User login
Pediatrics group stresses benefits of vitamin K shots for infants
After the American Academy of Pediatrics (AAP) began recommending vitamin K shots for newborns in 1961, infant bleeding as a result of vitamin K deficiency plummeted. The life-threatening disorder is so rare that some parents now question the need for injections to safeguard against it.
The situation amounts to “a failure of our success,” Ivan Hand, MD, a coauthor of a new AAP statement on vitamin K, told this news organization. Much like diseases that can be prevented with vaccines, vitamin K deficiency bleeding isn’t top of mind for parents. “It’s not something they’re aware of or afraid of,” he said.
In 2019, however, the AAP listed public education about the importance of the shots in its 10 most important priorities.
The policy update urges clinicians to bone up on the benefits and perceived risks of vitamin K deficiency, which is essential for clotting, and to “strongly advocate” for the shot in discussions with parents who may get competing messages from their social circles, the internet, and other health care professionals.
Dr. Hand, director of neonatology at NYC Health + Hospitals Kings County, Brooklyn, said clinicians walk a line between educating and alienating parents who favor natural birth processes. “We’re hoping that by talking to the families and answering their questions and explaining the risks, parents will accept vitamin K as a necessary treatment for their babies,” he said.
Vitamin K does not easily pass through the placenta and is not plentiful in breast milk, the preferred nutrition source for newborns. It takes months for babies to build their stores through food and gut bacteria.
Infants who do not receive vitamin K at birth are 81 times more likely to develop late-onset vitamin K deficiency bleeding, which occurs a week to 6 months after birth, according to the Centers for Disease Control and Prevention. One in five babies with the disorder dies, and about half have bleeding in the skull that can lead to brain damage.
New dosing for premature infants
The AAP’s new statement, published in the journal Pediatrics, reaffirms the administration of a 1-mg intramuscular dose for infants weighing more than 1,500 grams, or about 3 lb 5 oz, within 6 hours of birth. For premature infants who weigh less, the guidance recommends an intramuscular dose of 0.3 to 0.5 mg/kg.
The group notes that oral preparations of vitamin K have proven less effective because of malabsorption and challenges with adhering to dosing regimens.
The document also warns that breastfed babies can experience vitamin K deficiency bleeding even if they have received the shot, because concentration of vitamin K often wanes before a baby starts eating solid food. The disorder “should be considered when evaluating bleeding in the first 6 months of life, even in infants who received prophylaxis, and especially in exclusively breastfed infants,” it states.
Accounts of parental refusals date back to 2013, when the CDC reported four cases of deficiency bleeding in Tennessee. The infants’ parents said they declined vitamin K because they worried about increased risk of leukemia, thought the injection was unnecessary, or wanted to minimize the baby’s exposure to “toxins.” Leukemia concern stemmed from a 1992 report linking vitamin K to childhood cancer, an association that did not hold up in subsequent studies.
More recent research has documented parental concerns about preservatives and injection pain as well as distrust of medical and public health authorities. Some parents have been accused of neglect for refusing to allow their babies to receive the shots.
Phoebe Danziger, MD, a pediatrician and writer in rural Michigan who has studied parental refusal of standard-of-care interventions, called the document a “welcome update” to the AAP’s last statement on the topic, in 2003. She told this news organization that lower dosing for premature infants may reassure some vitamin K–hesitant parents who worry about one-size-fits-all dosing.
But Dr. Danziger added that “evidence is lacking to support the claim that pediatricians can really move the needle on parental hesitancy and refusal simply through better listening and more persuasive counseling.” She said the AAP should do more to address “the broader social climate of mistrust and misinformation” that fuels refusal.
Dr. Hand and Dr. Danziger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After the American Academy of Pediatrics (AAP) began recommending vitamin K shots for newborns in 1961, infant bleeding as a result of vitamin K deficiency plummeted. The life-threatening disorder is so rare that some parents now question the need for injections to safeguard against it.
The situation amounts to “a failure of our success,” Ivan Hand, MD, a coauthor of a new AAP statement on vitamin K, told this news organization. Much like diseases that can be prevented with vaccines, vitamin K deficiency bleeding isn’t top of mind for parents. “It’s not something they’re aware of or afraid of,” he said.
In 2019, however, the AAP listed public education about the importance of the shots in its 10 most important priorities.
The policy update urges clinicians to bone up on the benefits and perceived risks of vitamin K deficiency, which is essential for clotting, and to “strongly advocate” for the shot in discussions with parents who may get competing messages from their social circles, the internet, and other health care professionals.
Dr. Hand, director of neonatology at NYC Health + Hospitals Kings County, Brooklyn, said clinicians walk a line between educating and alienating parents who favor natural birth processes. “We’re hoping that by talking to the families and answering their questions and explaining the risks, parents will accept vitamin K as a necessary treatment for their babies,” he said.
Vitamin K does not easily pass through the placenta and is not plentiful in breast milk, the preferred nutrition source for newborns. It takes months for babies to build their stores through food and gut bacteria.
Infants who do not receive vitamin K at birth are 81 times more likely to develop late-onset vitamin K deficiency bleeding, which occurs a week to 6 months after birth, according to the Centers for Disease Control and Prevention. One in five babies with the disorder dies, and about half have bleeding in the skull that can lead to brain damage.
New dosing for premature infants
The AAP’s new statement, published in the journal Pediatrics, reaffirms the administration of a 1-mg intramuscular dose for infants weighing more than 1,500 grams, or about 3 lb 5 oz, within 6 hours of birth. For premature infants who weigh less, the guidance recommends an intramuscular dose of 0.3 to 0.5 mg/kg.
The group notes that oral preparations of vitamin K have proven less effective because of malabsorption and challenges with adhering to dosing regimens.
The document also warns that breastfed babies can experience vitamin K deficiency bleeding even if they have received the shot, because concentration of vitamin K often wanes before a baby starts eating solid food. The disorder “should be considered when evaluating bleeding in the first 6 months of life, even in infants who received prophylaxis, and especially in exclusively breastfed infants,” it states.
Accounts of parental refusals date back to 2013, when the CDC reported four cases of deficiency bleeding in Tennessee. The infants’ parents said they declined vitamin K because they worried about increased risk of leukemia, thought the injection was unnecessary, or wanted to minimize the baby’s exposure to “toxins.” Leukemia concern stemmed from a 1992 report linking vitamin K to childhood cancer, an association that did not hold up in subsequent studies.
More recent research has documented parental concerns about preservatives and injection pain as well as distrust of medical and public health authorities. Some parents have been accused of neglect for refusing to allow their babies to receive the shots.
Phoebe Danziger, MD, a pediatrician and writer in rural Michigan who has studied parental refusal of standard-of-care interventions, called the document a “welcome update” to the AAP’s last statement on the topic, in 2003. She told this news organization that lower dosing for premature infants may reassure some vitamin K–hesitant parents who worry about one-size-fits-all dosing.
But Dr. Danziger added that “evidence is lacking to support the claim that pediatricians can really move the needle on parental hesitancy and refusal simply through better listening and more persuasive counseling.” She said the AAP should do more to address “the broader social climate of mistrust and misinformation” that fuels refusal.
Dr. Hand and Dr. Danziger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
After the American Academy of Pediatrics (AAP) began recommending vitamin K shots for newborns in 1961, infant bleeding as a result of vitamin K deficiency plummeted. The life-threatening disorder is so rare that some parents now question the need for injections to safeguard against it.
The situation amounts to “a failure of our success,” Ivan Hand, MD, a coauthor of a new AAP statement on vitamin K, told this news organization. Much like diseases that can be prevented with vaccines, vitamin K deficiency bleeding isn’t top of mind for parents. “It’s not something they’re aware of or afraid of,” he said.
In 2019, however, the AAP listed public education about the importance of the shots in its 10 most important priorities.
The policy update urges clinicians to bone up on the benefits and perceived risks of vitamin K deficiency, which is essential for clotting, and to “strongly advocate” for the shot in discussions with parents who may get competing messages from their social circles, the internet, and other health care professionals.
Dr. Hand, director of neonatology at NYC Health + Hospitals Kings County, Brooklyn, said clinicians walk a line between educating and alienating parents who favor natural birth processes. “We’re hoping that by talking to the families and answering their questions and explaining the risks, parents will accept vitamin K as a necessary treatment for their babies,” he said.
Vitamin K does not easily pass through the placenta and is not plentiful in breast milk, the preferred nutrition source for newborns. It takes months for babies to build their stores through food and gut bacteria.
Infants who do not receive vitamin K at birth are 81 times more likely to develop late-onset vitamin K deficiency bleeding, which occurs a week to 6 months after birth, according to the Centers for Disease Control and Prevention. One in five babies with the disorder dies, and about half have bleeding in the skull that can lead to brain damage.
New dosing for premature infants
The AAP’s new statement, published in the journal Pediatrics, reaffirms the administration of a 1-mg intramuscular dose for infants weighing more than 1,500 grams, or about 3 lb 5 oz, within 6 hours of birth. For premature infants who weigh less, the guidance recommends an intramuscular dose of 0.3 to 0.5 mg/kg.
The group notes that oral preparations of vitamin K have proven less effective because of malabsorption and challenges with adhering to dosing regimens.
The document also warns that breastfed babies can experience vitamin K deficiency bleeding even if they have received the shot, because concentration of vitamin K often wanes before a baby starts eating solid food. The disorder “should be considered when evaluating bleeding in the first 6 months of life, even in infants who received prophylaxis, and especially in exclusively breastfed infants,” it states.
Accounts of parental refusals date back to 2013, when the CDC reported four cases of deficiency bleeding in Tennessee. The infants’ parents said they declined vitamin K because they worried about increased risk of leukemia, thought the injection was unnecessary, or wanted to minimize the baby’s exposure to “toxins.” Leukemia concern stemmed from a 1992 report linking vitamin K to childhood cancer, an association that did not hold up in subsequent studies.
More recent research has documented parental concerns about preservatives and injection pain as well as distrust of medical and public health authorities. Some parents have been accused of neglect for refusing to allow their babies to receive the shots.
Phoebe Danziger, MD, a pediatrician and writer in rural Michigan who has studied parental refusal of standard-of-care interventions, called the document a “welcome update” to the AAP’s last statement on the topic, in 2003. She told this news organization that lower dosing for premature infants may reassure some vitamin K–hesitant parents who worry about one-size-fits-all dosing.
But Dr. Danziger added that “evidence is lacking to support the claim that pediatricians can really move the needle on parental hesitancy and refusal simply through better listening and more persuasive counseling.” She said the AAP should do more to address “the broader social climate of mistrust and misinformation” that fuels refusal.
Dr. Hand and Dr. Danziger have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy not warranted in COVID-19 outpatients
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Antithrombotic therapy in clinically stable, nonhospitalized COVID-19 patients does not offer protection against adverse cardiovascular or pulmonary events, new randomized clinical trial results suggest.
Antithrombotic therapy has proven useful in acutely ill inpatients with COVID-19, but in this study, treatment with aspirin or apixaban (Eliquis) did not reduce the rate of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary causes in patients ill with COVID-19 but who were not hospitalized.
“Among symptomatic, clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome,” the authors conclude. “However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
The study, which was led by Jean M. Connors, MD, Brigham and Women’s Hospital, Boston, was published online October 11 in JAMA.
