User login
Introduction
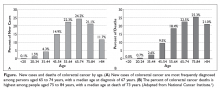
Colorectal cancer (CRC) is the fourth most common cancer in the United States and has a high prevalence among the older population.1 In 2017, there were an estimated 135,430 new cases of CRC and 50,260 deaths due to CRC. It is the second leading cause of cancer death in the United States, and the death rate for patients with CRC increases with age (Figure).2
Although elderly persons are more frequently diagnosed with CRC, they are underrepresented in clinical trials. This may be due in part to stringent eligibility criteria in prospective randomized controlled trials that exclude older patients with certain comorbidities and decreased functional status. Hutchins and colleagues compared the proportion of persons aged 65 years and older enrolled in Southwest Oncology Group (SWOG) clinical trials and the proportion of persons in this age group in the US population with the same cancer diagnoses.5 They found that while 72% of the US population with CRC were aged ≥ 65 years, persons in this age group comprised only 40% of patients enrolled in SWOG trials. An update on this study performed after Medicare policy changed in 2000 to include coverage of costs incurred due to clinical trials showed an upward trend in the accrual of older patients in SWOG trials, from 25% during the period 1993–1996 to 38% during the period 2001–2003; however, the percentage of older patients with CRC on clinical trials overall remained stable from 1993 to 2003.6
The underrepresentation of older adults with CRC in clinical trials presents oncologists with a challenging task when practicing evidence-based medicine in this patient population. Analysis of a large claims database demonstrated that the use of multi-agent chemotherapy for the treatment of metastatic CRC in older adults increased over time, while the use of single-agent 5-fluorouracil (5-FU) decreased.7 However, the adoption of combination therapy with irinotecan or oxaliplatin in older adults lagged behind the initial adoption of these agents in younger patients. This data demonstrates that as the field of medical oncology evolves, providers are becoming more comfortable treating older patients with multiple medical problems using standard approved regimens.
Geriatric Assessment
Before treating older patients with cancer, it is necessary to define the patient’s physiological age, ideally through a multidisciplinary team evaluation.
The Eastern Cooperative Oncology Group performance status (ECOG PS) and Karnofsky Performance Status (KPS) are crude measures of functional status.12 Generally, elderly patients with good ECOG PS or KPS scores are considered fit enough to receive standard therapy similar to their younger counterparts. Evaluation of functional status using these performance scores is often suboptimal, resulting in patients with a normal or adequate performance status score who may still experience poor outcomes, including decreased survival and inability to tolerate treatment. A study that explored parameters among older patients that predict for increased risk of chemotherapy-related toxicities found that physician-rated KPS score did not accurately predict the risk for adverse events.13 Therefore, a CGA represents a better way to evaluate functional status and other domains.
Functional status can also be evaluated by self-reported tools such as activities of daily living, which refer to basic self-care, and instrumental activities of daily living (IADLs), which are essential for independent living in the community.14,15 Mobility, gait, and balance can also be measured using the “Timed Get Up and Go” test and gait speed. Klepin et al found that faster gait speed was associated with overall survival (OS) in patients with metastatic cancer.16
Cognitive function is an important component of the geriatric assessment in older patients with cancer, as dementia is a prognostic factor for survival in the overall geriatric population. In a retrospective review, patients with dementia were less likely to have a biopsy-proven diagnosis and were twice as likely to have their CRC diagnosed postmortem.17 In addition, establishing that the patient has intact cognitive function prior to initiating treatment is essential to ensure that the patient can comply with treatment and understands when to report adverse effects. Nutritional status is an important portion of the geriatric assessment because malnutrition is associated with increased mortality and decreased tolerance for chemotherapy.18–20 Evaluating the patient’s psychosocial support is crucial as well because older patients are at greater risk of social isolation and depression.21 While the incidence of depression is lower in older adults with cancer than in younger adults with cancer, clinically significant depression is still noted in 3% to 25% of elderly cancer patients.22 Other critical components of the CGA are review of the patient’s comorbidities and medications to avoid complications of polypharmacy.
Both the Cancer and Aging Research Group (CARG) and Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) toxicity tools are valuable tools, as they predict chemotherapy tolerance in elderly patients.13,23 These tools can help guide discussions between oncologists and patients as well as the formulation of an appropriate treatment plan.24 Although toxicity tools can help to determine which patients are at risk for severe toxicity secondary to treatment, these tools do not replace the CGA. A prospective cohort study that evaluated the impact of CGA on tolerance to chemotherapy in older patients with cancer compared patients aged ≥ 70 years at the start of their treatment with chemotherapy (± radiation therapy) using geriatrician-delivered CGA versus standard care given by oncology.8 Patients who received geriatrician-guided CGA interventions tolerated chemotherapy better and completed treatments as planned (odds ratio 4.14 [95% confidence interval {CI} 1.50 to 11.42], P = 0.006) with fewer treatment modifications.
Unfortunately, the CGA is time-consuming to administer and difficult to incorporate into a busy oncology practice. Therefore, other screening models are used to identify patients who may benefit from a full CGA. The International Society of Geriatric Oncology performed a systematic review of screening tools used to identify older cancer patients in need of geriatric assessment and found that the 3 most studied screening tools are the G8, the Vulnerable Elders Survey-13 (VES-13), and the Flemish version of the Triage Risk Screening Tool.25 Another study found that the G8 was more sensitive than the VES-13 (76.5% versus 68.7%, P = 0.0046), whereas the VES-13 was more specific than the G8 (74.3% versus 64.4%, P < 0.0001).26 In addition to providing guidance to initiate a full geriatric assessment, these screening tools may assist in decision making for older cancer patients, especially those with advanced disease.
Surgery
Early-Stage Disease
When possible, surgical resection of colorectal tumors is the primary treatment in both the curative setting and to avoid complications, such as obstruction or perforation.27 Multiple studies have shown that fit elderly patients benefit from curative surgery similarly to their younger counterparts.27–29 With the growing population of persons aged 65 years or older, surgeons are becoming more comfortable with operating on the elderly.4 However, a large systematic review of 28 independent studies with a total of 34,194 patients showed that older patients were less likely to undergo curative surgery.30 Eligibility for surgery should not be determined by age alone, but rather should be based on a full assessment of the patient’s health, including comorbidities, functional status, nutrition, cognition, social support, and psychological status. The impact of age on short-term outcomes after colorectal surgery in terms of 30-day postoperative morbidity and mortality rates was explored in a study that divided patients into 2 groups: those aged ≥ 80 years (mean age 85) and those aged < 80 years (mean age 55.3).31 There were no statistical differences in 30-day postoperative morbidity and mortality rates between the 2 groups, and preexisting comorbidities and urgent nature of surgery were important predictors of colorectal surgery outcomes in the older adults, results that have been seen in several other studies.28,30 When possible, laparoscopic surgery is preferred as it is associated with less intraoperative blood loss, less postoperative pain, reduced postoperative ileus, a shorter hospital stay, and fewer cardiovascular and pulmonary complications.32 The Preoperative Assessment of Cancer in the Elderly (PACE), which combines surgical risk assessment tools with CGA tools, can assist surgeons in determining candidacy for surgery and help decrease unequal access to surgery in the geriatric population.33
Metastasectomy
A large international multicenter cohort study explored the outcomes of patients aged ≥ 70 years who underwent liver resection of colorectal metastases. The study investigatorsfound that neoadjuvant chemotherapy was used less frequently and less extensive surgery was performed in elderly patients than in younger patients.34 Sixty-day postoperative mortality was slightly higher (3.8% versus 1.6%, P < 0.001) and 3-year OS was slightly lower (57.1% versus 60.2%, P < 0.001) in the elderly group as compared to their younger counterparts, but overall the outcomes after liver surgery were similar. Therefore, the management of liver metastases in oligometastatic disease in elderly patients fit for surgery should be the same as that offered to younger patients. Since outcomes are comparable, older patients should be offered neoadjuvant chemotherapy, as several studies have shown similar response rates and OS in younger and older patients.35,36
Rectal Cancer
The standard of care for locally advanced rectal cancer is combined modality treatment with radiation and chemotherapy followed by total mesorectal excision. However, given conflicting data regarding the ability of elderly patients to tolerate neoadjuvant 5-FU-based chemotherapy and radiation, elderly patients are treated with trimodality therapy less often than their younger counterparts.37,38 A systematic review of 22 randomized trials involving 8507 patients with rectal cancer showed that adjuvant radiation therapy could reduce the risk of local recurrence and death from rectal cancer in patients of all ages.39 However, the risk of noncancer-related death was increased in the older population. The Stockholm II trial showed similar benefits of preoperative radiation overall, but this benefit did not extend to patients older than 68 years because of an increased risk of morbidity and mortality.40 In older patients, mortality from noncancer causes within the first 6 months after surgery was higher in the group that received perioperative radiation than in the group that did not receive radiation. Elderly patients (age > 68 years) accounted for most of the mortality, which was predominantly due to cardiovascular disease.
A retrospective study of 36 patients aged ≥ 70 years with rectal cancer evaluated the toxicity and feasibility of neoadjuvant 5-FU combined with pelvic radiation for treating locally advanced rectal cancer. Patients were classified as healthy and “fit” or “vulnerable” based on the presence of comorbidities.41 This study demonstrated that tolerability and response to neoadjuvant chemotherapy and radiation as well as ability to undergo surgery were similar in “vulnerable” patients and “fit” patients. Conversely, Margalit and colleagues studied the rate of treatment deviations in elderly patients with rectal cancer treated with combined modality therapy and found that most patients required early termination of treatment, treatment interruptions, or dose reductions.42 While trimodality treatment is the standard of care in rectal cancer, there is conflicting data from retrospective studies regarding the tolerability and feasibility of this approach. It is important to proceed with caution but to still consider fit older patients with locally advanced rectal cancer for neoadjuvant chemotherapy and radiation followed by surgery.
In patients who have a complete response (CR) to neoadjuvant chemoradiation, watchful waiting rather than proceeding to surgery may be a reasonable strategy, especially in older patients. A systematic review of 867 patients with locally advanced rectal cancer showed no statistically significant difference in OS between patients who were observed with watchful waiting and those who underwent surgery.43 The International Watch and Wait Database includes 679 patients who were managed with a watch-and-wait regimen because they had a clinical CR after chemoradiation. An outcomes analysis of these patients showed that 25% had local regrowth, with 3-year OS of 91% overall and 87% in patients with local regrowth.44 In most patients (84%), regrowth of the tumor occurred within the first 2 years of follow up.
