition is difficult to treat or the treatment puts patients at risk for mucormycosis (eg, malignancies). Surgery in addition to antifungal therapy should be pursued wherever possible for definitive therapy.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
1. Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13(2):236-301.
2. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. 2012;17(6):913-926.
3. Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18(3):556-569.
4. Prokopowicz GP, Bradley SF, Kauffman CA. Indolent zygomycosis associated with deferoxamine chelation therapy. Mycoses. 1994;37(11-12):427-431.
5. Hamilos G, Samonis G, Kontoyiannis DP. Pulmonary mucormycosis. Semin Respir Crit Care Med. 2011;32(6):693-702.
6. Chamilos G, Lewis RE, Kontoyiannis DP. Delaying amphotericin B-based frontline therapy significantly increases mortality among patients with hematologic malignancy who have zygomycosis. Clin Infect Dis. 2008;47(4):503-509.
7. Parfrey NA. Improved diagnosis and prognosis of mucormycosis. A clinicopathologic study of 33 cases. Medicine (Baltimore). 1986;65(2):113-1
8. Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. Ann Thorac Surg. 1994;57(4):1044-1050.
9.
10. Reed C, Bryant R, Ibrahim AS, et al. Combination polyene-caspofungin treatment of rhino-orbital-cerebral mucormycosis. Clin Infect Dis. 2008;47(3):364-371.
11. van Burik JA, Hare RS, Solomon HF, Corrado ML, Kontoyiannis DP. Posaconazole is effective as salvage therapy in zygomycosis: a retrospective summary of 91 cases. Clin Infect Dis. 2006;42(7):e61-e65.
12. Spellberg B, Ibrahim AS, Chin-Hong PV, et al. The Deferasirox-AmBisome Therapy for Mucormycosis (DEFEAT Mucor) study: a randomized, double-blinded, placebo-controlled trial. J Antimicrob Chemother. 2012;67(3):715-722.
13. de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994; 47(10):1843-1850.
Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.