The ACTIV-4B Outpatient Thrombosis Prevention Trial was a randomized, adaptive, double-blind, placebo-controlled trial that sought to compare anticoagulant and antiplatelet therapy among 7,000 symptomatic but clinically stable outpatients with COVID-19.
The trial was conducted at 52 sites in the U.S. between Sept. 2020 and June 2021, with final follow-up this past August 5, and involved minimal face-to-face interactions with study participants.
Patients were randomized in a 1:1:1:1 ratio to aspirin (81 mg orally once daily; n = 164 patients), prophylactic-dose apixaban (2.5 mg orally twice daily; n = 165), therapeutic-dose apixaban (5 mg orally twice daily; n = 164), or placebo (n = 164) for 45 days.
The primary endpoint was a composite of all-cause mortality, symptomatic venous or arterial thromboembolism, myocardial infarction, stroke, or hospitalization for cardiovascular or pulmonary cause.
The trial was terminated early this past June by the independent data monitoring committee because of lower than anticipated event rates. At the time, just 657 symptomatic outpatients with COVID-19 had been enrolled.
The median age of the study participants was 54 years (Interquartile Range [IQR] 46-59); 59% were women.
The median time from diagnosis to randomization was 7 days, and the median time from randomization to initiation of study medications was 3 days.
The trial’s primary efficacy and safety analyses were restricted to patients who received at least one dose of trial medication, for a final number of 558 patients.
Among these patients, the primary endpoint occurred in 1 patient (0.7%) in the aspirin group, 1 patient (0.7%) in the 2.5 mg apixaban group, 2 patients (1.4%) in the 5-mg apixaban group, and 1 patient (0.7%) in the placebo group.
The researchers found that the absolute risk reductions compared with placebo for the primary outcome were 0.0% (95% confidence interval not calculable) in the aspirin group, 0.7% (95% confidence interval, -2.1% to 4.1%) in the prophylactic-dose apixaban group, and 1.4% (95% CI, -1.5% to 5%) in the therapeutic-dose apixaban group.
No major bleeding events were reported.
The absolute risk differences compared with placebo for clinically relevant nonmajor bleeding events were 2% (95% CI, -2.7% to 6.8%) in the aspirin group, 4.5% (95% CI, -0.7% to 10.2%) in the prophylactic-dose apixaban group, and 6.9% (95% CI, 1.4% to 12.9%) in the therapeutic-dose apixaban group.
Safety and efficacy results were similar in all randomly assigned patients.
The researchers speculated that a combination of two demographic shifts over time may have led to the lower than anticipated rate of events in ACTIV-4B.
“First, the threshold for hospital admission has markedly declined since the beginning of the pandemic, such that hospitalization is no longer limited almost exclusively to those with severe pulmonary distress likely to require mechanical ventilation,” they write. “As a result, the severity of illness among individuals with COVID-19 and destined for outpatient care has declined.”
“Second, at least within the U.S., where the trial was conducted, individuals currently being infected with SARS-CoV-2 tend to be younger and have fewer comorbidities when compared with individuals with incident infection at the onset of the pandemic,” they add.
Further, COVID-19 testing was quite limited early in the pandemic, they note, “and it is possible that the anticipated event rates based on data from registries available at that time were overestimated because the denominator (that is, the number of infected individuals overall) was essentially unknown.”
Robust evidence
“The ACTIV-4B trial is the first randomized trial to generate robust evidence about the effects of antithrombotic therapy in outpatients with COVID-19,” Otavio Berwanger, MD, PhD, director of the Academic Research Organization, Hospital Israelita Albert Einstein, Sao Paulo-SP, Brazil, told this news organization.
“It should be noted that this was a well-designed trial with low risk of bias. On the other hand, the main limitation is the low number of events and, consequently, the limited statistical power,” said Dr. Berwanger, who wrote an accompanying editorial.
The ACTIV-4B trial has immediate implications for clinical practice, he added.
“In this sense, considering the neutral results for major cardiopulmonary outcomes, the use of aspirin or apixaban for the management of outpatients with COVID-19 should not be recommended.”
ACTIV-4B also provides useful information for the steering committees of other ongoing trials of antithrombotic therapy for patients with COVID-19 who are not hospitalized, Dr. Berwanger added.
“In this sense, probably issues like statistical power, outcome choices, recruitment feasibility, and even futility would need to be revisited. And finally, lessons learned from the implementation of an innovative, pragmatic, and decentralized trial design represent an important legacy for future trials in cardiovascular diseases and other common conditions,” he said.
The study was funded by the National Institutes of Health, and the National Heart, Lung, and Blood Institute. Dr. Connors reports financial relationships with Bristol-Myers Squibb, Pfizer, Abbott, Alnylam, Takeda, Roche, and Sanofi. Dr. Berwanger reports financial relationships with AstraZeneca, Amgen, Servier, Bristol-Myers Squibb, Bayer, Novartis, Pfizer, and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
Study suggests no added risk of blood clots in COVID-19 outpatients
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
The incidence of venous thromboembolism (VTE) in nonhospitalized patients with COVID-19 was not significantly different from patients without the infectious disease, according to a new study published in JAMA Internal Medicine.
National Institutes of Health guidelines recommend blood thinners to prevent blood clots in patients hospitalized with COVID-19. However, the new study provides more insight on the best treatment approach for COVID-19 outpatients.
“[COVID-19’s] rapid global progression and impact has caused us to make and modify treatment decisions at a pace that we never have in modern medicine,” study author Nareg Roubinian, MD, an investigator at Kaiser Permanente, Oakland, Calif., said in an interview.
“As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes,” Dr. Roubinian added.
The increased risk of blood clots in patients hospitalized with COVID-19 has been a major issue throughout the pandemic. In fact, one study published in November 2020 found that more than half of patients hospitalized with the illness have prothrombotic antiphospholipid (aPL) autoantibodies in their blood, which could contribute to venous and arterial thromboembolism.
Although it was clear many hospitalized patients diagnosed with COVID-19 were developing more clots, researchers of the current study were not sure if this trend would also be seen in outpatients.
“Most people with COVID-19 do not need to be hospitalized, and we needed to know how often patients outside the hospital were having blood clots,” said Dr. Roubinian.
For the study, Dr. Roubinian and colleagues examined data on 220,588 patients who were members of Kaiser Permanente Northern California health plan and were tested for COVID-19 between Feb. 25 and Aug. 31, 2020. They then reported on the 30-day incidence of outpatient and hospital-associated blood clots following the COVID-19 diagnosis. Patients who were asymptomatic at the time of testing or had received anticoagulants within the last year were excluded.
“We knew from other studies that patients with COVID-19 often get sicker in the first few weeks after infection. What we didn’t know was whether COVID-19 patients were developing blood clots but not pneumonia or were developing blood clots at the same time as they developed pneumonia,” said Dr. Roubinian, an intensive care doctor with the Permanente Medical Group in Oakland, Calif. “Following the patients for 30 days allowed us to focus on the time period from infection to when blood clots were most likely to develop.”
Researchers found that of the cohort who took the COVID-19 test, 11.8% had a positive result. Within 30 days of the COVID-19 test, 0.8% of patients with a positive result were diagnosed with VTE compared to 0.5% of those who received a negative test result. They also found that viral testing took place in an outpatient setting for 59.1% of the patients with a positive viral test who later developed VTE. Of those patients, 76.1% had to be hospitalized.
Dr. Roubinian said he was surprised to see that the blood clotting in outpatients with COVID-19 was similar in frequency to what he saw in patients without the infection.
“Our findings suggest that blood clots do occur in COVID-19 patients but not on a scale where we need to put all or many COVID outpatients on blood thinners,” he said. “As with other potential therapies for COVID-19, blood thinners need to be prospectively studied in a clinical trial to determine if they improve patient outcomes.”
In December 2020, three trials investigating the risk and benefits of increased levels of anticoagulation in hospitalized COVID-19 patients were paused because of safety issues. The trials would have enrolled critically ill COVID-19 patients for whom therapeutic doses of anticoagulation drugs showed no benefit.
Anticoagulants are associated with bleeding risks, including prolonged nosebleeds and vomiting or coughing up blood.
Instead of prescribing the routine use of thromboprophylactic drugs to COVID-19 outpatients, Dr. Roubinian believes it would be helpful to learn how to determine whether a patient at risk of becoming sick or being hospitalized would benefit from being treated with such drugs.
Dr. Roubinian reported receiving grants from the National Institutes of Health and the National Heart, Lung, and Blood Institute during the conduct of the study.
A Case Series of Catheter-Directed Thrombolysis With Mechanical Thrombectomy for Treating Severe Deep Vein Thrombosis
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
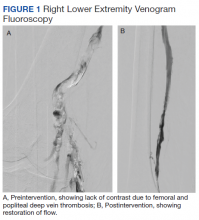
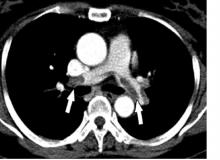
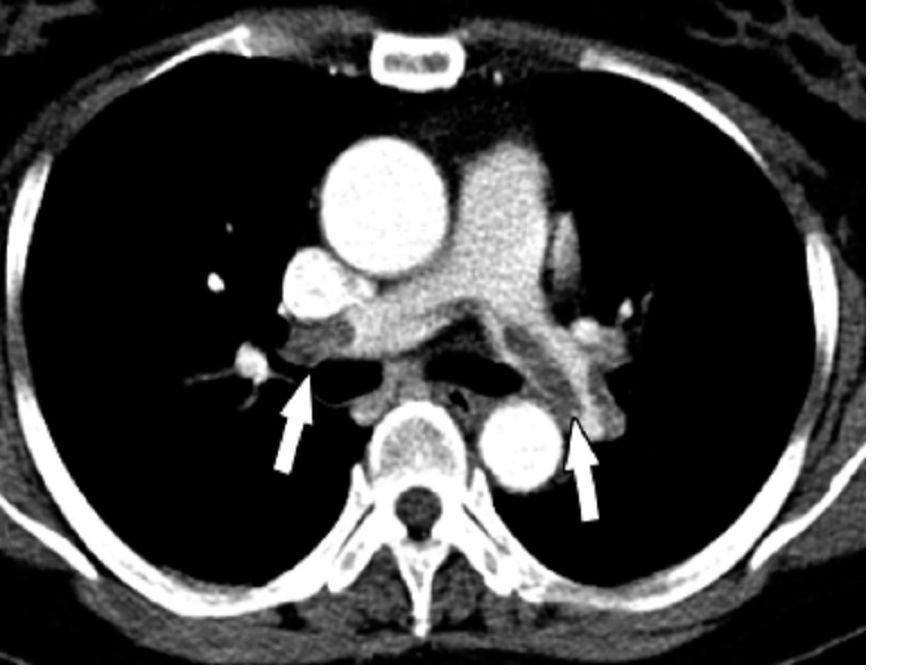
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
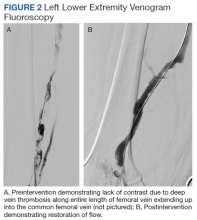
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
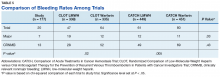
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Two cases of extensive symptomatic deep vein thrombosis without phlegmasia cerulea dolens were successfully treated with an endovascular technique that combines catheter-directed thrombolysis and mechanical thrombectomy.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
Deep vein thrombosis (DVT) is a frequently encountered medical condition with about 1 in 1,000 adults diagnosed annually.1,2 Up to one-half of patients who receive a diagnosis will experience long-term complications in the affected limb.1 Anticoagulation is the treatment of choice for DVT in the absence of any contraindications.3 Thrombolytic therapies (eg, systemic thrombolysis, catheter-directed thrombolysis with or without thrombectomy) historically have been reserved for patients who present with phlegmasia cerulea dolens (PCD), a severe condition involving venous obstruction within the extremities that causes impaired arterial blood supply and cyanosis that can lead to limb loss and death.4
The role of thrombolytic therapy is less clear in patients without PCD who present with extensive or symptomatic lower extremity DVT that causes significant pain, edema, and functional disability. Proximal lower extremity DVT (thrombus above the knee and above the popliteal vein) and particularly those involving the iliac or common femoral vein (ie, iliofemoral DVT) carry a significant risk of recurrent thromboembolism as well as postthrombotic syndrome (PTS), a complication of DVT resulting in chronic leg pain, edema, skin discoloration, and venous ulcers.5
The goal of thrombolytic therapy is to prevent thrombus propagation, recurrent thromboembolism, and PTS, in addition to providing more rapid pain relief and improvement in limb function.