In frail older adults, for whom longer courses of treatment are not feasible or chemotherapy is contraindicated, short-course radiation therapy can be considered either in the neoadjuvant setting or alone for palliation.45 A randomized trial of short-course radiation versus long-course chemoradiation in patients with T3 rectal cancer found that the difference in 3-year local recurrence rates was not statistically significant.46
Chemotherapy
An expected natural decline in function occurs with age, but given the great variability that exists between patients, it is important to focus on physiologic age rather than chronologic age to determine ability to receive and tolerate anticancer treatment. Decreases in renal and hepatic function, cognitive impairment, changes in gastrointestinal motility, decrements in cardiac and bone marrow reserves, as well as comorbidities and polypharmacy affect a patient’s ability to tolerate chemotherapy.47,48 Toxicity tools such as CARG and CRASH can help to predict severity of toxicity with chemotherapy.13,23 The information provided by these tools can help guide conversations between the oncologist and patient regarding treatment plans.
Adjuvant Chemotherapy for Early-Stage Disease
Stage II Disease
Defining treatment guidelines for older patients with stage II colon cancer is difficult due to lack of data that shows benefit in this population. The QUASAR (Quick and Simple and Reliable) group’s prospective study of adjuvant single-agent 5-FU in stage II colon cancer patients showed an absolute improvement in survival of 3.6% when 5-FU was given after surgery (95% CI 1.0 to 6.0).49 The subgroup analysis of patients aged ≥ 70 years showed a limited benefit of adjuvant 5-FU (hazard ratio [HR] 1.13 [95% CI 0.74 to 1.75]). Given the limited benefit, adjuvant 5-FU for elderly patients with stage II colon cancer should be used judiciously as patients may have competing causes of morbidity or mortality.
The use of oxaliplatin-based therapy in the adjuvant setting for stage II disease was evaluated in a subgroup analysis of the MOSAIC study (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer).50 Adjuvant oxaliplatin-based treatment may be offered to patients with stage II colon cancer that carries high-risk features (poorly differentiated histology, lymphovascular invasion, bowel obstruction and/or perforation, < 12 lymph nodes sampled, perineural invasion, or indeterminate or positive margins) due to a trend toward improved disease-free survival (DFS) at 5 years. Patients in this group who received adjuvant FOLFOX (leucovorin, oxaliplatin, 5-FU) versus 5-FU/leucovorin had a DFS of 82.3% versus 74.6%, respectively (HR 0.72 [95% CI 0.50 to 1.02]), a difference that was not statistically significant. A subgroup analysis of 315 patients aged 70 to 75 years with stage II colon cancer enrolled in the MOSAIC study found no statistically significant DFS or OS benefit with the addition of oxaliplatin to 5-FU/leucovorin.51 Therefore, use of this platinum/fluoropyrimidine combination for adjuvant therapy for high-risk stage II disease in older patients remains controversial given its associated risks and the lack of definitive data demonstrating a benefit in this patient group. Decisions regarding this therapy should be made through a shared discussion with patients about its risks and benefits.
Microsatellite status is an important biomarker in the evaluation of stage II CRC. Microsatellite stability is a marker of a functioning DNA mismatch repair system. In patients with colon cancer, tumor microsatellite stability is classified based on the percentage of abnormal microsatellite regions.52 Several studies have shown that patients with tumors that display high microsatellite instability (MSI-H) have an improved prognosis over patients with microsatellite stable tumors.53,54 While patients with stage II MSI-H colon cancer have better outcomes, MSI is associated with a reduced response to treatment with fluoropyrimidines, as demonstrated in a systematic review that found that patients with tumors with MSI obtained no benefit from adjuvant 5-FU (HR 1.24 [95% CI 0.72 to 2.14]).55 Aparicio and colleagues reported an increased prevalence of MSI-H tumors with increasing age.56 Therefore, mismatch repair phenotype should be considered when making adjuvant chemotherapy decisions in the older adult with colon cancer, as it may affect the decision to recommend single-agent 5-FU treatment.
Stage III Disease
The use of single-agent 5-FU for stage III resected CRC has been evaluated in multiple studies. Sargent et al performed a pooled analysis of 3351 patients from 7 randomized phase 3 trials comparing surgery and adjuvant 5-FU-based chemotherapy versus surgery alone in stage II or III colon cancer patients.57 Adjuvant chemotherapy was associated with improvement in both OS and time to tumor recurrence (HR 0.76 and 0.68, respectively). The 5-year OS was 71% for those who received adjuvant treatment and 64% for those who were treated with surgery alone. The benefit of adjuvant treatment was independent of age, and there was no difference in toxicity across age groups, except for 1 study which showed increased rates of leukopenia in the elderly. The oral fluoropyrimidine capecitabine was shown to be an effective alternative to 5-FU plus leucovorin as adjuvant treatment for those with resected stage III colon cancer.58 However, in the subgroup analysis of DFS in the intention-to-treat group, the improvement in DFS was not statistically significant in those aged ≥ 70 years. This study justified the phase 3 Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial, which compared capecitabine and 5-FU/leucovorin as adjuvant therapy in patients with resected stage III colon cancer.59 The X-ACT trial showed no significant effect of age on DFS or OS.
The addition of oxaliplatin to 5-FU in the adjuvant setting for stage III tumors has been studied and debated in the elderly population in multiple trials. The MOSAIC trial investigated FOLFOX versus 5-FU/leucovorin in the adjuvant setting.50 The addition of oxaliplatin was associated with a DFS and OS benefit, with a 20% reduction in risk of colon cancer recurrence and 16% reduction in risk of death in all patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial then studied 2409 patients with stage II or III colon cancer treated with weekly bolus 5-FU/leucovorin with or without oxaliplatin.60 In this study, OS was significantly improved with the addition of oxaliplatin in patients younger than 70 years, but OS at 5 years was 4.7% worse for patients aged ≥ 70 years treated with weekly 5-FU/leucovorin and oxaliplatin compared with those treated with weekly 5-FU/leucovorin (71.6% versus 76.3%, respectively). In contrast, the XELOXA trial (NO16968), which randomly assigned stage III colon cancer patients to capecitabine and oxaliplatin (XELOX) or bolus 5-FU/leucovorin (standard of care at study start), showed an efficacy benefit, albeit not statistically significant, in patients aged ≥ 70 years (HR 0.87 [95% CI 0.63 to 1.18]).61–63
The Adjuvant Colon Cancer Endpoints (ACCENT) database included 7 randomized trials totaling 14,528 patients with stage II or III colon cancer treated with adjuvant 5-FU with or without oxaliplatin or irinotecan.64 Subgroup analysis of patients aged ≥ 70 years (n = 2575) showed no benefit with an oxaliplatin-based regimen in DFS (HR 0.94 [95% CI 0.78 to 1.13]) or OS (HR 1.04 [95% CI, 0.85 to 1.27]). Based on these studies and the increased toxicity with oxaliplatin, oxaliplatin-based adjuvant chemotherapy is utilized less often than single-agent 5-FU in geriatric patients with early-stage colon cancer.65 Conversely, a recent pooled analysis of individual patient data from 4 randomized trials (NSABP C-08, XELOXA, X-ACT, and AVANT) showed improved DFS and OS with adjuvant XELOX or FOLFOX over single-agent 5-FU in patients aged ≥ 70 years (DFS HR 0.77 [95% CI 0.62 to 0.95], P = 0.014; OS HR 0.78 [95% CI 0.61 to 0.99], P = 0.045).66 This analysis also showed that grade 3 and 4 adverse events related to oxaliplatin were similar across age groups.
These data come from post-hoc analyses, and there is no prospective data to steer decision making in elderly patients with early-stage CRC (Table).
It is well established that patients with stage III colon cancer benefit from oxaliplatin-based adjuvant chemotherapy after curative surgical resection.68 However, older patients are less likely to be referred to oncology as compared with their younger counterparts, due to the conflicting data regarding the benefit of this approach in older adults. Studies have shown that when the referral is placed, the geriatric population is less likely to receive chemotherapy.69 Sanoff et al analyzed 4 data sets (SEER-Medicare, National Comprehensive Cancer Network, New York State Cancer Registry, and Cancer Care Outcomes Research and Surveillance Consortium) to assess the benefit of adjuvant chemotherapy for resected stage III CRC among patients aged ≥ 75 years. Their analysis showed that only 40% of patients evaluated received adjuvant chemotherapy for stage III CRC after surgical resection.70
Summary
Prospective data to guide the treatment of older patients with early-stage CRC in the adjuvant setting is lacking. For fit older patients with stage II disease, limited benefit will be derived from single-agent 5-FU. For those with stage III CRC, the benefit and toxicities of fluoropyrimidines as adjuvant therapy appear to be similar regardless of age. The addition of oxaliplatin to fluoropyrimidines in patients aged ≥ 70 years has not been proven to improve DFS or OS and could result in an incremental toxicity profile. Therefore, treatment plans must be individualized, and decisions should be made through an informed discussion evaluating the overall risk/benefit ratio of each approach.
Metastatic Disease
Palliative Chemotherapy
Approximately 20% of patients with CRC are diagnosed with metastatic disease at presentation, and 35% to 40% develop metastatic disease following surgery and adjuvant therapy.2 The mainstay of treatment in this population is systemic therapy in the form of chemotherapy with or without biologic agents. In this setting, several prospective studies specific to older adults have been completed, providing more evidence-based guidance to oncologists who see these patients. Folprecht et al retrospectively reviewed data from 22 clinical trials evaluating 5-FU-based palliative chemotherapy in 3825 patients with metastatic CRC, including 629 patients aged ≥ 70 years.71 OS in elderly patients (10.8 months [95% CI 9.7 to 11.8]) was equivalent to that in younger patients (11.3 months [95% CI 10.9 to 11.7], P = 0.31). Similarly, relative risk and progression-free survival (PFS) were comparable irrespective of age.
Standard of care for most patients with metastatic colon cancer consists of 5-FU/leucovorin in combination with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with a monoclonal antibody.72 A retrospective pooled analysis of patients with metastatic CRC compared the safety and efficacy of FOLFOX4 in patients aged < 70 years versus those aged ≥ 70 years.73 While age ≥ 70 years was associated with an increased rate of grade ≥ 3 hematologic toxicity, it was not associated with increased rates of severe neurologic events, diarrhea, nausea, vomiting, infection, 60-day mortality, or overall incidence of grade ≥ 3 toxicity. The benefit of treatment was consistent across both age groups; therefore, age alone should not exclude an otherwise healthy individual from receiving FOLFOX.
