User login
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
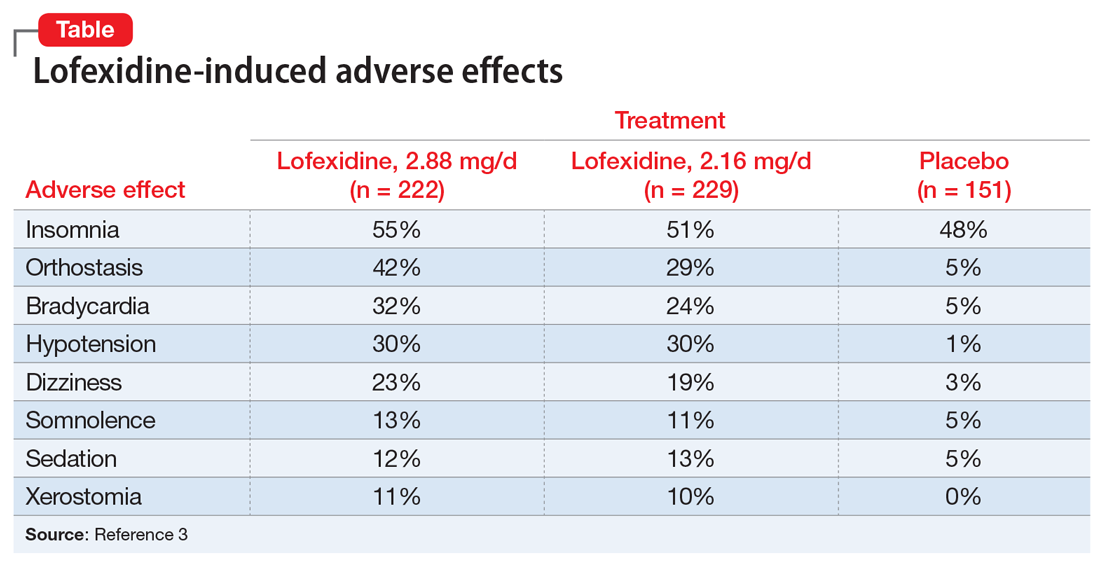
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
Opioid use disorder (OUD) and deaths by opioid overdose are a major public health concern, especially with the advent of synthetic opioids such as fentanyl.1 Enrolling patients with OUD into substance abuse treatment programs can be a difficult hurdle to cross because patients do not want to experience withdrawal. The fear of withdrawal leads many individuals to refuse appropriate interventions. For these patients, consider the alpha-2 agonist lofexidine, which was FDA-approved in 2018 to help diminish the signs and symptoms of opioid withdrawal.1-3 Use of lofexidine might encourage more patients with OUD to accept substance abuse treatment.1,4,5
How to prescribe lofexidine
For decades, clinicians in Britain have prescribed lofexidine to attenuate opioid withdrawal.1An analog of clonidine, lofexidine is reportedly less likely than clonidine to induce hypotension.1,4 While this agent does not diminish drug toxicity, it can provide symptomatic relief for patients undergoing opioid withdrawal, and is efficacious as a supplement to and/or replacement for methadone, buprenorphine, clonidine, or other symptomatic pharmacotherapies.1,4,5
Lofexidine is available in 0.18-mg tablets. For patients experiencing overt symptoms of opioid withdrawal, initially prescribe 3 0.18-mg tablets, 4 times a day.3 The recommended maximum dosage is 2.88 mg/d, and each dose generally should not exceed 0.72 mg/d. Lofexidine may be continued for up to 14 days, with dosing guided by symptoms. Initiate a taper once the patient no longer experiences withdrawal symptoms.3
Adverse effects. Lofexidine’s efficacy and safety were evaluated in 3 randomized, double-blind, placebo-controlled trials that included 935 participants dependent on short-acting opioids who were experiencing abrupt opioid withdrawal and received lofexidine, 2.16 or 2.88 mg/d, or placebo.3 The most common adverse effects of lofexidine were insomnia, orthostatic hypotension, bradycardia, hypotension, dizziness, somnolence, sedation, and dry mouth.3 In the 3 trials, these effects were reported by ≥10% of patients receiving lofexidine, and occurred more frequently compared with placebo (Table3).
Take precautions when prescribing lofexidine because it can cause QT prolongation and CNS depression, especially when co-administered with sedative agents.3 It also can result in rebound hypertension once discontinued. This may be minimized by gradually reducing the dosage.3
A pathway to OUD treatment
Lofexidine can help relieve symptoms of opioid withdrawal, such as stomach cramps, muscle spasms or twitching, feeling cold, muscular tension, and aches and pains.1-5 This new option might help clinicians encourage more patients with OUD to fully engage in substance abuse treatment.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.
1. Rehman SU, Maqsood MH, Bajwa H, et al. Clinical efficacy and safety profile of lofexidine hydrochloride in treating opioid withdrawal symptoms: a review of literature. Cureus. 2019;11(6):e4827. doi: 10.7759/cureus.4827.
2. FDA approves the first non-opioid treatment for management of opioid withdrawal symptoms in adults. US Food & Drug Administration. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm607884.htm. Published May 16, 2018. Accessed December 13, 2019.
3. Lucemyra [package insert]. Louisville, KY: US WorldMeds, LLC; 2018.
4. Carnwath T, Hardman J. Randomized double-blind comparison of lofexidine and clonidine in the out-patient treatment of opiate withdrawal. Drug Alcohol Depend. 1998;50(3):251-254.
5. Gonzalez G, Oliveto A, Kosten TR. Combating opiate dependence: a comparison among the available pharmacological options. Exp Opin Pharmacother. 2004;5(4):713-725.