Catheter-directed thrombolysis can be combined with catheter-directed thrombectomy using the same endovascular technique. This combination is called a pharmacomechanical thrombectomy or a pharmacomechanical thromobolysis and can offer more rapid removal of thrombus and decreased infusion times of thrombolytic drug.8 Pharmacomechanical thrombolysis is a relatively new technique, so the choice of thrombolytic therapy will depend on procedural expertise and resource availability. Early interventional radiology consultation (or vascular surgery in some centers) can assist in determining appropriate candidates for thrombolytic therapies. Here we present 2 cases of extensive symptomatic DVT successfully treated with catheter-directed pharmacomechanical thrombolysis.
Case 1
A 61-year-old male current smoker with a history of obesity and hypertension presented to the West Los Angeles Veterans Affairs Medical Center emergency department (ED) with 2 days of progressive pain and swelling in the right lower extremity (RLE) after sustaining a calf injury the preceding week. The patient rated pain as 9 on a 10-point scale and reported no other symptoms. He reported no prior history of venous thromboembolism (VTE) or family history of thrombophilia.
A physical examination was notable for stable vital signs and normal cardiopulmonary examination. There was extensive RLE edema below the knee with tenderness to palpation and shiny taut skin. The neurovascular examination of the RLE was normal. Laboratory studies were notable only for a mild leukocytosis. Compression ultrasound with Doppler of the RLE demonstrated an acute thrombus of the right femoral vein extending to the popliteal vein.
The patient was prescribed enoxaparin 90 mg every 12 hours for anticoagulation. After 36 hours of anticoagulation, he continued to experience severe RLE pain and swelling limiting ambulation. Interventional radiology was consulted, and catheter-directed pharmacomechanical thrombolysis of the RLE was pursued given the persistence of significant symptoms. Intraprocedure venogram demonstrated thrombi filling the entirety of the right femoral and popliteal veins (Figure 1A). This was treated with catheter-directed pulse-spray thrombolysis with 12 mg of tissue plasminogen activator (tPA).
After a 20-minute incubation period, a thrombectomy was performed several times along the femoral vein and popliteal vein, using an AngioJet device. A follow-up venogram revealed a small amount of residual thrombi in the right suprageniculate popliteal vein and right femoral vein. This entire segment was further treated with angioplasty, and a postintervention venogram demonstrated patency of the right suprageniculate popliteal vein and right femoral vein with minimal residual thrombi and with brisk venous flow (Figure 1B). Immediately after the procedure, the patient’s RLE pain significantly improved. On day 2 postprocedure, the patient’s RLE edema resolved, and the patient was able to resume normal ambulation. There were no bleeding complications. The patient was discharged with oral anticoagulation therapy.
Case 2
A male aged 78 years with a history of hypertension, hyperlipidemia, and benign prostatic hypertrophy presented to the ED with 10 days of progressive pain and swelling in the left lower extremity (LLE). The patient noted decreased mobility over recent months and was using a front wheel walker while recovering from surgical repair of a hamstring tendon injury. He reported taking a transcontinental flight around the same time that his LLE pain began. The patient reported no prior history of VTE or family history of thrombophilia.
A physical examination was notable for stable vital signs with a normal cardiopulmonary examination. There was extensive LLE edema up to the proximal thigh without erythema or cyanosis, and his skin was taut and tender. Neurovascular examination of the LLE was normal. Laboratory studies were unremarkable. Compression ultrasonography with Doppler of the LLE demonstrated an extensive acute occlusive thrombus within the left common femoral, entire left femoral, and left popliteal veins.
After evaluating the patient, the Vascular Surgery service did not feel there was evidence of compartment syndrome nor PCD. The patient received unfractionated heparin anticoagulation therapy and the LLE was elevated continuously. After 24 hours of anticoagulation therapy, the patient continued to have significant pain and was unable to ambulate. The case was presented in a joint Interventional Radiology/Vascular Surgery conference and the decision was made to pursue pharmacomechanic thrombolysis given the significant extent of thrombotic burden.
The patient underwent successful catheter-directed pharmacomechanic thrombolysis via pulse-spray thrombolysis of 15 mg of tPA using the Boston Scientific AngioJet Thrombectomy System, and angioplasty with no immediate complications (Figure 2). The patient noted dramatic improvement in LLE pain and swelling 1 day postprocedure and was able to ambulate. He developed mild asymptomatic hematuria, which resolved within 12 hours and without an associated drop in hemoglobin. The patient was transitioned to oral anticoagulation and discharged to an acute rehabilitation unit on postprocedure day 2.
Discussion
Anticoagulation is the preferred therapy for most patients with acute uncomplicated lower extremity DVT. PCD is the only widely accepted indication for thrombolytic therapy in patients with acute lower extremity DVT. However, in the absence of PCD, management of complicated DVT where there are either significant symptoms, extensive clot burden, or proximal location is less clear due to the paucity of clinical data. For example, in the case of iliofemoral DVT, thrombosis of the iliofemoral region is associated with an increased risk of pulmonary embolism, limb malperfusion, and PTS when compared with other types of DVT.5,6
Earlier retrospective observational studies in patients with acute DVT found that the addition of either systemic thrombolysis or catheter-directed thrombolysis to anticoagulation increased rates of clot lysis but did not lead to a reduction in clinical outcomes such as recurrent thromboembolism, mortality, or the rate of PTS.10-12 Additionally, both systemic thrombolytic therapy and catheter-directed thrombolytic therapy were associated with higher rates of major bleeding. However, these studies included all patients with acute DVT without selecting for criteria, such as proximal location of DVT, severe symptoms, or extensive clot burden. Because thrombolytic therapy is proven to provide more rapid and immediate clot lysis (whereas conventional anticoagulation prevents thrombus extension and recurrence but does not dissolve the clot), it is reasonable to suggest that a subpopulation of patients with extensive or symptomatic DVT may benefit from immediate clot lysis, thereby restoring limb perfusion and avoiding limb gangrene while preserving venous function and preventing PTS.
Mixed Study Results
The 2012 CaVenT study is one of the few randomized controlled trials to assess outcomes comparing conventional anticoagulation alone to anticoagulation with catheter-directed thrombolysis in patients with acute lower extremity DVT.13 Study patients did not undergo catheter-directed mechanical thrombectomy. Patients in this study consisted solely of those with first-time iliofemoral DVT. Long-term outcomes at 24-month follow-up showed that additional catheter-directed thrombolysis reduced the risk of PTS when compared with those who were treated with anticoagulation alone (41.1% vs 55.6%, P = .047). The difference in PTS corresponded to an absolute risk reduction of 14.4% (95% CI, 0.2-27.9), and the number needed to treat was 7 (95% CI, 4-502). There was a clinically relevant bleeding complication rate of 8.9% in the thrombolysis group with none leading to a permanently impaired outcome.
These results could not be confirmed by a more recent randomized control trial in 2017 conducted by Vedantham and colleagues.14 In this trial, patients with acute proximal DVT (femoral and iliofemoral DVT) were randomized to receive either anticoagulation alone or anticoagulation plus pharmacomechanical thrombolysis. In the pharmacomechanic thrombolysis group, the overall incidence of PTS and recurrent VTE was not reduced over the 24-month follow-up period. Those who developed PTS in the pharmacomechanical thrombolysis group had lower severity scores, as there was a significant reduction in moderate-to-severe PTS in this group. There also were more early major bleeds in the pharmacomechanic thrombolysis group (1.7%, with no fatal or intracranial bleeds) when compared with the control group; however, this bleeding complication rate was much less than what was noted in the CaVenT study. Additionally, there was a significant decrease in both lower extremity pain and edema in the pharmacomechanical thrombolysis group at 10 days and 30 days postintervention.
Given the mixed results of these 2 randomized controlled trials, further studies are warranted to clarify the role of thrombolytic therapies in preventing major events such as recurrent VTE and PTS, especially given the increased risk of bleeding observed with thrombolytic therapies. The 2016 American College of Chest Physicians guidelines recommend anticoagulation as monotherapy vs thrombolytics, systemic or catheter-directed thrombolysis as designated treatment modalities.3 These guidelines are rated “Grade 2C”, which reflect a weak recommendation based on low-quality evidence. While these recommendations do not comment on additional considerations, such as DVT clot burden, location, or severity of symptoms, the guidelines do state that patients who attach a high value to the prevention of PTS and a lower value to the risk of bleeding with catheter-directed therapy are likely to choose catheter-directed therapy over anticoagulation alone.
Case Studies Analyses
In our first case presentation, pharma-comechanic thrombolysis was pursued because the patient presented with severesymptoms and did not experience any symptomatic improvement after 36 hours of anticoagulation. It is unclear whether a longer duration of anticoagulation might have improved the severity of his symptoms. When considering the level of pain, edema, and inability to ambulate, thrombolytic therapy was considered the most appropriate choice for treatment. Pharmacomechanic thrombolysis was successful, resulting in complete clot lysis, significant decrease in pain and edema with total recovery of ambulatory abilities, no bleeding complications, and prevention of any potential clinical deterioration, such as phlegmasia cerulea dolens. The patient is now 12 months postprocedure without symptoms of PTS or recurrent thromboembolic events. Continued follow-up that monitors the development of PTS will be necessary for at least 2 years postprocedure.
In the second case, our patient experienced some improvement in pain after 24 hours of anticoagulation alone. However, considering the extensive proximal clot burden involving the entire femoral and common femoral veins, the treatment teams believed it was likely that this patient would experience a prolonged recovery time and increased morbidity on anticoagulant therapy alone. Pharmacomechanic thrombolysis was again successful with almost immediate resolution of pain and edema, and recovery of ambulatory abilities on postprocedure day 1. The patient is now 6 months postprocedure without any symptoms of PTS or recurrent thromboembolic events.
In both case presentations, the presenting symptoms, methods of treatment, and immediate symptomatic improvement postintervention were similar. The patient in Case 2 had more extensive clot burden, a more proximal location of clot, and was classified as having an iliofemoral DVT because the thrombus included the common femoral vein; the decision for intervention in this case was more weighted on clot burden and location rather than on the significant symptoms of severe pain and difficulty with ambulation seen in Case 1. However, it is noteworthy that in Case 2 our patient also experienced significant improvement in pain, swelling, and ambulation postintervention. Complications were minimal and limited to Case 2 where our patient experienced mild asymptomatic hematuria likely related to the catheter-directed tPA that resolved spontaneously within hours and did not cause further complications. Additionally, it is likely that the length of hospital stay was decreased significantly in both cases given the rapid improvement in symptoms and recovery of ambulatory abilities.