These post-hoc analyses show that fit older patients who were candidates for trial participation tolerated these treatments well; however, these treatments may be more challenging for less fit older adults. The UK Medical Research Council FOCUS2 (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing) study was a prospective phase 3 trial that included 459 patients with metastatic CRC who were deemed too frail or not fit enough for standard-dose chemotherapy by their oncologists.74 In this group, 43% of patients were older than 75 years and 13% were older than 80 years. Patients were randomly assigned to receive infusional 5-FU with levofolinate; oxaliplatin and 5-FU; capecitabine; or oxaliplatin and capecitabine; all regimens were initiated with an empiric 20% dose reduction. The addition of oxaliplatin suggested some improvement in PFS, but this was not significant (5.8 months versus 4.5 months, HR 0.84 [95% CI 0.69 to 1.01], P = 0.07). Oxaliplatin was not associated with increased grade 3 or 4 toxicities. Capecitabine is often viewed as less toxic because it is taken by mouth, but this study found that replacement of 5-FU with capecitabine did not improve quality of life. Grade 3 or 4 toxicities were seen more frequently in those receiving capecitabine than in those receiving 5-FU (40% versus 30%, P = 0.03) in this older and frailer group of patients. As the patients on this study were frail and treatment dose was reduced, this data may not apply to fit older adults who are candidates for standard therapy.
When managing an older patient with metastatic CRC, it is important to tailor therapy based on goals of care, toxicity of proposed treatment, other comorbidities, and the patient’s functional status. One approach to minimizing toxicity in the older population is the stop-and-go strategy. The OPTIMOX1 study showed that stopping oxaliplatin after 6 cycles of FOLFOX7 and continuing maintenance therapy with infusional 5-FU/leucovorin alone for 12 cycles prior to reintroducing FOLFOX7 achieved efficacy similar to continuous FOLFOX4 with decreased toxicity.75 Figer et al studied an exploratory cohort of 37 patients aged 76 to 80 years who were included in the OPTIMOX1 study.76 The overall relative risk, median PFS, and median OS did not differ between the older patients in this cohort and younger patients studied in the original study. Older patients did experience more neutropenia, neurotoxicity, and overall grade 3 to 4 toxicity, but there were no toxic deaths in patients older than 75 years. The approach of giving treatment breaks, as in OPTIMOX2, may also provide patients with better quality of life, but perhaps at the expense of cancer-related survival.77
The combination of irinotecan and 5-FU has also been studied as treatment for patients with metastatic CRC. A pooled analysis of 2691 patients aged ≥ 70 years with metastatic CRC across 4 phase 3 randomized trials investigating irinotecan and 5-FU demonstrated that irinotecan-containing chemotherapy provided similar benefits to both older and younger patients with similar risk of toxicity.78 A phase 2 trial studying FOLFIRI as first-line treatment in older metastatic CRC patients showed this to be a safe and active regimen with manageable toxicity.79 Another randomized phase 3 trial for older patients compared 5-FU/leucovorin with or without irinotecan for first-line treatment of metastatic CRC (FFCD 2001-02).80 The study accrued 282 patients aged ≥ 75 years (median age 80 years), and found that the addition of irinotecan to infusional 5-FU–based chemotherapy did not significantly increase either PFS or OS. Aparicio et al performed a substudy of baseline geriatric evaluation prior to treatment in the FFCD 2001-02 study and assessed the value of geriatric parameters for predicting outcomes (objective response rate [ORR], PFS, and OS).81 Multivariate analysis showed that none of the geriatric parameters were predictive of ORR or PFS but that normal IADL was associated with better OS. This combination may still be appropriate for some older patients with metastatic disease, while single- agent 5-FU may be more appropriate in frail patients.
Biologic Agents
VEGF Inhibitors
Targeted biologic agents have been studied in the treatment of metastatic CRC. Bevacizumab is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is approved in the first-line setting for treatment of metastatic CRC. A pooled analysis examined 439 patients 65 years of age and older with metastatic CRC who received bevacizumab plus chemotherapy versus placebo plus chemotherapy.82 In this analysis, the addition of bevacizumab was associated with an improvement in OS (19.3 months versus 14.3 months, HR 0.7 [95% CI 0.55 to 0.90], P = 0.006) and in PFS (9.2 months versus 6.2 months, HR 0.52 [95% CI 0.40 to 0.67], P < 0.0001). Known adverse events associated with bevacizumab were seen in the bevacizumab plus chemotherapy group but not at increased rates in the older population compared to their younger counterparts. Conversely, another pooled analysis found that while there was a PFS and OS benefit in older patients receiving bevacizumab, there was an increased incidence of thrombotic events in patients older than 65 years.83 The BEAT (Bevacizumab Expanded Access Trial) and BRiTE (Bevacizumab Regimens Investigation of Treatment Effects) studies showed similar clinical outcomes across all age groups.84,85 While older patients experienced more arterial thromboembolic events with the addition of bevacizumab, other factors such as ECOG PS, prior anticoagulation, and history of arterial disease were more predictive of these adverse events than age.
The randomized phase 3 AVEX study explored the efficacy and tolerability of capecitabine plus bevacizumab versus capecitabine alone in 280 frail patients aged ≥ 70 years.86 PFS in the capecitabine/bevacizumab arm was 9.1 months versus 5.1 months in the capecitabine alone arm. While the OS difference was not statistically significant, patients in the capecitabine/bevacizumab arm had an OS of 20.7 months versus 16.8 months in the capecitabine alone group. As reported in prior studies, patients in the capecitabine/bevacizumab arm had increased rates of toxic events (40%) compared with those who received capecitabine alone (22%), with reports of hypertension, hand-foot syndrome, bleeding, and thrombotic events. More recently, the phase 2 PRODIGE 20 trial studied the addition of bevacizumab to chemotherapy (5-FU, FOLFOX, or FOLFIRI) based on physician choice in untreated metastatic CRC patients aged ≥ 75 years (median age 80 years).87 They found that the addition of bevacizumab to standard of care chemotherapy was both safe and effective. The adverse events seen with bevacizumab, such as hypertension and thrombotic events, were consistent with prior studies.
A newer antiangiogenic agent, ziv-aflibercept, has been approved for the second-line treatment of metastatic CRC. The VELOUR trial demonstrated that the addition of ziv-aflibercept to FOLFIRI benefited patients across all age groups compared with FOLFIRI plus placebo in patients who had failed prior oxaliplatin-based chemotherapy.88,89 Ramucirumab is a human IgG-1 monoclonal antibody approved in second-line treatment in combination with FOLFIRI. A subgroup analysis of the RAISE study showed that the survival benefit was similar in patients aged ≥ 65 years versus those < 65 years.90 Based on the above data, the use of a VEGF inhibitor in combination with chemotherapy should be considered in older patients with metastatic CRC. Furthermore, based on the conflicting data regarding the benefit of FOLFOX/FOLFIRI over single-agent 5-FU discussed above, the combination of capecitabine plus bevacizumab may be considered a front-line treatment option in older patients based on the AVEX study.
EGFR Inhibitors
Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) antibodies approved for the treatment of RAS wild-type metastatic CRC. Data regarding the use of EGFR inhibitors in the geriatric population is scarce and the data that does exist is conflicting.91,92 The PRIME study demonstrated that panitumumab plus FOLFOX had a PFS benefit compared to FOLFOX alone in KRAS wild-type metastatic CRC patients.92 While the study met its primary endpoint, the benefit did not translate to patients aged ≥ 65 years in subgroup analysis. Conversely, a retrospective study of the efficacy and safety of cetuximab in elderly patients with heavily pretreated metastatic CRC found similar efficacy in older and younger patients as well as no increased adverse events in the older population.91 A phase 2 trial investigating cetuximab as single-agent first-line treatment of metastatic CRC in fit older patients found cetuximab to be safe with moderate activity in this population, but did not support the use of cetuximab as first-line single-agent treatment in fit geriatric patients who may be candidates for combination therapy.93 Our group studied the patterns of use and tolerance of anti-EGFR antibodies in 117 older adults with metastatic CRC with a median age of 73 years.94 The study showed that older age at the time of treatment was associated with administration of anti-EGFR antibody as monotherapy rather than in combination with chemotherapy (P = 0.0009). We found no association between age and presence of grade 3 or higher toxicity. In addition, the toxicity profile seen in older patients was similar to what has been demonstrated in prior studies involving a younger patient population. Given the discordance seen between studies, additional prospective trials are needed to elucidate the efficacy and safety of EGFR inhibitors in the geriatric population.
Other Agents
Two newer agents approved in the treatment of metastatic CRC are regorafenib, a multikinase inhibitor, and trifluridine/tipiracil (TFD/TPI), a nucleoside analog combined with an inhibitor of thymidine phosphorylase. The phase 3 CORRECT trial studied regorafenib as monotherapy in previously treated metastatic CRC and found an OS benefit of 1.4 months and minimal PFS benefit.95 Van Cutsem et al performed a subgroup analysis by age and found similar OS benefit in patients < 65 years of age and ≥ 65 years.96 The most frequent adverse events grade 3 or higher were hand-foot syndrome, fatigue, diarrhea, hypertension, and desquamation/rash, which were seen at similar rates in both age groups. More recently, the phase 2 Regorafenib Dose Optimization Study (ReDOS) found that weekly dose escalation of regorafenib from 80 mg to 160 mg daily over 3 weeks was superior to the standard 160 mg daily dosing in patients with metastatic CRC.97 The dose escalation group had a longer median OS, although this difference was not statistically significant, as well as a more favorable toxicity profile. Therefore, this new dosing strategy may be a reasonable option for older patients with pretreated metastatic CRC. A study of TFD/TPI versus placebo in refractory metastatic CRC found an OS benefit of 7.1 months versus 5.3 months.98 In subgroup analyses, the OS benefit extended to both patients < 65 years and ≥ 65 years. Given the sparse data on these newer agents in the geriatric population and the modest benefit they provide to those with refractory metastatic CRC, more data is needed to determine their utility in elderly patients. The decision to use these agents in the older patients warrants a thorough discussion with the patient regarding risks, benefit, and treatment goals.
Immunotherapy
Between 3.5% and 6.5% of stage IV colorectal cancers are MSI-H and have deficient mismatch repair (dMMR).99–101 A recent phase 2 trial studied the use of pembrolizumab, an IgG4 monoclonal antibody against PD-1 (programmed cell death-1), in heavily pretreated patients with dMMR metastatic CRC, MMR-proficient (pMMR) metastatic CRC, and noncolorectal dMMR metastatic cancer.102 Patients with dMMR metastatic CRC had a 50% ORR and 89% disease control rate (DCR), as compared with an ORR of 0% and DCR of 16% in patients with pMMR metastatic CRC. There was also an OS and PFS benefit seen in the dMMR CRC group as compared with the pMMR CRC group. Another phase 2 study, CheckMate 142, studied the anti-PD-1 monoclonal antibody nivolumab with or without ipilimumab (a monoclonal antibody against cytotoxic T-lymphocyte antigen 4) in patients with dMMR and pMMR metastatic CRC.103 In the interim analysis, nivolumab was found to provide both disease control and durable response in patients with dMMR metastatic CRC.