High-Risk Patients
Given the successful treatment results in these 2 cases, we believe that there is a subset of higher-risk patients with severe symptomatic proximal DVT but without PCD that may benefit from the addition of thrombolytic therapies to anticoagulation. These patients may present with significant pain, difficulty ambulating, and will likely have extensive proximal clot burden. Immediate thrombolytic intervention can achieve rapid symptom relief, which, in turn, can decrease morbidity by decreasing length of hospitalization, improving ambulation, and possibly decreasing the incidence or severity of future PTS. Positive outcomes may be easier to predict for those with obvious features of pain, edema, and difficulty ambulating, which may be more readily reversed by rapid clot reversal/removal.
These patients should be considered on a case-by-case basis. For example, the severity of pain can be balanced against the patient’s risk factors for bleeding because rapid thrombus lysis or immediate thrombus removal will likely reduce the pain. Patients who attach a high value to functional quality (eg, both patients in this case study experienced significant difficulty ambulating), quicker recovery, and decreased hospitalization duration may be more likely to choose the addition of thrombolytic therapies over anticoagulation alone and accept the higher risk of bleed.
Finally, additional studies involving variations in methodology should be examined, including whether pharmacomechanic thrombolysis may be safer in terms of bleeding than catheter-directed thrombolysis alone, as suggested by the lower bleeding rates seen in the pharmacomechanic study by Vedantham and colleagues when compared with the CaVenT study.13,14 Patients in the CaVenT study received an infusion of 20 mg of alteplase over a maximum of 96 hours. Patients in the pharmacomechanic study by Vedanthem and colleagues received either a rapid pulsed delivery of alteplase over a single procedural session (
Conclusions
There is a relative lack of high-quality data examining thrombolytic therapies in the setting of acute lower extremity DVT. Recent studies have prioritized evaluation of the posttreatment incidence of PTS, recurrent thromboembolism, and risk of bleeding caused by thrombolytic therapies. Results are mixed thus far, and further studies are necessary to clarify a more definitive role for thrombolytic therapies, particularly in established higher-risk populations with proximal DVT. In this case series, we highlighted 2 patients with extensive proximal DVT burden with significant symptoms who experienced almost complete resolution of symptoms immediately following thrombolytic therapies. We postulate that even in the absence of PCD, there is a subset of patients with severe symptoms in the setting of acute proximal lower extremity DVT that clearly benefit from thrombolytic therapies.
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
1. Centers for Disease Control and Prevention. Venous Thromboembolism (Blood Clots). Updated February 7, 2020. Accessed January 11, 2021. https://www.cdc.gov/ncbddd/dvt/data.html
2. White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107(23 Suppl 1):I4-I8. doi:10.1161/01.CIR.0000078468.11849.66
3. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report [published correction appears in Chest. 2016 Oct;150(4):988]. Chest. 2016;149(2):315-352. doi:10.1016/j.chest.2015.11.026
4. Sarwar S, Narra S, Munir A. Phlegmasia cerulea dolens. Tex Heart Inst J. 2009;36(1):76-77.
5. Nyamekye I, Merker L. Management of proximal deep vein thrombosis. Phlebology. 2012;27 Suppl 2:61-72. doi:10.1258/phleb.2012.012s37
6. Abhishek M, Sukriti K, Purav S, et al. Comparison of catheter-directed thrombolysis vs systemic thrombolysis in pulmonary embolism: a propensity match analysis. Chest. 2017;152(4): A1047. doi:10.1016/j.chest.2017.08.1080
7. Sista AK, Kearon C. Catheter-directed thrombolysis for pulmonary embolism: where do we stand? JACC Cardiovasc Interv. 2015;8(10):1393-1395. doi:10.1016/j.jcin.2015.06.009
8. Robertson L, McBride O, Burdess A. Pharmacomechanical thrombectomy for iliofemoral deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD011536. Published 2016 Nov 4. doi:10.1002/14651858.CD011536.pub2
9. Kahn SR, Shbaklo H, Lamping DL, et al. Determinants of health-related quality of life during the 2 years following deep vein thrombosis. J Thromb Haemost. 2008;6(7):1105-1112. doi:10.1111/j.1538-7836.2008.03002.x
10. Kearon C, Akl EA, Comerota AJ, et al. Antithrombotic therapy for VTE disease: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines [published correction appears in Chest. 2012 Dec;142(6):1698-1704]. Chest. 2012;141(2 Suppl):e419S-e496S. doi:10.1378/chest.11-2301
11. Bashir R, Zack CJ, Zhao H, Comerota AJ, Bove AA. Comparative outcomes of catheter-directed thrombolysis plus anticoagulation vs anticoagulation alone to treat lower-extremity proximal deep vein thrombosis. JAMA Intern Med. 2014;174(9):1494-1501. doi:10.1001/jamainternmed.2014.3415
12. Watson L, Broderick C, Armon MP. Thrombolysis for acute deep vein thrombosis. Cochrane Database Syst Rev. 2016;11(11):CD002783. Published 2016 Nov 10. doi:10.1002/14651858.CD002783.pub4
13. Enden T, Haig Y, Kløw NE, et al; CaVenT Study Group. Long-term outcome after additional catheter-directed thrombolysis versus standard treatment for acute iliofemoral deep vein thrombosis (the CaVenT study): a randomised controlled trial. Lancet. 2012;379(9810):31-38. doi:10.1016/S0140-6736(11)61753-4
14. Vedantham S, Goldhaber SZ, Julian JA, et al; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377(23):2240-2252. doi:10.1056/NEJMoa1615066
Heparin Drug Shortage Conservation Strategies
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
Heparin is the anticoagulant of choice when a rapid anticoagulant is indicated: Onset of action is immediate when administered IV as a bolus.1 The major anticoagulant effect of heparin is mediated by heparin/antithrombin (AT) interaction. Heparin/AT inactivates factor IIa (thrombin) and factors Xa, IXa, XIa, and XIIa. Heparin is approved for multiple indications, such as venous thromboembolism (VTE) treatment and prophylaxis of medical and surgical patients; stroke prevention in atrial fibrillation (AF); acute coronary syndrome (ACS); vascular and cardiac surgeries; and various interventional procedures (eg, diagnostic angiography and percutaneous coronary intervention [PCI]). It also is used as an anticoagulant in blood transfusions, extracorporeal circulation, and for maintaining patency of central vascular access devices (CVADs).
About 60% of the crude heparin used to manufacture heparin in the US originates in China, derived from porcine mucosa. African swine fever, a contagious virus with no cure, has eliminated about 25% to 35% of China’s pig population, or about 150 million pigs. In July 2019, members of the US House of Representatives Committee on Energy and Commerce sent a letter to the US Food and Drug Administration asking for details on the potential impact of African swine fever on the supply of heparin.2
The US Department of Veterans Affairs (VA) heath care system is currently experiencing a shortage of heparin vials and syringes. It is unclear when resolution of this shortage will occur as it could resolve within several weeks or as late as January 2020.3 Although vials and syringes are the current products that are affected, it is possible the shortage may eventually include IV heparin bags as well.
Since the foremost objective of VA health care providers is to provide timely access to medications for veterans, strategies to conserve unfractionated heparin (UfH) must be used since it is a first-line therapy where few evidence-based alternatives exist. Conservation strategies may include drug rationing, therapeutic substitution, and compounding of needed products using the limited stock available in the pharmacy.4 It is important that all staff are educated on facility strategies in order to be familiar with alternatives and limit the potential for near misses, adverse events, and provider frustration.
In shortage situations, the VA-Pharmacy Benefits Management (PBM) defers decisions regarding drug preservation, processes to shift to viable alternatives, and the best practice for safe transitions to local facilities and their subject matter experts.5 At the VA Tennessee Valley Healthcare System, a 1A, tertiary, dual campus health care system, a pharmacy task force has formed to track drug shortages impacting the facility’s efficiencies and budgets. This group communicates with the Pharmacy and Therapeutics committee about potential risks to patient care and develops shortage briefs (following an SBAR [situation, background, assessment, recommendation] design) generally authored and championed by at least 1 clinical pharmacy specialist and supervising physicians who are field experts. Prior to dissemination, the SBAR undergoes a rapid peer-review process.
To date, VA PBM has not issued specific guidance on how pharmacists should proceed in case of a shortage. However, we recommend strategies that may be considered for implementation during a potential UfH shortage. For example, pharmacists can use therapeutic alternatives for which best available evidence suggests no disadvantage.4 The Table lists alternative agents according to indication and patient-specific considerations that may preclude use. Existing UfH products may also be used for drug compounding (eg, use current stock to provide an indicated aliquot) to meet the need of prioritized patients.4 In addition, we suggest prioritizing current UfH/heparinized saline for use for the following groups of patients4:
- Emergent/urgent cardiac surgery1,6;
- Hemodialysis patients1,7-9 for which the low-molecular-weight heparin (LMWH) dalteparin is deemed inappropriate or the patient is not monitored in the intensive care unit for regional citrate administration;
- VTE prophylaxis for patients with epidurals or chest tubes for which urgent invasive management may occur, recent cardiac or neurosurgery, or for patients with a creatine clearance < 15 mL/min or receiving hemodialysis10-12;
- Vascular surgery (eg, limb ischemia) and interventions (eg, carotid stenting, endarterectomy)13,14;
- Mesenteric ischemia (venous thrombosis) with a potential to proceed to laparotomy15;
- Critically ill patients with arterial lines for which normal saline is deemed inappropriate for line flushing16;
- Electrophysiology procedures (eg, AF ablation)17; and
- Contraindication to use of a long-acting alternative listed in the table or a medical necessity exists for using a rapidly reversible agent. Examples for this category include but are not limited to recent gastrointestinal bleeding, central nervous system lesion, and select neurologic diagnoses (eg, cerebral venous sinus thrombosis with hemorrhage, thrombus in vertebral basilar system or anterior circulation, intraparenchymal hemorrhage plus mechanical valve, medium to large cardioembolic stroke with intracardiac thrombus).
Conclusion
The UfH drug shortage represents a significant threat to public health and is a major challenge for US health care systems, including the Veterans Health Administration. Overreliance on a predominant source of crude heparin has affected multiple UfH manufacturers and products. Current alternatives to UfH include low-molecular-weight heparins, IV direct thrombin inhibitors, and SC fondaparinux, with selection supported by guidelines or evolving literature. However, the shortage has the potential to expand to other injectables, such as dalteparin and enoxaparin, and severely limit care for veterans. It is vital that clinicians rapidly address the current shortage by creating a plan to develop efficient and equitable access to UfH, continue to assess supply and update stakeholders, and select evidence-based alternatives while maintaining focus on efficacy and safety.
Acknowledgments
The authors thank Ashley Yost, PharmD, for her coordination of the multidisciplinary task force assigned to efficiently manage the heparin drug shortage. This material is the result of work supported with resources and the use of facilities at the VA Tennessee Valley Healthcare System in Nashville, Tennessee.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
1. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest. 2001;119(1):64S-94S.
2. Bipartisan E&C leaders request FDA briefing on threat to U.S. heparin supply [press release]. Washington, DC: House Committee on Energy and Commerce; July 30, 2019. https://energycommerce.house.gov/newsroom/press-releases/bipartisan-ec-leaders-request-fda-briefing-on-threat-to-us-heparin-supply. Accessed September 19, 2019.
3. American Society of Health-System Pharmacists. Drug Shortages. Heparin injection. https://www.ashp.org/Drug-Shortages/Current-Shortages/Drug-Shortages-List?page=CurrentShortages. Accessed September 19, 2019.
4. Reed BN, Fox ER, Konig M, et al. The impact of drug shortages on patients with cardiovascular disease: causes, consequences, and a call to action. Am Heart J. 2016;175:130-141.
5. US Department of Veterans Affairs. Pharmacy Benefits Management Services, Medical Advisory Panel, VISN Pharmacist Executives, The Center For Medication Safety. Heparin supply status: frequently asked questions. PBM-2018-02. https://www.pbm.va.gov/PBM/vacenterformedicationsafety/HeparinandSalineSyringeRecallDuetoContamination_NationalPBMPati.pdf. Published May 3, 2018. Accessed September 11, 2019.
6. Shore-Lesserson I, Baker RA, Ferraris VA, et al. The Society of Thoracic Surgeons, The Society of Cardiovascular Anesthesiologists, and the American Society of ExtraCorporeal Technology: Clinical Practice Guidelines-anticoagulation during cardiopulmonary bypass. Ann Thorac Surg. 2018;105(2):650-662.
7. Soroka S, Agharazii M, Donnelly S, et al. An adjustable dalteparin sodium dose regimen for the prevention of clotting in the extracorporeal circuit in hemodialysis: a clinical trial of safety and efficacy (the PARROT Study). Can J Kidney Health Dis. 2018;5:1-12.
8. Shantha GPS, Kumar AA, Sethi M, Khanna RC, Pancholy SB. Efficacy and safety of low molecular weight heparin compared to unfractionated heparin for chronic outpatient hemodialysis in end stage renal disease: systematic review and meta-analysis. Peer J. 2015;3:e835.
9. Kessler M, Moureau F, and Nguyen P. Anticoagulation in chronic hemodialysis: progress toward an optimal approach. Semin Dial. 2015;28(5):474-489.
10. Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e227s-e277S.
11. Kaye AD, Brunk AJ, Kaye AJ, et al. Regional anesthesia in patients on anticoagulation therapies—evidence-based recommendations. Curr Pain Headache Rep. 2019;23(9):67.
12. Kahn SR, Lim W, Dunn AS, et al. Prevention of VTE in nonsurgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e195S-e226S.
13. Naylor AR, Ricco JB, de Borst GJ, et al. Management of atherosclerotic carotid and vertebral artery disease: 2017 clinical practice guidelines of the European Society for Vascular Surgery. Eur J Vasc Endovasc Surg. 2018;55:3-81.
14. Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the Management of Patients With Lower Extremity Peripheral Artery Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. JACC. 2017;69(11): e71-e126.
15. Bjorck M, Koelemaya M, Acosta S, et al. Management of diseases of mesenteric arteries and veins. Eur J Vasc Endovasc Surg. 2017;53(4):460-510.
16. Gorski L, Hadaway L, Hagle ME, McGoldrick M, Orr M, Doellman D. Infusion therapy standards of practice. J Infusion Nurs. 2016;39:S1-S156.
17. Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14(10):e275-e444.
18. Spyropoulos AC, Al-Badri A, Sherwood MW, Douketis JD. Periprocedural management of patients receiving a vitamin K antagonist or a direct oral anticoagulant requiring an elective procedure or surgery. J Thromb Haemost. 2016;14(5):875-885.
, . Periprocedural bridging management of anticoagulation. Circulation. 2012;126(4):486-490.
20. Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e326S-e350S.
21. Sousa-Uva M, Neumann F-J, Ahlsson A, et al; ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Developed with a special contribution of the European Association for Percutaneous Cardiovascular Interventions (EAPCI). Eur J Cardiothorac Surg. 2019;55(1):4-90.
22. Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes. JACC. 2014;64(24):e139-e228.
23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of patients with ST-elevation myocardial infarction. JACC. 2013;61(4):e78-e140.
24. Angiomax [package insert]. Parsippany, NJ: The Medicines Company; March 2016.
25. Sousa-Uva, Head SJ, Milojevic M, et al. 2017 EACTS guidelines on perioperative medication in adult cardiac surgery. Eur J Cardiothorac Surg. 2018;53(1):5-33.
26. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018: 2(22):3257-3291
27. Kearon C, Akl EA, Blaivas A, et al. Antithrombotic therapy for VTE disease: Chest guideline and expert panel report. Chest. 2016;149(2):315-352.
28. US Department of Veterans Affairs, Pharmacy Benefits Manager Service. Direct oral anticoagulants criteria for use and algorithm for venous thromboembolism treatment. https://www.pbm.va.gov/PBM/clinicalguidance/criteriaforuse.asp. Updated December 2016. [Source not verified]
29. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2)(suppl):e278S-e325S.
30. Raja S, Idrees JJ, Blackstone EH, et al. Routine venous thromboembolism screening after pneumonectomy: the more you look, the more you see. J Thorac Cardiovasc Surg. 2016;152(2):524-532.e2.
31. Schünemann HJ, Cushman M, Burnett AE, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: prophylaxis for hospitalized and nonhospitalized patients. Blood Adv. 2018;2(22):3198-3225.
32. Naidu SS, Aronow HD, Box LC, et al. SCAI expert consensus statement: 2016 best practices in the cardiac catheterization laboratory:(endorsed by the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencionista; affirmation of value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention). Catheter Cardiovasc Interv. 2016;88(3):407-423.
33. Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. JACC. 2011;58(24):e44-e122.
34. Mason PJ, Shah B, Tamis-Holland JE, et al; American Heart Association Interventional Cardiovascular Care Committee of the Council on Clinical Cardiology; Council on Cardiovascular and Stroke Nursing; Council on Peripheral Vascular Disease; and Council on Genomic and Precision Medicine. AHA scientific statement: an update on radial artery access and best practices for transradial coronary angiography and intervention in acute coronary syndrome. Circ Cardiovasc Interv. 2018;11(9):e000035.
35. Rao SV, Tremmel JA, Gilchrist IC, et al; Society for Cardiovascular Angiography and Intervention’s Transradial Working Group. Best practices for transradial angiography and intervention: a consensus statement from the society for cardiovascular angiography and interventions’ transradial working group. Catheter Cardiovasc Interv. 2014;83(2):228-236. 36. Moran JE, Ash SR. Locking solutions for hemodialysis catheters; heparin and citrate: a position paper by ASDIN. Semin Dial. 2008;21(5):490-492.
Antibody hierarchy may drive development of SLE vs. antiphospholipid syndrome
according to study findings presented at the European Congress of Rheumatology.
Spanish researchers found that the number of antiphospholipid (aPL) antibodies present was important for the development of antiphospholipid syndrome (APS) and that lupus anticoagulant (LA) was the major aPL antibody linked to systemic lupus erythematosus (SLE)–related organ involvement.
“aPL [antibodies] has been extensively associated with an increased risk of thrombosis and poor pregnancy outcomes, mainly in patients with primary APS,” study investigator Leyre Riancho-Zarrabeitia, MD, PhD, explained in an interview ahead of the congress.
“Moreover, aPL [antibody] positivity in SLE has been proposed to be associated with higher damage accrual and with certain manifestations such as valvular heart disease, pulmonary hypertension, and neuropsychiatric manifestations,” she added.
Anticardiolipin antibodies – notably IgG rather than IgM isotypes – also seemed to play an important role in APS and SLE manifestations, Dr. Riancho-Zarrabeitia, of Hospital Sierrallana, Instituto De Investigación Marqués De Valdecilla, and the University of Cantabria (Spain), noted during her oral presentation.
She reported data on 3,651 patients included in the RELESSER registry between October 2011 and August 2012. This large, multicenter, hospital-based registry retrospectively collects immunologic, clinical and demographic data from unselected adult patients with SLE who are attending 45 Spanish rheumatology services within the country’s national health system.
Over one-third (37.5%) of patients, who had a mean age of 47 years and were mostly (90%) women, were positive for aPL. The most frequent aPL detected was IgG anticardiolipin (aCL) antibodies, seen in 25% of patients, followed by LA in 24%, and IgM aCL in 20%.
Of the aPL-positive patients, 20.6% were positive for only one antibody, 12.1% were positive for two antibodies, and 4.8% were positive for three antibodies.
“All types of aPL were associated with classic APS manifestations,” Dr. Riancho-Zarrabeitia said. The associations were strongest for thrombotic events, such as arterial and venous small-vessel thrombosis and recurrent early pregnancy losses.
aCL antibodies conferred the highest risk for arterial thrombosis, she noted (odds ratio, 5.7), whereas LA conferred the highest risk for venous thrombosis (OR, 4.7). Both IgG and IgM isotypes were associated with thrombotic events, fetal death and recurrent pregnancy loss, but the association was stronger with the IgG isotypes.
Having more than one aPL was particularly associated with a higher risk of these APS manifestations. For example, when one antibody was present the OR for arterial thrombosis was 4.45, but when two or more aPL were detected, the ORs rose to 9.23 and 15.6, respectively.
aCL and LA also were associated with thrombocytopenia and hemolytic anemia, with ORs of around 1-2 and 2-3 respectively. There also were antibody associations with cognitive impairments.
Similar results were seen in patients with SLE. “aPL [antibody] positivity in SLE patients influenced the risk for thrombotic and obstetric manifestations,” Dr. Riancho-Zarrabeitia said. LA and aCL were associated with an increased risk of neuropsychiatric manifestations, and LA was linked to an increased risk for renal disease.
The risk for specific SLE manifestations was again higher with IgG isotypes of aCL, notably an increased risk for cardiac and respiratory events.
While increased antibody numbers generally led to a higher risk of complications, the risk for cutaneous manifestations decreased.
“The load of aPL [antibodies] confers a higher risk for APS,” Dr. Riancho-Zarrabeitia said during her conclusion. “Regarding systemic lupus erythematosus, the number of positive antibodies is directly associated with neurological and ophthalmological manifestations, and inversely associated with cutaneous manifestations.”
What these findings show, said Dr. Riancho-Zarrabeitia in the precongress interview, is that individuals who test positive for aPL antibodies need careful monitoring to prevent and treat severe manifestations. “The next step would be to confirm our findings with a prospective study.”
Dr. Riancho-Zarrabeitia has received travel grants from AbbVie, Pfizer, UCB, Merck, GlaxoSmithKline, Amgen, and Roche.
SOURCE: Riancho-Zarrabeitia L et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):136-7. Abstract OP0124. doi: 10.1136/annrheumdis-2019-eular.2485.
according to study findings presented at the European Congress of Rheumatology.
Spanish researchers found that the number of antiphospholipid (aPL) antibodies present was important for the development of antiphospholipid syndrome (APS) and that lupus anticoagulant (LA) was the major aPL antibody linked to systemic lupus erythematosus (SLE)–related organ involvement.
“aPL [antibodies] has been extensively associated with an increased risk of thrombosis and poor pregnancy outcomes, mainly in patients with primary APS,” study investigator Leyre Riancho-Zarrabeitia, MD, PhD, explained in an interview ahead of the congress.