While these studies led to the FDA approval of pembrolizumab and nivolumab for management of previously treated MSI-H or dMMR metastatic CRC, data on the use of immunotherapy in older adults is scarce. Immunosenescence, or the gradual deterioration of the immune system that comes with aging, may impact the efficacy of immune checkpoint inhibitors (ICI) in older patients with advanced cancer.104 There is conflicting data on the efficacy of PD-1 and programmed death ligand-1) PD-L1 inhibitors in older patients across different cancers. A meta-analysis of immunotherapy in older adults with a variety of malignancies showed overall efficacy comparable to that seen in adults younger than 65 years.105 However, another review found ICIs to be less effective in older patients with head and neck, non-small cell lung cancer, and renal cell carcinoma compared with their younger counterparts.104 Regarding the toxicity profile of ICIs in the elderly, similar rates of grade 3 or higher adverse events in patients younger than 65 years and older than 65 years have been reported.106 However, patients aged ≥ 70 years had increased rates of grade 3 to 5 adverse events as compared to patients younger than 65 years (71.7% versus 58.4%, respectively). Given the scant data on ICIs in older patients with MSI-H or dMMR metastatic CRC, more clinical trials inclusive of this population are needed in order to determine the efficacy and safety of immunotherapy.
Palliative Care
The incorporation of palliative care early following the diagnosis of cancer has been shown to improve quality of life, decrease depression, and help with symptom management.107 The triggers for geriatric patients to initiate palliative care may be different from those of younger patients, as older patients may have different goals of care.108 Older patients will often choose quality over quantity of life when making treatment decisions.109 The ideal medical treatment for the frail patient with colorectal cancer would focus on treating disease while providing palliative measures to help support the patient and improve quality of life. It is paramount that patients maintain functional independence as loss of independence is recognized as a major threat to an older patient’s quality of life.110 The optimal way to achieve these goals is through the efforts of a multidisciplinary care team including not only physicians and nurses, but also social workers, nutritionists, physical therapists, and family who can provide support for the patient’s psychosocial, cognitive, and medical needs.111 Although cancer and noncancer–related death occur more frequently in the geriatric population, data to guide a specific palliative care approach to the elderly population is lacking.108
Conclusion
Colorectal cancer is a disease of older adults with a median age at diagnosis of 67 years.1 With the aging population, oncologists will be faced with treating increasing numbers of older patients, and must adjust their practice to accommodate this population of patients. Treating geriatric patients is challenging given the lack of available data to guide the treatment approach. Although several prospective elderly-specific studies have been conducted evaluating treatments for metastatic CRC, most treatment decisions are made based on the available retrospective studies and pooled analyses. Oncologists must carefully consider and evaluate each patient based on physiologic age rather than chronologic age.112 Overall, older patients should be given the opportunity to receive standard of care treatments in the appropriate setting. The decision to modify treatment plans should be made after a thorough evaluation by a multidisciplinary team and a discussion with the patient regarding their goals and the risks and benefits of the treatment. Geriatric assessment tools can help the care team identify patients with various geriatric syndromes that may not be detected on routine oncology evaluation. This type of evaluation is time consuming and is rarely done in a busy oncology practice. Ongoing studies are aiming to develop a method to incorporate geriatric assessments into the care of older adults.Additional prospective trials targeting older, more frail patients are essential to improve upon our knowledge so we can provide best care for this growing elderly population.
1. National Cancer Institute. SEER cancer stat facts: colorectal cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed March 1, 2018.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
3. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017:1-8.
4. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States, current population reports, P25-1140. Washington, DC: U.S. Census Bureau; 2014.
5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7.
6. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4.
7. Vijayvergia N, Li T, Wong YN, et al. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer 2016;122:3191–8.
8. Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435–44.
9. National Comprehensive Cancer Network. Older adult oncology (Version 2.2017). Accessed March 1, 2018,
10. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72.
11. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J 2014;20:358–66.
12. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
13. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
14. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
15. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
16. Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
17. Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 2004;52:1681–7.
18. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7.
19. Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218–26.
20. Martucci RB, Barbosa MV, D’Almeida CA, et al. Undernutrition as independent predictor of early mortality in elderly cancer patients. Nutrition 2017;34:65–70.
21. Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. J Clin Oncol 2014;32:2627–34.
22. Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore 2005;34:250–6.
23. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.
24. Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013;11:1494-502.
25. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300.
26. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014; 9:e115060.
27. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg 2009;13:100–4.
28. Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: surgical treatment and long-term survival. Int J Colorectal Dis 2008;23:601–10.
29. Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg 2008;195:344–8.
30. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–74.
31. Shalaby M, Di Lorenzo N, Franceschilli L, et al. Outcome of colorectal surgery in elderly populations. Ann Coloproctol 2016;32:139–43.
32. Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300.
33. PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63.
34. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76.
35. de Liguori Carino N, van Leeuwen BL, Ghaneh P, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol 2008;67:273–8.
36. Tamandl D, Gruenberger B, Herberger B, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol 2009;100:364–71.
37. Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015–21.
38. Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215–21.
39. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
40. Martling A, Holm T, Johansson H, et al, Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902.
41. Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–23.
42. Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735–41.
43. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–13.
44. van der Valk M. The International Watch & Wait database (IWWD) for rectal cancer: An update. J Clin Oncol 2017;35 suppl:521.
45. Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol 2003;45:305–11.
46. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–33.
47. McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 2014;32:2570–80.
48. Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204–20.
49. Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.
50. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
51. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
52. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010;36:550–6.
53. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
54. Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
55. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
56. Aparicio T, Schischmanoff O, Poupardin C, et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis 2013;45:245–50.
57. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7.
58. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
59. Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
60. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
61. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
62. Haller DG, Cassidy J, Tabernero J, et al. Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): impact of age on disease-free survival (DFS) [abstract]. J Clin Oncol 2010;28:3521.
63. Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
64. McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
65. Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
66. Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24.
67. Aparicio T, Francois E, Cristol-Dalstein L, et al. PRODIGE 34-FFCD 1402-ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig Liver Dis 2016;48:206–7.
68. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
69. Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000;135:182–5.
70. Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
71. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol 2004;15:1330–8.
72. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1–9.
73. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
74. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749–59.
75. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400.
76. Figer A, Perez-Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007;110:2666–71.
77. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33.
78. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008;26:1443–51.
79. Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology 2005;69:384–90.
80. Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol 2016;27:121–7.
81. Aparicio T, Gargot D, Teillet L, et al. Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108.
82. Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
83. Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 2010;136:737–43.
84. Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
85. Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology 2010;78:329–39.
86. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85.
87. Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol 2018;29:133–8.
88. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506.
89. Ruff P, Van Cutsem E, Lakomy R, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–9.
90. Obermannova R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 2016;27:2082–90.
91. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol 2008;67:255-62.
92. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55.
93. Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339–45.
94. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer 2014;13:192–8.
95. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12.
96. Van Cutsem E, Sobrero A, Siena S, et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial [abstract]. J Clin Oncol 2013;31(15 suppl):3636-3636.
97. Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): an ACCRU Network study [abstract]. J Clin Oncol 2018;36(4 suppl):611-611.
98. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19.
99. Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73.
100. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30.
101. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
102. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20.
103. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.
104. Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155–66.
105. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26.
106. Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers [abstract]. J Clin Oncol 2016;34(15 suppl):10010-10010.
107. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41.
108. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014;5:197–203.
109. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer 2008;113:3459–66.
110. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82.
111. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center. Clin J Oncol Nurs 2007;11:929–33.
112. Sheridan J, Walsh P, Kevans D, et al. Determinants of short- and long-term survival from colorectal cancer in very elderly patients. J Geriatr Oncol 2014;5:376–83.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the United States and has a high prevalence among the older population.1 In 2017, there were an estimated 135,430 new cases of CRC and 50,260 deaths due to CRC. It is the second leading cause of cancer death in the United States, and the death rate for patients with CRC increases with age (Figure).2
Although elderly persons are more frequently diagnosed with CRC, they are underrepresented in clinical trials. This may be due in part to stringent eligibility criteria in prospective randomized controlled trials that exclude older patients with certain comorbidities and decreased functional status. Hutchins and colleagues compared the proportion of persons aged 65 years and older enrolled in Southwest Oncology Group (SWOG) clinical trials and the proportion of persons in this age group in the US population with the same cancer diagnoses.5 They found that while 72% of the US population with CRC were aged ≥ 65 years, persons in this age group comprised only 40% of patients enrolled in SWOG trials. An update on this study performed after Medicare policy changed in 2000 to include coverage of costs incurred due to clinical trials showed an upward trend in the accrual of older patients in SWOG trials, from 25% during the period 1993–1996 to 38% during the period 2001–2003; however, the percentage of older patients with CRC on clinical trials overall remained stable from 1993 to 2003.6
The underrepresentation of older adults with CRC in clinical trials presents oncologists with a challenging task when practicing evidence-based medicine in this patient population. Analysis of a large claims database demonstrated that the use of multi-agent chemotherapy for the treatment of metastatic CRC in older adults increased over time, while the use of single-agent 5-fluorouracil (5-FU) decreased.7 However, the adoption of combination therapy with irinotecan or oxaliplatin in older adults lagged behind the initial adoption of these agents in younger patients. This data demonstrates that as the field of medical oncology evolves, providers are becoming more comfortable treating older patients with multiple medical problems using standard approved regimens.
Geriatric Assessment
Before treating older patients with cancer, it is necessary to define the patient’s physiological age, ideally through a multidisciplinary team evaluation.
The Eastern Cooperative Oncology Group performance status (ECOG PS) and Karnofsky Performance Status (KPS) are crude measures of functional status.12 Generally, elderly patients with good ECOG PS or KPS scores are considered fit enough to receive standard therapy similar to their younger counterparts. Evaluation of functional status using these performance scores is often suboptimal, resulting in patients with a normal or adequate performance status score who may still experience poor outcomes, including decreased survival and inability to tolerate treatment. A study that explored parameters among older patients that predict for increased risk of chemotherapy-related toxicities found that physician-rated KPS score did not accurately predict the risk for adverse events.13 Therefore, a CGA represents a better way to evaluate functional status and other domains.