“Moreover, aPL [antibody] positivity in SLE has been proposed to be associated with higher damage accrual and with certain manifestations such as valvular heart disease, pulmonary hypertension, and neuropsychiatric manifestations,” she added.
Anticardiolipin antibodies – notably IgG rather than IgM isotypes – also seemed to play an important role in APS and SLE manifestations, Dr. Riancho-Zarrabeitia, of Hospital Sierrallana, Instituto De Investigación Marqués De Valdecilla, and the University of Cantabria (Spain), noted during her oral presentation.
She reported data on 3,651 patients included in the RELESSER registry between October 2011 and August 2012. This large, multicenter, hospital-based registry retrospectively collects immunologic, clinical and demographic data from unselected adult patients with SLE who are attending 45 Spanish rheumatology services within the country’s national health system.
Over one-third (37.5%) of patients, who had a mean age of 47 years and were mostly (90%) women, were positive for aPL. The most frequent aPL detected was IgG anticardiolipin (aCL) antibodies, seen in 25% of patients, followed by LA in 24%, and IgM aCL in 20%.
Of the aPL-positive patients, 20.6% were positive for only one antibody, 12.1% were positive for two antibodies, and 4.8% were positive for three antibodies.
“All types of aPL were associated with classic APS manifestations,” Dr. Riancho-Zarrabeitia said. The associations were strongest for thrombotic events, such as arterial and venous small-vessel thrombosis and recurrent early pregnancy losses.
aCL antibodies conferred the highest risk for arterial thrombosis, she noted (odds ratio, 5.7), whereas LA conferred the highest risk for venous thrombosis (OR, 4.7). Both IgG and IgM isotypes were associated with thrombotic events, fetal death and recurrent pregnancy loss, but the association was stronger with the IgG isotypes.
Having more than one aPL was particularly associated with a higher risk of these APS manifestations. For example, when one antibody was present the OR for arterial thrombosis was 4.45, but when two or more aPL were detected, the ORs rose to 9.23 and 15.6, respectively.
aCL and LA also were associated with thrombocytopenia and hemolytic anemia, with ORs of around 1-2 and 2-3 respectively. There also were antibody associations with cognitive impairments.
Similar results were seen in patients with SLE. “aPL [antibody] positivity in SLE patients influenced the risk for thrombotic and obstetric manifestations,” Dr. Riancho-Zarrabeitia said. LA and aCL were associated with an increased risk of neuropsychiatric manifestations, and LA was linked to an increased risk for renal disease.
The risk for specific SLE manifestations was again higher with IgG isotypes of aCL, notably an increased risk for cardiac and respiratory events.
While increased antibody numbers generally led to a higher risk of complications, the risk for cutaneous manifestations decreased.
“The load of aPL [antibodies] confers a higher risk for APS,” Dr. Riancho-Zarrabeitia said during her conclusion. “Regarding systemic lupus erythematosus, the number of positive antibodies is directly associated with neurological and ophthalmological manifestations, and inversely associated with cutaneous manifestations.”
What these findings show, said Dr. Riancho-Zarrabeitia in the precongress interview, is that individuals who test positive for aPL antibodies need careful monitoring to prevent and treat severe manifestations. “The next step would be to confirm our findings with a prospective study.”
Dr. Riancho-Zarrabeitia has received travel grants from AbbVie, Pfizer, UCB, Merck, GlaxoSmithKline, Amgen, and Roche.
SOURCE: Riancho-Zarrabeitia L et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):136-7. Abstract OP0124. doi: 10.1136/annrheumdis-2019-eular.2485.
according to study findings presented at the European Congress of Rheumatology.
Spanish researchers found that the number of antiphospholipid (aPL) antibodies present was important for the development of antiphospholipid syndrome (APS) and that lupus anticoagulant (LA) was the major aPL antibody linked to systemic lupus erythematosus (SLE)–related organ involvement.
“aPL [antibodies] has been extensively associated with an increased risk of thrombosis and poor pregnancy outcomes, mainly in patients with primary APS,” study investigator Leyre Riancho-Zarrabeitia, MD, PhD, explained in an interview ahead of the congress.
“Moreover, aPL [antibody] positivity in SLE has been proposed to be associated with higher damage accrual and with certain manifestations such as valvular heart disease, pulmonary hypertension, and neuropsychiatric manifestations,” she added.
Anticardiolipin antibodies – notably IgG rather than IgM isotypes – also seemed to play an important role in APS and SLE manifestations, Dr. Riancho-Zarrabeitia, of Hospital Sierrallana, Instituto De Investigación Marqués De Valdecilla, and the University of Cantabria (Spain), noted during her oral presentation.
She reported data on 3,651 patients included in the RELESSER registry between October 2011 and August 2012. This large, multicenter, hospital-based registry retrospectively collects immunologic, clinical and demographic data from unselected adult patients with SLE who are attending 45 Spanish rheumatology services within the country’s national health system.
Over one-third (37.5%) of patients, who had a mean age of 47 years and were mostly (90%) women, were positive for aPL. The most frequent aPL detected was IgG anticardiolipin (aCL) antibodies, seen in 25% of patients, followed by LA in 24%, and IgM aCL in 20%.
Of the aPL-positive patients, 20.6% were positive for only one antibody, 12.1% were positive for two antibodies, and 4.8% were positive for three antibodies.
“All types of aPL were associated with classic APS manifestations,” Dr. Riancho-Zarrabeitia said. The associations were strongest for thrombotic events, such as arterial and venous small-vessel thrombosis and recurrent early pregnancy losses.
aCL antibodies conferred the highest risk for arterial thrombosis, she noted (odds ratio, 5.7), whereas LA conferred the highest risk for venous thrombosis (OR, 4.7). Both IgG and IgM isotypes were associated with thrombotic events, fetal death and recurrent pregnancy loss, but the association was stronger with the IgG isotypes.
Having more than one aPL was particularly associated with a higher risk of these APS manifestations. For example, when one antibody was present the OR for arterial thrombosis was 4.45, but when two or more aPL were detected, the ORs rose to 9.23 and 15.6, respectively.
aCL and LA also were associated with thrombocytopenia and hemolytic anemia, with ORs of around 1-2 and 2-3 respectively. There also were antibody associations with cognitive impairments.
Similar results were seen in patients with SLE. “aPL [antibody] positivity in SLE patients influenced the risk for thrombotic and obstetric manifestations,” Dr. Riancho-Zarrabeitia said. LA and aCL were associated with an increased risk of neuropsychiatric manifestations, and LA was linked to an increased risk for renal disease.
The risk for specific SLE manifestations was again higher with IgG isotypes of aCL, notably an increased risk for cardiac and respiratory events.
While increased antibody numbers generally led to a higher risk of complications, the risk for cutaneous manifestations decreased.
“The load of aPL [antibodies] confers a higher risk for APS,” Dr. Riancho-Zarrabeitia said during her conclusion. “Regarding systemic lupus erythematosus, the number of positive antibodies is directly associated with neurological and ophthalmological manifestations, and inversely associated with cutaneous manifestations.”
What these findings show, said Dr. Riancho-Zarrabeitia in the precongress interview, is that individuals who test positive for aPL antibodies need careful monitoring to prevent and treat severe manifestations. “The next step would be to confirm our findings with a prospective study.”
Dr. Riancho-Zarrabeitia has received travel grants from AbbVie, Pfizer, UCB, Merck, GlaxoSmithKline, Amgen, and Roche.
SOURCE: Riancho-Zarrabeitia L et al. Ann Rheum Dis. Jun 2019;78(Suppl 2):136-7. Abstract OP0124. doi: 10.1136/annrheumdis-2019-eular.2485.
REPORTING FROM EULAR 2019 CONGRESS
Occurrence of pulmonary embolisms in hospitalized patients nearly doubled during 2004-2015
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
At my center, Allegheny General Hospital, we often rely on catheter-directed therapy to treat major pulmonary embolism. We now perform more catheter-directed interventions than surgical embolectomies. Generally, when treating patients with major pulmonary embolism it comes down to a choice between those two options. We rarely use systemic thrombolysis for major pulmonary embolism any more.
Raymond L. Benza, MD , is professor of medicine at Temple University College of Medicine and program director for advanced heart failure at the Allegheny Health Network in Pittsburgh. He has been a consultant to Actelion, Gilead, and United Therapeutics, and he has received research funding from Bayer. He made these comments in an interview.
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
NEW ORLEANS –
During 2004-2015 the incidence of all diagnosed pulmonary embolism (PE), based on discharge diagnoses, rose from 5.4 cases/1,000 hospitalized patients in 2004 to 9.7 cases/1,000 hospitalized patients in 2015, an 80% increase, Joshua B. Goldberg, MD said at the annual meeting of the American College of Cardiology. The incidence of major PE – defined as a patient who needed vasopressor treatment, mechanical ventilation, or had nonseptic shock – rose from 7.9% of all hospitalized PE diagnoses in 2004 to 9.7% in 2015, a 23% relative increase.
The data also documented a shifting pattern of treatment for all hospitalized patients with PE, and especially among patients with major PE. During the study period, treatment with systemic thrombolysis for all PE rose nearly threefold, and catheter-directed therapy began to show a steady rise in use from 0.2% of all patients in 2011 (and before) to 1% of all patients by 2015. Surgical intervention remained lightly used throughout, with about 0.2% of all PE patients undergoing surgery annually.
Most of these intervention options focused on patients with major PE. Among patients in this subgroup with more severe disease, use of one of these three types of interventions rose from 6% in 2004 to 12% in 2015, mostly driven by a rise in systemic thrombolysis, which jumped from 3% of major PE in 2004 to 9% in 2015. However, the efficacy of systemic thrombolysis in patients with major PE remains suspect. In 2004, 39% of patients with major PE treated with systemic thrombolysis died in hospital; in 2015 the number was 47%. “The data don’t support using systemic thrombolysis to treat major PE; the mortality is high,” noted Dr. Goldberg, a cardiothoracic surgeon at Westchester Medical Center in Valhalla, N.Y.
Although catheter-directed therapy began to be much more widely used in U.S. practice starting in about 2015, during the period studied its use for major PE held fairly steady at roughly 2%-3%, but this approach also showed substantial shortcomings for the major PE population. These sicker patients treated with catheter-directed therapy had 37% mortality in 2004 and a 31% mortality in 2015, a difference that was not statistically significant. In general, PE patients enrolled in the catheter-directed therapy trials were not as sick as the major PE patients who get treated with surgery in routine practice, Dr. Goldberg said in an interview.
The data showed much better performance using surgery, although only 1,237 patients of the entire group of 713,083 PE patients studied in the database underwent surgical embolectomy. Overall, in-hospital mortality in these patients was 22%, but in a time trend analysis, mortality among all PE patients treated with surgery fell from 32% in 2004 to 14% in 2015; among patients with major PE treated with surgery, mortality fell from 52% in 2004 to 21% in 2015.
Dr. Goldberg attributed the success of surgery in severe PE patients to the definitive nature of embolectomy and the concurrent use of extracorporeal membrane oxygenation that helps stabilize acutely ill PE patients. He also cited refinements that surgery underwent during the 2004-2015 period based on the experience managing chronic thromboembolic pulmonary hypertension, including routine use of cardiopulmonary bypass during surgery. “Very high risk [PE] patients should go straight to surgery, unless the patient is at high risk for surgery because of conditions like prior sternotomy or very advanced age, in which case catheter-directed therapy may be a safer option, he said. He cited a recent 5% death rate after surgery at his center among patients with major PE who did not require cardiopulmonary resuscitation.
The database Dr. Goldberg and his collaborator reviewed included 12,735 patients treated by systemic thrombolysis, and 2,595 treated by catheter-directed therapy. Patients averaged 63 years old. The most common indicator of major PE was mechanical ventilation, used on 8% of all PE patients in the study. Non-septic shock occurred in 2%, and just under 1% needed vasopressor treatment.
Published guidelines on PE management from several medical groups are “vague and have numerous caveats,” Dr. Goldberg said. He is participating in an update to the 2011 PE management statement from the American College of Cardiology and American Heart Association (Circulation. 2011 April 26;123[16]:1788-1830).
The study received no commercial funding. Dr. Goldberg had no disclosures.
SOURCE: Haider A et al. J Amer Coll Cardiol. 2019 March;73:9[suppl 1]: doi: 10.1016/S0735-1097(19)32507-0
REPORTING FROM ACC 2019
Algorithm ruled out PE, averts radiation exposure in pregnant women
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
A diagnostic algorithm adapted for use in pregnancy safely ruled out acute pulmonary embolism in nearly 500 women with suspected pulmonary embolism enrolled in a recent prospective study, investigators are reporting.
Using the adapted algorithm, there was only one deep-vein thrombosis (DVT) and no pulmonary embolism (PE) in follow-up among those women, according to the investigators, including senior author Menno V. Huisman, MD, PhD, of the department of thrombosis and hemostasis at Leiden (Netherlands) University Medical Center and his coauthors.
The main advantage of the algorithm is that it averted CT pulmonary angiography in nearly 40% of patients, thus sparing radiation exposure to mother and fetus in many cases, the investigators added.
“Our algorithm provides solid evidence for the safe management of suspected PE in pregnant women, with selective use of CT pulmonary angiography,” Dr. Huisman and colleagues said in their March 21 report in the New England Journal of Medicine.
In a previous clinical trial, known as the YEARS study, a specialized diagnostic algorithm had a low incidence of failure in men and women with clinically suspected PE, as shown by a venous thromboembolism (VTE) rate of just 0.61% at 3 months and by use of CT pulmonary angiography that was 14 percentage points lower than with a conventional algorithmic approach.
For the current study, Dr. Huisman and his coinvestigators took the YEARS algorithm and adapted it for use in pregnant women with suspected PE presenting at 1 of 18 centers in the Netherlands, France, and Ireland.
Their adapted algorithm was based on the three criteria investigators said were most predictive in the YEARS trial, namely, clinical signs of symptoms of DVT, hemoptysis, and PE as the most likely diagnosis. Patients also underwent D-dimer testing, and if they had clinical signs and symptoms of DVT, underwent compression utrasonography of the symptomatic leg.
Pulmonary embolism was considered ruled out in patients who met none of the three YEARS criteria and had a D-dimer under 1,000 ng/mL, or if they met one to three YEARS criteria and had a D-dimer under 500 ng/mL. Otherwise, patients underwent CT pulmonary angiography and started anticoagulant treatment if results of that test indicated PE.
The primary endpoint of the study was the cumulative 3-month incidence of symptomatic VTE among patients with PE ruled out by this algorithm.
Of 498 patients participating in the study, 477 (96%) had a negative result on the adapted YEARS algorithm at baseline, while 20 (4.0%) received a diagnosis of PE, according to results of the study. One patient was lost to follow-up.
Of the 477 patients with negative results, 1 patient (0.21%) had a diagnosis of symptomatic DVT over the 3 months of follow-up, investigators reported, adding that there were no PE diagnoses over the follow-up period.
That patient with the DVT diagnosis met none of the three YEARS criteria and had a D-dimer level of 480 ng/mL, and so did not undergo CT pulmonary angiography, investigators said.
In the worst-case scenario, the VTE incidence would have been 0.42%, assuming the one patient lost to follow-up would have had a VTE diagnosis over the 3-month follow-up period, they added.
“These data meet the proposed criteria for assessing the safety of diagnostic methods in VTE, even in the context of a low baseline prevalence of disease,” Dr. Huisman and his colleagues wrote.
Overall, CT pulmonary angiography was avoided – avoiding potential radiation exposure-related harms– in 39% of the patients, the investigators said, noting that the proportion of women avoiding the diagnostic test decreased from 65% for those evaluated in the third trimester, 46% in the second trimester, and 32% in the third.
“This decreasing specificity can be explained by the physiological rise in the D-dimer level that commonly occurs during pregnancy,” said Dr. Huisman and his coauthors.
The study was supported by unrestricted grants from Leiden University Medical Center and 17 other participating hospitals. Many authors reported financial ties to the pharmaceutical industry.
SOURCE: van der Pol LM et al. N Engl J Med. 2019;380:1139-49
FROM The New England Journal of Medicine
Repeat VTE risk heightened in HIV patients
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
SEATTLE – HIV infection is associated with increased risk of recurrent venous thromboembolism, especially within 1 year of the initial episode. The finding, presented during a poster session at the Conference on Retroviruses & Opportunistic Infections, follows up on an earlier study that found that first-time VTE risk also is higher among HIV-positive individuals than in the general population.
The conclusion about first-time VTE risk, published earlier this year in Lancet HIV, came from a comparison between the ATHENA (AIDS Therapy Evaluation in the Netherlands) cohort and European population-level of studies of VTE. It found a crude incidence of 2.33 VTE events per 1,000 person-years In HIV patients, with heightened odds when CD4 cell counts were below 200 cells/mcL (adjusted hazard ratio, 3.40).
The new work represents a follow-up and compared results from ATHENA (153 patients with HIV and first VTE) and the Dutch MEGA cohort (4,005 patients without HIV, with first VTE), which includes the general population. Overall, 26% of patients in the ATHENA cohort experienced a second VTE event, compared with 16% of the general population. At 1 year after anticoagulation withdrawal, HIV-positive individuals were at 67% increased risk (HR, 1.67). At 6-years after withdrawal, the relationship was not statistically significant (HR, 1.22).
Researchers also found that CD4 cell-count recovery was associated with lowered risk, with every 100 cell-count increase between initial VTE diagnosis and anticoagulant withdrawal linked to a 20% reduction in risk (HR, 0.80).
“The clinical question is: If it’s true you have an increased risk of recurrence, should you be continuing anticoagulant therapy longer in people with HIV? This poster doesn’t answer that question and you probably need a randomized, controlled trial to look at that,” Peter Reiss, MD, professor of medicine at Amsterdam University Medical Center, said in an interview during the conference.
In the absence of a clear answer, it’s sensible for clinicians to be aware of the potential increased risk, much as clinicians have internalized the increased risk of atherosclerotic vascular disease in HIV patients. “I think the publication [in Lancet HIV] as well as this poster suggest that on the venous side of things there may also be an accentuated risk,” said Dr. Reiss.
Heidi Crane, MD, a professor of medicine at the University of Washington, Seattle, presented a poster examining the underlying factors that may predispose HIV patients to first-time VTE events. Her team performed an adjudicated review of VTE cases among HIV patients at six institutions and found that the risk factors appeared to be distinct from those seen in the general population.
The traditional long plane ride was less common in this population, while factors such as injected drug use and pneumonia were more common. The VTE events occurred at a median age of 49 years; 30% of the patients had a detectable viral load. “We’re seeing a little more (VTE) than you might expect, and in a younger population than you might have guessed,” said Dr. Crane in an interview.
The most frequent predisposing risk factors were recent hospitalization (40%), infection (40%), or immobilization/bed rest (24%) within the past 90 days, and injectable drug use (22%). “It’s not just the traditional risk factors. Some HIV-specific risk factors are driving this,” said Dr. Crane.
She also aims to learn more about the specifics of risk factors, such as catheter-associated thromboses. The team is working to increase the sample size in order to parse out the relationships with specific outcomes.
In the meantime, the data further characterize the health challenges facing people living with HIV. “This is another example demonstrating that comorbid conditions among patients with HIV that are often considered age related occur at much younger ages in our population,” said Dr. Crane.
SOURCE: Rokx C et al. CROI 2019, Abstract 636; and Tenforde MW et al. CROI 2019, Abstract 637.
.
REPORTING FROM CROI 2019
Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants (FULL)
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
Patients with cancer are at an increased risk of both venous thromboembolism (VTE) and bleeding complications. Risk factors for development of cancer-associated thrombosis (CAT) include indwelling lines, antineoplastic therapies, lack of mobility, and physical/chemical damage from the tumor.1 Venous thromboembolism may manifest as either deep vein thrombosis (DVT) or pulmonary embolism (PE). Cancer-associated thrombosis can lead to significant mortality in patients with cancer and may increase health care costs for additional medications and hospitalizations.
Zullig and colleagues estimated that 46,666 veterans received cancer care from the US Department of Veteran Affairs (VA) health care system in 2010. This number equates to about 3% of all patients with cancer in the US who receive at least some of their health care from the VA health care system.2 In addition to cancer care, these veterans receive treatment for various comorbid conditions. One such condition that is of concern in a prothrombotic state is atrial fibrillation (AF). For this condition, patients often require anticoagulation therapy with aspirin, warfarin, or one of the recently approved direct oral anticoagulant agents (DOACs), depending on risk factors.
Background
Due to their ease of administration, limited monitoring requirements, and proven safety and efficacy in patients with AF requiring anticoagulation, the American Heart Association (AHA) and American College of Cardiology recently switched their recommendations for rivaroxaban and dabigatran for oral stroke prevention to a class 1/level B recommendation.3
The American College of Chest Physicians (ACCP) recommends treatment with DOACs over warfarin therapy for acute VTE in patients without cancer; however, the ACCP prefers low molecular-weight heparin (LMWH) over the DOACs for treatment of CAT.4 Recently, the National Comprehensive Cancer Network (NCCN) updated its guidelines for the treatment of cancer-associated thromboembolic disease to recommend 2 of the DOACs (apixaban, rivaroxaban) for treatment of acute VTE over warfarin. These guidelines also recommend LMWH over DOACs for treatment of acute VTE in patients with cancer.5 These NCCN recommendations are largely based on prespecified subgroup meta-analyses of the DOACs compared with those of LMWH or warfarin in the cancer population.
In addition to stroke prevention in patients with AF, DOACs have additional FDA-approved indications, including treatment of acute VTE, prevention of recurrent VTE, and postoperative VTE treatment and prophylaxis. Due to a lack of head-to-head, randomized controlled trials comparing LMWH with DOACs in patients with cancer, these agents have not found their formal place in the treatment or prevention of CAT. Several meta-analyses have suggested similar efficacy and safety outcomes in patients with cancer compared with those of LMWH.6-8 These meta-analysis studies largely looked at subpopulations and compared the outcomes with those of the landmark CLOT (Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer Investigators) and CATCH (Comparison of Acute Treatments in Cancer Hemostasis) trials.9,10
As it is still unclear whether the DOACs are effective and safe for treatment/prevention of CAT, some confusion remains regarding the best management of these at-risk patients. In patients with cancer on DOAC therapy for an approved indication, it is assumed that the therapeutic benefit seen in approved indications would translate to treatment and prevention of CAT. This study aims to determine the incidence of VTE and rates of major and clinically relevant nonmajor bleeding (CRNMB) in veterans with cancer who received a DOAC.