Functional status can also be evaluated by self-reported tools such as activities of daily living, which refer to basic self-care, and instrumental activities of daily living (IADLs), which are essential for independent living in the community.14,15 Mobility, gait, and balance can also be measured using the “Timed Get Up and Go” test and gait speed. Klepin et al found that faster gait speed was associated with overall survival (OS) in patients with metastatic cancer.16
Cognitive function is an important component of the geriatric assessment in older patients with cancer, as dementia is a prognostic factor for survival in the overall geriatric population. In a retrospective review, patients with dementia were less likely to have a biopsy-proven diagnosis and were twice as likely to have their CRC diagnosed postmortem.17 In addition, establishing that the patient has intact cognitive function prior to initiating treatment is essential to ensure that the patient can comply with treatment and understands when to report adverse effects. Nutritional status is an important portion of the geriatric assessment because malnutrition is associated with increased mortality and decreased tolerance for chemotherapy.18–20 Evaluating the patient’s psychosocial support is crucial as well because older patients are at greater risk of social isolation and depression.21 While the incidence of depression is lower in older adults with cancer than in younger adults with cancer, clinically significant depression is still noted in 3% to 25% of elderly cancer patients.22 Other critical components of the CGA are review of the patient’s comorbidities and medications to avoid complications of polypharmacy.
Both the Cancer and Aging Research Group (CARG) and Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) toxicity tools are valuable tools, as they predict chemotherapy tolerance in elderly patients.13,23 These tools can help guide discussions between oncologists and patients as well as the formulation of an appropriate treatment plan.24 Although toxicity tools can help to determine which patients are at risk for severe toxicity secondary to treatment, these tools do not replace the CGA. A prospective cohort study that evaluated the impact of CGA on tolerance to chemotherapy in older patients with cancer compared patients aged ≥ 70 years at the start of their treatment with chemotherapy (± radiation therapy) using geriatrician-delivered CGA versus standard care given by oncology.8 Patients who received geriatrician-guided CGA interventions tolerated chemotherapy better and completed treatments as planned (odds ratio 4.14 [95% confidence interval {CI} 1.50 to 11.42], P = 0.006) with fewer treatment modifications.
Unfortunately, the CGA is time-consuming to administer and difficult to incorporate into a busy oncology practice. Therefore, other screening models are used to identify patients who may benefit from a full CGA. The International Society of Geriatric Oncology performed a systematic review of screening tools used to identify older cancer patients in need of geriatric assessment and found that the 3 most studied screening tools are the G8, the Vulnerable Elders Survey-13 (VES-13), and the Flemish version of the Triage Risk Screening Tool.25 Another study found that the G8 was more sensitive than the VES-13 (76.5% versus 68.7%, P = 0.0046), whereas the VES-13 was more specific than the G8 (74.3% versus 64.4%, P < 0.0001).26 In addition to providing guidance to initiate a full geriatric assessment, these screening tools may assist in decision making for older cancer patients, especially those with advanced disease.
Surgery
Early-Stage Disease
When possible, surgical resection of colorectal tumors is the primary treatment in both the curative setting and to avoid complications, such as obstruction or perforation.27 Multiple studies have shown that fit elderly patients benefit from curative surgery similarly to their younger counterparts.27–29 With the growing population of persons aged 65 years or older, surgeons are becoming more comfortable with operating on the elderly.4 However, a large systematic review of 28 independent studies with a total of 34,194 patients showed that older patients were less likely to undergo curative surgery.30 Eligibility for surgery should not be determined by age alone, but rather should be based on a full assessment of the patient’s health, including comorbidities, functional status, nutrition, cognition, social support, and psychological status. The impact of age on short-term outcomes after colorectal surgery in terms of 30-day postoperative morbidity and mortality rates was explored in a study that divided patients into 2 groups: those aged ≥ 80 years (mean age 85) and those aged < 80 years (mean age 55.3).31 There were no statistical differences in 30-day postoperative morbidity and mortality rates between the 2 groups, and preexisting comorbidities and urgent nature of surgery were important predictors of colorectal surgery outcomes in the older adults, results that have been seen in several other studies.28,30 When possible, laparoscopic surgery is preferred as it is associated with less intraoperative blood loss, less postoperative pain, reduced postoperative ileus, a shorter hospital stay, and fewer cardiovascular and pulmonary complications.32 The Preoperative Assessment of Cancer in the Elderly (PACE), which combines surgical risk assessment tools with CGA tools, can assist surgeons in determining candidacy for surgery and help decrease unequal access to surgery in the geriatric population.33
Metastasectomy
A large international multicenter cohort study explored the outcomes of patients aged ≥ 70 years who underwent liver resection of colorectal metastases. The study investigatorsfound that neoadjuvant chemotherapy was used less frequently and less extensive surgery was performed in elderly patients than in younger patients.34 Sixty-day postoperative mortality was slightly higher (3.8% versus 1.6%, P < 0.001) and 3-year OS was slightly lower (57.1% versus 60.2%, P < 0.001) in the elderly group as compared to their younger counterparts, but overall the outcomes after liver surgery were similar. Therefore, the management of liver metastases in oligometastatic disease in elderly patients fit for surgery should be the same as that offered to younger patients. Since outcomes are comparable, older patients should be offered neoadjuvant chemotherapy, as several studies have shown similar response rates and OS in younger and older patients.35,36
Rectal Cancer
The standard of care for locally advanced rectal cancer is combined modality treatment with radiation and chemotherapy followed by total mesorectal excision. However, given conflicting data regarding the ability of elderly patients to tolerate neoadjuvant 5-FU-based chemotherapy and radiation, elderly patients are treated with trimodality therapy less often than their younger counterparts.37,38 A systematic review of 22 randomized trials involving 8507 patients with rectal cancer showed that adjuvant radiation therapy could reduce the risk of local recurrence and death from rectal cancer in patients of all ages.39 However, the risk of noncancer-related death was increased in the older population. The Stockholm II trial showed similar benefits of preoperative radiation overall, but this benefit did not extend to patients older than 68 years because of an increased risk of morbidity and mortality.40 In older patients, mortality from noncancer causes within the first 6 months after surgery was higher in the group that received perioperative radiation than in the group that did not receive radiation. Elderly patients (age > 68 years) accounted for most of the mortality, which was predominantly due to cardiovascular disease.
A retrospective study of 36 patients aged ≥ 70 years with rectal cancer evaluated the toxicity and feasibility of neoadjuvant 5-FU combined with pelvic radiation for treating locally advanced rectal cancer. Patients were classified as healthy and “fit” or “vulnerable” based on the presence of comorbidities.41 This study demonstrated that tolerability and response to neoadjuvant chemotherapy and radiation as well as ability to undergo surgery were similar in “vulnerable” patients and “fit” patients. Conversely, Margalit and colleagues studied the rate of treatment deviations in elderly patients with rectal cancer treated with combined modality therapy and found that most patients required early termination of treatment, treatment interruptions, or dose reductions.42 While trimodality treatment is the standard of care in rectal cancer, there is conflicting data from retrospective studies regarding the tolerability and feasibility of this approach. It is important to proceed with caution but to still consider fit older patients with locally advanced rectal cancer for neoadjuvant chemotherapy and radiation followed by surgery.
In patients who have a complete response (CR) to neoadjuvant chemoradiation, watchful waiting rather than proceeding to surgery may be a reasonable strategy, especially in older patients. A systematic review of 867 patients with locally advanced rectal cancer showed no statistically significant difference in OS between patients who were observed with watchful waiting and those who underwent surgery.43 The International Watch and Wait Database includes 679 patients who were managed with a watch-and-wait regimen because they had a clinical CR after chemoradiation. An outcomes analysis of these patients showed that 25% had local regrowth, with 3-year OS of 91% overall and 87% in patients with local regrowth.44 In most patients (84%), regrowth of the tumor occurred within the first 2 years of follow up.
In frail older adults, for whom longer courses of treatment are not feasible or chemotherapy is contraindicated, short-course radiation therapy can be considered either in the neoadjuvant setting or alone for palliation.45 A randomized trial of short-course radiation versus long-course chemoradiation in patients with T3 rectal cancer found that the difference in 3-year local recurrence rates was not statistically significant.46
Chemotherapy
An expected natural decline in function occurs with age, but given the great variability that exists between patients, it is important to focus on physiologic age rather than chronologic age to determine ability to receive and tolerate anticancer treatment. Decreases in renal and hepatic function, cognitive impairment, changes in gastrointestinal motility, decrements in cardiac and bone marrow reserves, as well as comorbidities and polypharmacy affect a patient’s ability to tolerate chemotherapy.47,48 Toxicity tools such as CARG and CRASH can help to predict severity of toxicity with chemotherapy.13,23 The information provided by these tools can help guide conversations between the oncologist and patient regarding treatment plans.
Adjuvant Chemotherapy for Early-Stage Disease
Stage II Disease
Defining treatment guidelines for older patients with stage II colon cancer is difficult due to lack of data that shows benefit in this population. The QUASAR (Quick and Simple and Reliable) group’s prospective study of adjuvant single-agent 5-FU in stage II colon cancer patients showed an absolute improvement in survival of 3.6% when 5-FU was given after surgery (95% CI 1.0 to 6.0).49 The subgroup analysis of patients aged ≥ 70 years showed a limited benefit of adjuvant 5-FU (hazard ratio [HR] 1.13 [95% CI 0.74 to 1.75]). Given the limited benefit, adjuvant 5-FU for elderly patients with stage II colon cancer should be used judiciously as patients may have competing causes of morbidity or mortality.
The use of oxaliplatin-based therapy in the adjuvant setting for stage II disease was evaluated in a subgroup analysis of the MOSAIC study (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer).50 Adjuvant oxaliplatin-based treatment may be offered to patients with stage II colon cancer that carries high-risk features (poorly differentiated histology, lymphovascular invasion, bowel obstruction and/or perforation, < 12 lymph nodes sampled, perineural invasion, or indeterminate or positive margins) due to a trend toward improved disease-free survival (DFS) at 5 years. Patients in this group who received adjuvant FOLFOX (leucovorin, oxaliplatin, 5-FU) versus 5-FU/leucovorin had a DFS of 82.3% versus 74.6%, respectively (HR 0.72 [95% CI 0.50 to 1.02]), a difference that was not statistically significant. A subgroup analysis of 315 patients aged 70 to 75 years with stage II colon cancer enrolled in the MOSAIC study found no statistically significant DFS or OS benefit with the addition of oxaliplatin to 5-FU/leucovorin.51 Therefore, use of this platinum/fluoropyrimidine combination for adjuvant therapy for high-risk stage II disease in older patients remains controversial given its associated risks and the lack of definitive data demonstrating a benefit in this patient group. Decisions regarding this therapy should be made through a shared discussion with patients about its risks and benefits.
Microsatellite status is an important biomarker in the evaluation of stage II CRC. Microsatellite stability is a marker of a functioning DNA mismatch repair system. In patients with colon cancer, tumor microsatellite stability is classified based on the percentage of abnormal microsatellite regions.52 Several studies have shown that patients with tumors that display high microsatellite instability (MSI-H) have an improved prognosis over patients with microsatellite stable tumors.53,54 While patients with stage II MSI-H colon cancer have better outcomes, MSI is associated with a reduced response to treatment with fluoropyrimidines, as demonstrated in a systematic review that found that patients with tumors with MSI obtained no benefit from adjuvant 5-FU (HR 1.24 [95% CI 0.72 to 2.14]).55 Aparicio and colleagues reported an increased prevalence of MSI-H tumors with increasing age.56 Therefore, mismatch repair phenotype should be considered when making adjuvant chemotherapy decisions in the older adult with colon cancer, as it may affect the decision to recommend single-agent 5-FU treatment.
Stage III Disease
The use of single-agent 5-FU for stage III resected CRC has been evaluated in multiple studies. Sargent et al performed a pooled analysis of 3351 patients from 7 randomized phase 3 trials comparing surgery and adjuvant 5-FU-based chemotherapy versus surgery alone in stage II or III colon cancer patients.57 Adjuvant chemotherapy was associated with improvement in both OS and time to tumor recurrence (HR 0.76 and 0.68, respectively). The 5-year OS was 71% for those who received adjuvant treatment and 64% for those who were treated with surgery alone. The benefit of adjuvant treatment was independent of age, and there was no difference in toxicity across age groups, except for 1 study which showed increased rates of leukopenia in the elderly. The oral fluoropyrimidine capecitabine was shown to be an effective alternative to 5-FU plus leucovorin as adjuvant treatment for those with resected stage III colon cancer.58 However, in the subgroup analysis of DFS in the intention-to-treat group, the improvement in DFS was not statistically significant in those aged ≥ 70 years. This study justified the phase 3 Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial, which compared capecitabine and 5-FU/leucovorin as adjuvant therapy in patients with resected stage III colon cancer.59 The X-ACT trial showed no significant effect of age on DFS or OS.
The addition of oxaliplatin to 5-FU in the adjuvant setting for stage III tumors has been studied and debated in the elderly population in multiple trials. The MOSAIC trial investigated FOLFOX versus 5-FU/leucovorin in the adjuvant setting.50 The addition of oxaliplatin was associated with a DFS and OS benefit, with a 20% reduction in risk of colon cancer recurrence and 16% reduction in risk of death in all patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial then studied 2409 patients with stage II or III colon cancer treated with weekly bolus 5-FU/leucovorin with or without oxaliplatin.60 In this study, OS was significantly improved with the addition of oxaliplatin in patients younger than 70 years, but OS at 5 years was 4.7% worse for patients aged ≥ 70 years treated with weekly 5-FU/leucovorin and oxaliplatin compared with those treated with weekly 5-FU/leucovorin (71.6% versus 76.3%, respectively). In contrast, the XELOXA trial (NO16968), which randomly assigned stage III colon cancer patients to capecitabine and oxaliplatin (XELOX) or bolus 5-FU/leucovorin (standard of care at study start), showed an efficacy benefit, albeit not statistically significant, in patients aged ≥ 70 years (HR 0.87 [95% CI 0.63 to 1.18]).61–63
The Adjuvant Colon Cancer Endpoints (ACCENT) database included 7 randomized trials totaling 14,528 patients with stage II or III colon cancer treated with adjuvant 5-FU with or without oxaliplatin or irinotecan.64 Subgroup analysis of patients aged ≥ 70 years (n = 2575) showed no benefit with an oxaliplatin-based regimen in DFS (HR 0.94 [95% CI 0.78 to 1.13]) or OS (HR 1.04 [95% CI, 0.85 to 1.27]). Based on these studies and the increased toxicity with oxaliplatin, oxaliplatin-based adjuvant chemotherapy is utilized less often than single-agent 5-FU in geriatric patients with early-stage colon cancer.65 Conversely, a recent pooled analysis of individual patient data from 4 randomized trials (NSABP C-08, XELOXA, X-ACT, and AVANT) showed improved DFS and OS with adjuvant XELOX or FOLFOX over single-agent 5-FU in patients aged ≥ 70 years (DFS HR 0.77 [95% CI 0.62 to 0.95], P = 0.014; OS HR 0.78 [95% CI 0.61 to 0.99], P = 0.045).66 This analysis also showed that grade 3 and 4 adverse events related to oxaliplatin were similar across age groups.
These data come from post-hoc analyses, and there is no prospective data to steer decision making in elderly patients with early-stage CRC (Table).
It is well established that patients with stage III colon cancer benefit from oxaliplatin-based adjuvant chemotherapy after curative surgical resection.68 However, older patients are less likely to be referred to oncology as compared with their younger counterparts, due to the conflicting data regarding the benefit of this approach in older adults. Studies have shown that when the referral is placed, the geriatric population is less likely to receive chemotherapy.69 Sanoff et al analyzed 4 data sets (SEER-Medicare, National Comprehensive Cancer Network, New York State Cancer Registry, and Cancer Care Outcomes Research and Surveillance Consortium) to assess the benefit of adjuvant chemotherapy for resected stage III CRC among patients aged ≥ 75 years. Their analysis showed that only 40% of patients evaluated received adjuvant chemotherapy for stage III CRC after surgical resection.70
Summary
Prospective data to guide the treatment of older patients with early-stage CRC in the adjuvant setting is lacking. For fit older patients with stage II disease, limited benefit will be derived from single-agent 5-FU. For those with stage III CRC, the benefit and toxicities of fluoropyrimidines as adjuvant therapy appear to be similar regardless of age. The addition of oxaliplatin to fluoropyrimidines in patients aged ≥ 70 years has not been proven to improve DFS or OS and could result in an incremental toxicity profile. Therefore, treatment plans must be individualized, and decisions should be made through an informed discussion evaluating the overall risk/benefit ratio of each approach.
Metastatic Disease
Palliative Chemotherapy
Approximately 20% of patients with CRC are diagnosed with metastatic disease at presentation, and 35% to 40% develop metastatic disease following surgery and adjuvant therapy.2 The mainstay of treatment in this population is systemic therapy in the form of chemotherapy with or without biologic agents. In this setting, several prospective studies specific to older adults have been completed, providing more evidence-based guidance to oncologists who see these patients. Folprecht et al retrospectively reviewed data from 22 clinical trials evaluating 5-FU-based palliative chemotherapy in 3825 patients with metastatic CRC, including 629 patients aged ≥ 70 years.71 OS in elderly patients (10.8 months [95% CI 9.7 to 11.8]) was equivalent to that in younger patients (11.3 months [95% CI 10.9 to 11.7], P = 0.31). Similarly, relative risk and progression-free survival (PFS) were comparable irrespective of age.
Standard of care for most patients with metastatic colon cancer consists of 5-FU/leucovorin in combination with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with a monoclonal antibody.72 A retrospective pooled analysis of patients with metastatic CRC compared the safety and efficacy of FOLFOX4 in patients aged < 70 years versus those aged ≥ 70 years.73 While age ≥ 70 years was associated with an increased rate of grade ≥ 3 hematologic toxicity, it was not associated with increased rates of severe neurologic events, diarrhea, nausea, vomiting, infection, 60-day mortality, or overall incidence of grade ≥ 3 toxicity. The benefit of treatment was consistent across both age groups; therefore, age alone should not exclude an otherwise healthy individual from receiving FOLFOX.
These post-hoc analyses show that fit older patients who were candidates for trial participation tolerated these treatments well; however, these treatments may be more challenging for less fit older adults. The UK Medical Research Council FOCUS2 (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing) study was a prospective phase 3 trial that included 459 patients with metastatic CRC who were deemed too frail or not fit enough for standard-dose chemotherapy by their oncologists.74 In this group, 43% of patients were older than 75 years and 13% were older than 80 years. Patients were randomly assigned to receive infusional 5-FU with levofolinate; oxaliplatin and 5-FU; capecitabine; or oxaliplatin and capecitabine; all regimens were initiated with an empiric 20% dose reduction. The addition of oxaliplatin suggested some improvement in PFS, but this was not significant (5.8 months versus 4.5 months, HR 0.84 [95% CI 0.69 to 1.01], P = 0.07). Oxaliplatin was not associated with increased grade 3 or 4 toxicities. Capecitabine is often viewed as less toxic because it is taken by mouth, but this study found that replacement of 5-FU with capecitabine did not improve quality of life. Grade 3 or 4 toxicities were seen more frequently in those receiving capecitabine than in those receiving 5-FU (40% versus 30%, P = 0.03) in this older and frailer group of patients. As the patients on this study were frail and treatment dose was reduced, this data may not apply to fit older adults who are candidates for standard therapy.
When managing an older patient with metastatic CRC, it is important to tailor therapy based on goals of care, toxicity of proposed treatment, other comorbidities, and the patient’s functional status. One approach to minimizing toxicity in the older population is the stop-and-go strategy. The OPTIMOX1 study showed that stopping oxaliplatin after 6 cycles of FOLFOX7 and continuing maintenance therapy with infusional 5-FU/leucovorin alone for 12 cycles prior to reintroducing FOLFOX7 achieved efficacy similar to continuous FOLFOX4 with decreased toxicity.75 Figer et al studied an exploratory cohort of 37 patients aged 76 to 80 years who were included in the OPTIMOX1 study.76 The overall relative risk, median PFS, and median OS did not differ between the older patients in this cohort and younger patients studied in the original study. Older patients did experience more neutropenia, neurotoxicity, and overall grade 3 to 4 toxicity, but there were no toxic deaths in patients older than 75 years. The approach of giving treatment breaks, as in OPTIMOX2, may also provide patients with better quality of life, but perhaps at the expense of cancer-related survival.77
The combination of irinotecan and 5-FU has also been studied as treatment for patients with metastatic CRC. A pooled analysis of 2691 patients aged ≥ 70 years with metastatic CRC across 4 phase 3 randomized trials investigating irinotecan and 5-FU demonstrated that irinotecan-containing chemotherapy provided similar benefits to both older and younger patients with similar risk of toxicity.78 A phase 2 trial studying FOLFIRI as first-line treatment in older metastatic CRC patients showed this to be a safe and active regimen with manageable toxicity.79 Another randomized phase 3 trial for older patients compared 5-FU/leucovorin with or without irinotecan for first-line treatment of metastatic CRC (FFCD 2001-02).80 The study accrued 282 patients aged ≥ 75 years (median age 80 years), and found that the addition of irinotecan to infusional 5-FU–based chemotherapy did not significantly increase either PFS or OS. Aparicio et al performed a substudy of baseline geriatric evaluation prior to treatment in the FFCD 2001-02 study and assessed the value of geriatric parameters for predicting outcomes (objective response rate [ORR], PFS, and OS).81 Multivariate analysis showed that none of the geriatric parameters were predictive of ORR or PFS but that normal IADL was associated with better OS. This combination may still be appropriate for some older patients with metastatic disease, while single- agent 5-FU may be more appropriate in frail patients.