Methods
This retrospective, single-center chart review was approved by the local institutional review board and research safety committee. A search within the VA Corporate Data Warehouse identified patients who had an active prescription for one of the DOACs (apixaban, dabigatran, edoxaban, and rivaroxaban) along with an ICD 9 or ICD 10 code corresponding to a malignancy.
Patients were included in the final analysis if they were aged 18 to 89 years at time of DOAC receipt, undergoing active treatment for malignancy, had evidence of a history of malignancy (either diagnostic or charted evidence of previous treatment), or received cancer-related surgery within 30 days of DOAC prescription with curative intent. Patients were excluded from the final analysis if they did not receive a DOAC prescription or have any clear evidence of malignancy documented in the medical chart.
Patients’ charts were evaluated for the following clinical endpoints: patient age, height (cm), weight (kg), type of malignancy, type of treatment for malignancy, serum creatinine (SCr), creatinine clearance (CrCl) calculated with the Cockcroft-Gault equation using actual body weight, serum hemoglobin, aspartate aminotransferase, alanine aminotransferase, total bilirubin, indication for DOAC, type of VTE, presence of a prior VTE, and diagnostic test performed for VTE. Major bleeding and CRNMB criteria were based on the definitions provided by the International Society on Thrombosis and Haemostasis (ISTH).11 All laboratory values and demographic information were gathered at the time of initial DOAC prescription.
The primary endpoint for this study was incidence of VTE. The secondary endpoints included major bleeding and CRNMB. All data collection and statistical analysis were done using Microsoft Excel 2016 (Redmond, WA). Comparisons of data between trials were done using the chi-squared calculation.
Results
From initial FDA approval of dabigatran (first DOAC on the market) on October 15, 2012, to January 1, 2017, there were 343 patients who met initial inclusion criteria. Of those, 115 did not have any clear evidence of malignancy, 22 did not have any records of DOAC receipt, 15 did not receive a DOAC within the date range, and 23 patients’ charts were unavailable.
The majority of the patients were males (96.6%), with an average age of 74.5 years. The average weight of all patients was 92.5 kg, with an average SCr of 1.1 mg/dL. This equated to an average CrCl of 85.5 mL/min based on the Cockcroft-Gault equation using actual bodyweight. Of the 177 patients evaluated, 30 (16.9%) were receiving active cancer treatment at time of DOAC initiation.
Two (1.1%) patients developed a VTE while receiving a DOAC.
Among the 177 evaluable patients in this study, there were 7 patients (4%) who developed a major bleed and 13 patients (7.3%) who developed a clinically relevant nonmajor bleed according to the definitions provided by ISTH.11
As previously mentioned, only 30 of the patients were actively receiving treatment during DOAC administration. Most of the documented cases of malignancy were either a history of nonmelanoma skin cancer (NMSC) or prostate cancer. The most common method of treatment was surgical resection for both malignancies. Of the 30 patients who received active malignancy treatment while on a DOAC, there were 4 patients with multiple myeloma, 6 patients with NMSC, 4 patients with colon cancer, 1 patient with chronic lymphocytic leukemia (CLL), 1 patient with chronic myelogenous leukemia (CML), 1 patient with small lymphocytic leukemia (SLL), 4 patients with non-small cell lung cancer (NSCLC), 1 patient with unspecified brain cancer, and 1 patient with breast cancer. The various characteristics of these patients are presented in Table 6.
Discussion
The CLOT and CATCH trials were chosen as historic comparators. Although the active treatment interventions and comparator arms were not similar between the patients included in this study and the CLOT and CATCH trials, the authors felt the comparison was appropriate as these trials were designed specifically for patients with malignancy. Additionally, these trials sought to assess rates of VTE formation and bleeding in the patient with malignancies—outcomes that aligned with this study. Alternative trials for comparison are the subgroup analyses of patients with malignancies in the AMPLIFY, RE-COVER, and EINSTEIN trials.12-14 Although these trials were designed to stratify patients based on presence of malignancy, they were not powered to account for increased risk of VTE in patients with malignancies.
There are multiple risk factors that increase the risk of CAT. Khoranna and colleagues identified primary stomach, pancreas, brain, lung, lymphoma, gynecologic, bladder, testicular, and renal carcinomas as a high risk of VTE formation.15 Additionally, Khoranna and colleagues noted that elderly patients and patients actively receiving treatment are at an increased risk of VTE formation.15 The low rate of VTE formation (1.1%) in the patients in this study may be due to the low risk for VTE formation. As previously mentioned, only 30 of the patients (16.9%) in this study were receiving active treatment.
Additionally, there were only 42 patients (23.7%) who had a high-risk malignancy. The increased age of the patient population (74.5 years old) in this study is one risk factor that could largely skew the risks of VTE formation in the patient population. In addition to age, the average body mass index (BMI) of this study’s patient population (30 kg/m2) may further increase risk of VTE. Although Khoranna and colleagues identified a BMI of 35 kg/m2 as the cutoff for increased risk of CAT, the increased risk based on a BMI of 30 kg/m2 cannot be ignored in the patients in this study.15
Another risk inherent in the treatment of patients with cancer is pancytopenia, which may lead to increased risks of bleeding and infection. When patients are exposed to an anticoagulant agent in the setting of decreased platelets and hemoglobin (from treatment or disease process), the risk for major bleeds and CRNMB are increased drastically. In this patient population, the combined rate of bleeding (11.3%) was relatively decreased compared with that of the CLOT (16.5% for all bleeding events) and CATCH (15.7% for all bleeding events) trials.9,10
Compared with the oncology subgroup analysis of the AMPLIFY, RE-COVER, and EINSTEIN trials, the differences are more noticeable. The AMPLIFY trial reported a 1.1% incidence of bleeding in patients with cancer on apixaban, whereas the RE-COVER trial did not report bleeding rates, and the EINSTEIN trial reported a 14% incidence of bleeding in all patients with cancer on rivaroxaban for VTE treatment.12-14 This study found a bleeding incidence of 12.2% with apixaban, 5.7% with dabigatran, and 14.7% with rivaroxaban. In this trial the incidence of bleeding with rivaroxaban were similar; however, the incidence of bleeding with apixaban was markedly higher. There is no obvious explanation for this, as the dosing of apixaban was appropriate in all patients in this trial except for one. There was no documented bleed in this patient’s medical chart.
A meta-analysis conducted by Vedovati and colleagues identified 6 studies in which patients with cancer received either a DOAC (with or without a heparin product) or vitamin K antagonist.16 That analysis found a nonsignificant reduction in VTE recurrence (odds ratio [OR], 0.63; 95% confidence interval [CI], 0.31-1.1), major bleeding (OR, 0.77; 95% CI, 0.41-1.44), and CRNMB (OR, 0.85; 95% CI, 0.62-1.18).16 The meta-analysis adds to the growing body of evidence in support of both safety and efficacy of DOACs in patients with cancer. Although the Vedovati and colleagues study does not directly compare rates between 2 treatment groups, the findings of similar rates of VTE recurrence, major bleed, and CRNMB are consistent with the current study. Despite differing patient characteristics, the meta-analysis by Vedovati and colleagues supports the ongoing use of DOACs in patients with malignancy, as does the current study.16
Limitations
Although it seems that apixaban, dabigatran, and rivaroxaban are effective in reducing the risk of VTE in veterans with malignancy, there are some inherent weaknesses in the current study. Most notably is the choice of comparator trials. The authors’ believe that the CLOT and CATCH trials were the most appropriate based on similarities in population and outcomes. Considering the CLOT and CATCH trials compared LMWH to coumarin products for treatment of VTE, future studies should compare use of these agents with DOACs in the cancer population. In addition, the study did not include outcomes that would adequately assess risks of VTE and bleeding formation. This information would have been beneficial to more effectively categorize this study’s patient population based on risks of each of its predetermined outcomes. Understanding safety and efficacy of DOACs in patients at various risks would help practitioners to choose more appropriate agents in practice. Last, this study did not assess the incidence of stroke in study patients. This is important because the DOACs were used mostly for stroke prevention in AF and atrial flutter. The increased risk of VTE in patients with cancer cannot directly correlate to risk of stroke with a comorbid cardiac condition, but the hypercoagulable state cannot be ignored in these patients.
Conclusion
This study provided some preliminary evidence for the safety and efficacy of DOACs in patients with cancer. The low incidence of VTE formation and similar rates of bleeding among other clinical trials indicate that DOACs are safe alternatives to currently recommended anticoagulation medication in patients with cancer.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.
1. Motykie GD, Zebala LP, Caprini JA, et al. A guide to venous thromboembolism risk factor assessment. J Thromb Thrombolysis. 2000;9(3):253-262.
2. Zullig LL, Sims KJ, McNeil R, et al. Cancer incidence among patients of the U.S. Veterans Affairs Health Care System: 2010 update. Mil Med. 2017;182(7):e1883-e1891.
3. January CT, Wann S, Alpert JS, et al; ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. Circulation. 2014;130(23):2071-2104.
4. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149(2):315-352.
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Cancer-associated venous thromboembolic disease. Version 1.2018. https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/vte.pdf. Updated March 22, 2018. Accessed April 9, 2018.
6. Brunetti ND, Gesuete E, De Gennaro L, et al. Direct-acting oral anticoagulants compared to vitamin K inhibitors and low molecular weight heparin for the prevention of venous thromboembolism in patients with cancer: a meta-analysis study. Int J Cardiol. 2017;230:214-221.
7. Posch F, Konigsbrügge O, Zielinski C, Pabinger I, Ay C. Treatment of venous thromboembolism in patients with cancer: a network meta-analysis comparing efficacy and safety of anticoagulants. Thromb Res. 2015;136(3):582-589.
8. van Es N, Coppens M, Schulman S, Middledorp S, Büller HR. Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood. 2014;124(12):1968-1975.
9. Lee AY, Levine MN, Baker RI, et al; Randomized Comparison of Low-Molecular-Weight Heparin versus Oral Anticoagulant Therapy for the Prevention of Recurrent Venous Thromboembolism in Patients with Cancer (CLOT) Investigators. Low molecular weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349(2):146-153.
10. Lee AY, Kamphuisen PW, Meyer G, et al; CATCH Investigators. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677-686.
11. Kaatz S, Ahmad D, Spyropoulos AC, Schulman S; Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;13(11):2119-2126.
12. Agnelli G, Büller HR, Cohen A, et al. Oral apixaban for the treatment of venous thromboembolism in cancer patients: results from the AMPLIFY trial. J Thromb Haemost. 2015;13(12):2187-2191.
13. Schulman S, Goldhaber SZ, Kearon C, et al. Treatment with dabigatran or warfarin in patients with venous thromboembolism and cancer. Thromb Haemost. 2015;114(1):150-157.
14. Prins MH, Lensing AW, Brighton TA, et al. Oral rivaroxaban versus enoxaparin with vitamin K antagonist for the treatment of symptomatic venous thromboembolism in patients with cancer (EINSTEIN-DVT and EINSTEIN-PF): a pooled subgroup analysis of two randomised controlled trials. Lancet Haematol. 2014;1(1):e37-e46.
15. Khoranna AA, Connolly GC. Assessing risk of venous thromboembolism in the patient with cancer. J Clin Oncol. 2009;27(9):4839-4847.
16. Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147(2):475-483.