Biologic Agents
VEGF Inhibitors
Targeted biologic agents have been studied in the treatment of metastatic CRC. Bevacizumab is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is approved in the first-line setting for treatment of metastatic CRC. A pooled analysis examined 439 patients 65 years of age and older with metastatic CRC who received bevacizumab plus chemotherapy versus placebo plus chemotherapy.82 In this analysis, the addition of bevacizumab was associated with an improvement in OS (19.3 months versus 14.3 months, HR 0.7 [95% CI 0.55 to 0.90], P = 0.006) and in PFS (9.2 months versus 6.2 months, HR 0.52 [95% CI 0.40 to 0.67], P < 0.0001). Known adverse events associated with bevacizumab were seen in the bevacizumab plus chemotherapy group but not at increased rates in the older population compared to their younger counterparts. Conversely, another pooled analysis found that while there was a PFS and OS benefit in older patients receiving bevacizumab, there was an increased incidence of thrombotic events in patients older than 65 years.83 The BEAT (Bevacizumab Expanded Access Trial) and BRiTE (Bevacizumab Regimens Investigation of Treatment Effects) studies showed similar clinical outcomes across all age groups.84,85 While older patients experienced more arterial thromboembolic events with the addition of bevacizumab, other factors such as ECOG PS, prior anticoagulation, and history of arterial disease were more predictive of these adverse events than age.
The randomized phase 3 AVEX study explored the efficacy and tolerability of capecitabine plus bevacizumab versus capecitabine alone in 280 frail patients aged ≥ 70 years.86 PFS in the capecitabine/bevacizumab arm was 9.1 months versus 5.1 months in the capecitabine alone arm. While the OS difference was not statistically significant, patients in the capecitabine/bevacizumab arm had an OS of 20.7 months versus 16.8 months in the capecitabine alone group. As reported in prior studies, patients in the capecitabine/bevacizumab arm had increased rates of toxic events (40%) compared with those who received capecitabine alone (22%), with reports of hypertension, hand-foot syndrome, bleeding, and thrombotic events. More recently, the phase 2 PRODIGE 20 trial studied the addition of bevacizumab to chemotherapy (5-FU, FOLFOX, or FOLFIRI) based on physician choice in untreated metastatic CRC patients aged ≥ 75 years (median age 80 years).87 They found that the addition of bevacizumab to standard of care chemotherapy was both safe and effective. The adverse events seen with bevacizumab, such as hypertension and thrombotic events, were consistent with prior studies.
A newer antiangiogenic agent, ziv-aflibercept, has been approved for the second-line treatment of metastatic CRC. The VELOUR trial demonstrated that the addition of ziv-aflibercept to FOLFIRI benefited patients across all age groups compared with FOLFIRI plus placebo in patients who had failed prior oxaliplatin-based chemotherapy.88,89 Ramucirumab is a human IgG-1 monoclonal antibody approved in second-line treatment in combination with FOLFIRI. A subgroup analysis of the RAISE study showed that the survival benefit was similar in patients aged ≥ 65 years versus those < 65 years.90 Based on the above data, the use of a VEGF inhibitor in combination with chemotherapy should be considered in older patients with metastatic CRC. Furthermore, based on the conflicting data regarding the benefit of FOLFOX/FOLFIRI over single-agent 5-FU discussed above, the combination of capecitabine plus bevacizumab may be considered a front-line treatment option in older patients based on the AVEX study.
EGFR Inhibitors
Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) antibodies approved for the treatment of RAS wild-type metastatic CRC. Data regarding the use of EGFR inhibitors in the geriatric population is scarce and the data that does exist is conflicting.91,92 The PRIME study demonstrated that panitumumab plus FOLFOX had a PFS benefit compared to FOLFOX alone in KRAS wild-type metastatic CRC patients.92 While the study met its primary endpoint, the benefit did not translate to patients aged ≥ 65 years in subgroup analysis. Conversely, a retrospective study of the efficacy and safety of cetuximab in elderly patients with heavily pretreated metastatic CRC found similar efficacy in older and younger patients as well as no increased adverse events in the older population.91 A phase 2 trial investigating cetuximab as single-agent first-line treatment of metastatic CRC in fit older patients found cetuximab to be safe with moderate activity in this population, but did not support the use of cetuximab as first-line single-agent treatment in fit geriatric patients who may be candidates for combination therapy.93 Our group studied the patterns of use and tolerance of anti-EGFR antibodies in 117 older adults with metastatic CRC with a median age of 73 years.94 The study showed that older age at the time of treatment was associated with administration of anti-EGFR antibody as monotherapy rather than in combination with chemotherapy (P = 0.0009). We found no association between age and presence of grade 3 or higher toxicity. In addition, the toxicity profile seen in older patients was similar to what has been demonstrated in prior studies involving a younger patient population. Given the discordance seen between studies, additional prospective trials are needed to elucidate the efficacy and safety of EGFR inhibitors in the geriatric population.
Other Agents
Two newer agents approved in the treatment of metastatic CRC are regorafenib, a multikinase inhibitor, and trifluridine/tipiracil (TFD/TPI), a nucleoside analog combined with an inhibitor of thymidine phosphorylase. The phase 3 CORRECT trial studied regorafenib as monotherapy in previously treated metastatic CRC and found an OS benefit of 1.4 months and minimal PFS benefit.95 Van Cutsem et al performed a subgroup analysis by age and found similar OS benefit in patients < 65 years of age and ≥ 65 years.96 The most frequent adverse events grade 3 or higher were hand-foot syndrome, fatigue, diarrhea, hypertension, and desquamation/rash, which were seen at similar rates in both age groups. More recently, the phase 2 Regorafenib Dose Optimization Study (ReDOS) found that weekly dose escalation of regorafenib from 80 mg to 160 mg daily over 3 weeks was superior to the standard 160 mg daily dosing in patients with metastatic CRC.97 The dose escalation group had a longer median OS, although this difference was not statistically significant, as well as a more favorable toxicity profile. Therefore, this new dosing strategy may be a reasonable option for older patients with pretreated metastatic CRC. A study of TFD/TPI versus placebo in refractory metastatic CRC found an OS benefit of 7.1 months versus 5.3 months.98 In subgroup analyses, the OS benefit extended to both patients < 65 years and ≥ 65 years. Given the sparse data on these newer agents in the geriatric population and the modest benefit they provide to those with refractory metastatic CRC, more data is needed to determine their utility in elderly patients. The decision to use these agents in the older patients warrants a thorough discussion with the patient regarding risks, benefit, and treatment goals.
Immunotherapy
Between 3.5% and 6.5% of stage IV colorectal cancers are MSI-H and have deficient mismatch repair (dMMR).99–101 A recent phase 2 trial studied the use of pembrolizumab, an IgG4 monoclonal antibody against PD-1 (programmed cell death-1), in heavily pretreated patients with dMMR metastatic CRC, MMR-proficient (pMMR) metastatic CRC, and noncolorectal dMMR metastatic cancer.102 Patients with dMMR metastatic CRC had a 50% ORR and 89% disease control rate (DCR), as compared with an ORR of 0% and DCR of 16% in patients with pMMR metastatic CRC. There was also an OS and PFS benefit seen in the dMMR CRC group as compared with the pMMR CRC group. Another phase 2 study, CheckMate 142, studied the anti-PD-1 monoclonal antibody nivolumab with or without ipilimumab (a monoclonal antibody against cytotoxic T-lymphocyte antigen 4) in patients with dMMR and pMMR metastatic CRC.103 In the interim analysis, nivolumab was found to provide both disease control and durable response in patients with dMMR metastatic CRC.
While these studies led to the FDA approval of pembrolizumab and nivolumab for management of previously treated MSI-H or dMMR metastatic CRC, data on the use of immunotherapy in older adults is scarce. Immunosenescence, or the gradual deterioration of the immune system that comes with aging, may impact the efficacy of immune checkpoint inhibitors (ICI) in older patients with advanced cancer.104 There is conflicting data on the efficacy of PD-1 and programmed death ligand-1) PD-L1 inhibitors in older patients across different cancers. A meta-analysis of immunotherapy in older adults with a variety of malignancies showed overall efficacy comparable to that seen in adults younger than 65 years.105 However, another review found ICIs to be less effective in older patients with head and neck, non-small cell lung cancer, and renal cell carcinoma compared with their younger counterparts.104 Regarding the toxicity profile of ICIs in the elderly, similar rates of grade 3 or higher adverse events in patients younger than 65 years and older than 65 years have been reported.106 However, patients aged ≥ 70 years had increased rates of grade 3 to 5 adverse events as compared to patients younger than 65 years (71.7% versus 58.4%, respectively). Given the scant data on ICIs in older patients with MSI-H or dMMR metastatic CRC, more clinical trials inclusive of this population are needed in order to determine the efficacy and safety of immunotherapy.
Palliative Care
The incorporation of palliative care early following the diagnosis of cancer has been shown to improve quality of life, decrease depression, and help with symptom management.107 The triggers for geriatric patients to initiate palliative care may be different from those of younger patients, as older patients may have different goals of care.108 Older patients will often choose quality over quantity of life when making treatment decisions.109 The ideal medical treatment for the frail patient with colorectal cancer would focus on treating disease while providing palliative measures to help support the patient and improve quality of life. It is paramount that patients maintain functional independence as loss of independence is recognized as a major threat to an older patient’s quality of life.110 The optimal way to achieve these goals is through the efforts of a multidisciplinary care team including not only physicians and nurses, but also social workers, nutritionists, physical therapists, and family who can provide support for the patient’s psychosocial, cognitive, and medical needs.111 Although cancer and noncancer–related death occur more frequently in the geriatric population, data to guide a specific palliative care approach to the elderly population is lacking.108
Conclusion
Colorectal cancer is a disease of older adults with a median age at diagnosis of 67 years.1 With the aging population, oncologists will be faced with treating increasing numbers of older patients, and must adjust their practice to accommodate this population of patients. Treating geriatric patients is challenging given the lack of available data to guide the treatment approach. Although several prospective elderly-specific studies have been conducted evaluating treatments for metastatic CRC, most treatment decisions are made based on the available retrospective studies and pooled analyses. Oncologists must carefully consider and evaluate each patient based on physiologic age rather than chronologic age.112 Overall, older patients should be given the opportunity to receive standard of care treatments in the appropriate setting. The decision to modify treatment plans should be made after a thorough evaluation by a multidisciplinary team and a discussion with the patient regarding their goals and the risks and benefits of the treatment. Geriatric assessment tools can help the care team identify patients with various geriatric syndromes that may not be detected on routine oncology evaluation. This type of evaluation is time consuming and is rarely done in a busy oncology practice. Ongoing studies are aiming to develop a method to incorporate geriatric assessments into the care of older adults.Additional prospective trials targeting older, more frail patients are essential to improve upon our knowledge so we can provide best care for this growing elderly population.
Introduction
Colorectal cancer (CRC) is the fourth most common cancer in the United States and has a high prevalence among the older population.1 In 2017, there were an estimated 135,430 new cases of CRC and 50,260 deaths due to CRC. It is the second leading cause of cancer death in the United States, and the death rate for patients with CRC increases with age (Figure).2
Although elderly persons are more frequently diagnosed with CRC, they are underrepresented in clinical trials. This may be due in part to stringent eligibility criteria in prospective randomized controlled trials that exclude older patients with certain comorbidities and decreased functional status. Hutchins and colleagues compared the proportion of persons aged 65 years and older enrolled in Southwest Oncology Group (SWOG) clinical trials and the proportion of persons in this age group in the US population with the same cancer diagnoses.5 They found that while 72% of the US population with CRC were aged ≥ 65 years, persons in this age group comprised only 40% of patients enrolled in SWOG trials. An update on this study performed after Medicare policy changed in 2000 to include coverage of costs incurred due to clinical trials showed an upward trend in the accrual of older patients in SWOG trials, from 25% during the period 1993–1996 to 38% during the period 2001–2003; however, the percentage of older patients with CRC on clinical trials overall remained stable from 1993 to 2003.6
The underrepresentation of older adults with CRC in clinical trials presents oncologists with a challenging task when practicing evidence-based medicine in this patient population. Analysis of a large claims database demonstrated that the use of multi-agent chemotherapy for the treatment of metastatic CRC in older adults increased over time, while the use of single-agent 5-fluorouracil (5-FU) decreased.7 However, the adoption of combination therapy with irinotecan or oxaliplatin in older adults lagged behind the initial adoption of these agents in younger patients. This data demonstrates that as the field of medical oncology evolves, providers are becoming more comfortable treating older patients with multiple medical problems using standard approved regimens.
Geriatric Assessment
Before treating older patients with cancer, it is necessary to define the patient’s physiological age, ideally through a multidisciplinary team evaluation.
The Eastern Cooperative Oncology Group performance status (ECOG PS) and Karnofsky Performance Status (KPS) are crude measures of functional status.12 Generally, elderly patients with good ECOG PS or KPS scores are considered fit enough to receive standard therapy similar to their younger counterparts. Evaluation of functional status using these performance scores is often suboptimal, resulting in patients with a normal or adequate performance status score who may still experience poor outcomes, including decreased survival and inability to tolerate treatment. A study that explored parameters among older patients that predict for increased risk of chemotherapy-related toxicities found that physician-rated KPS score did not accurately predict the risk for adverse events.13 Therefore, a CGA represents a better way to evaluate functional status and other domains.
Functional status can also be evaluated by self-reported tools such as activities of daily living, which refer to basic self-care, and instrumental activities of daily living (IADLs), which are essential for independent living in the community.14,15 Mobility, gait, and balance can also be measured using the “Timed Get Up and Go” test and gait speed. Klepin et al found that faster gait speed was associated with overall survival (OS) in patients with metastatic cancer.16
Cognitive function is an important component of the geriatric assessment in older patients with cancer, as dementia is a prognostic factor for survival in the overall geriatric population. In a retrospective review, patients with dementia were less likely to have a biopsy-proven diagnosis and were twice as likely to have their CRC diagnosed postmortem.17 In addition, establishing that the patient has intact cognitive function prior to initiating treatment is essential to ensure that the patient can comply with treatment and understands when to report adverse effects. Nutritional status is an important portion of the geriatric assessment because malnutrition is associated with increased mortality and decreased tolerance for chemotherapy.18–20 Evaluating the patient’s psychosocial support is crucial as well because older patients are at greater risk of social isolation and depression.21 While the incidence of depression is lower in older adults with cancer than in younger adults with cancer, clinically significant depression is still noted in 3% to 25% of elderly cancer patients.22 Other critical components of the CGA are review of the patient’s comorbidities and medications to avoid complications of polypharmacy.
Both the Cancer and Aging Research Group (CARG) and Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) toxicity tools are valuable tools, as they predict chemotherapy tolerance in elderly patients.13,23 These tools can help guide discussions between oncologists and patients as well as the formulation of an appropriate treatment plan.24 Although toxicity tools can help to determine which patients are at risk for severe toxicity secondary to treatment, these tools do not replace the CGA. A prospective cohort study that evaluated the impact of CGA on tolerance to chemotherapy in older patients with cancer compared patients aged ≥ 70 years at the start of their treatment with chemotherapy (± radiation therapy) using geriatrician-delivered CGA versus standard care given by oncology.8 Patients who received geriatrician-guided CGA interventions tolerated chemotherapy better and completed treatments as planned (odds ratio 4.14 [95% confidence interval {CI} 1.50 to 11.42], P = 0.006) with fewer treatment modifications.
Unfortunately, the CGA is time-consuming to administer and difficult to incorporate into a busy oncology practice. Therefore, other screening models are used to identify patients who may benefit from a full CGA. The International Society of Geriatric Oncology performed a systematic review of screening tools used to identify older cancer patients in need of geriatric assessment and found that the 3 most studied screening tools are the G8, the Vulnerable Elders Survey-13 (VES-13), and the Flemish version of the Triage Risk Screening Tool.25 Another study found that the G8 was more sensitive than the VES-13 (76.5% versus 68.7%, P = 0.0046), whereas the VES-13 was more specific than the G8 (74.3% versus 64.4%, P < 0.0001).26 In addition to providing guidance to initiate a full geriatric assessment, these screening tools may assist in decision making for older cancer patients, especially those with advanced disease.
Surgery
Early-Stage Disease
When possible, surgical resection of colorectal tumors is the primary treatment in both the curative setting and to avoid complications, such as obstruction or perforation.27 Multiple studies have shown that fit elderly patients benefit from curative surgery similarly to their younger counterparts.27–29 With the growing population of persons aged 65 years or older, surgeons are becoming more comfortable with operating on the elderly.4 However, a large systematic review of 28 independent studies with a total of 34,194 patients showed that older patients were less likely to undergo curative surgery.30 Eligibility for surgery should not be determined by age alone, but rather should be based on a full assessment of the patient’s health, including comorbidities, functional status, nutrition, cognition, social support, and psychological status. The impact of age on short-term outcomes after colorectal surgery in terms of 30-day postoperative morbidity and mortality rates was explored in a study that divided patients into 2 groups: those aged ≥ 80 years (mean age 85) and those aged < 80 years (mean age 55.3).31 There were no statistical differences in 30-day postoperative morbidity and mortality rates between the 2 groups, and preexisting comorbidities and urgent nature of surgery were important predictors of colorectal surgery outcomes in the older adults, results that have been seen in several other studies.28,30 When possible, laparoscopic surgery is preferred as it is associated with less intraoperative blood loss, less postoperative pain, reduced postoperative ileus, a shorter hospital stay, and fewer cardiovascular and pulmonary complications.32 The Preoperative Assessment of Cancer in the Elderly (PACE), which combines surgical risk assessment tools with CGA tools, can assist surgeons in determining candidacy for surgery and help decrease unequal access to surgery in the geriatric population.33
Metastasectomy
A large international multicenter cohort study explored the outcomes of patients aged ≥ 70 years who underwent liver resection of colorectal metastases. The study investigatorsfound that neoadjuvant chemotherapy was used less frequently and less extensive surgery was performed in elderly patients than in younger patients.34 Sixty-day postoperative mortality was slightly higher (3.8% versus 1.6%, P < 0.001) and 3-year OS was slightly lower (57.1% versus 60.2%, P < 0.001) in the elderly group as compared to their younger counterparts, but overall the outcomes after liver surgery were similar. Therefore, the management of liver metastases in oligometastatic disease in elderly patients fit for surgery should be the same as that offered to younger patients. Since outcomes are comparable, older patients should be offered neoadjuvant chemotherapy, as several studies have shown similar response rates and OS in younger and older patients.35,36
Rectal Cancer
The standard of care for locally advanced rectal cancer is combined modality treatment with radiation and chemotherapy followed by total mesorectal excision. However, given conflicting data regarding the ability of elderly patients to tolerate neoadjuvant 5-FU-based chemotherapy and radiation, elderly patients are treated with trimodality therapy less often than their younger counterparts.37,38 A systematic review of 22 randomized trials involving 8507 patients with rectal cancer showed that adjuvant radiation therapy could reduce the risk of local recurrence and death from rectal cancer in patients of all ages.39 However, the risk of noncancer-related death was increased in the older population. The Stockholm II trial showed similar benefits of preoperative radiation overall, but this benefit did not extend to patients older than 68 years because of an increased risk of morbidity and mortality.40 In older patients, mortality from noncancer causes within the first 6 months after surgery was higher in the group that received perioperative radiation than in the group that did not receive radiation. Elderly patients (age > 68 years) accounted for most of the mortality, which was predominantly due to cardiovascular disease.
A retrospective study of 36 patients aged ≥ 70 years with rectal cancer evaluated the toxicity and feasibility of neoadjuvant 5-FU combined with pelvic radiation for treating locally advanced rectal cancer. Patients were classified as healthy and “fit” or “vulnerable” based on the presence of comorbidities.41 This study demonstrated that tolerability and response to neoadjuvant chemotherapy and radiation as well as ability to undergo surgery were similar in “vulnerable” patients and “fit” patients. Conversely, Margalit and colleagues studied the rate of treatment deviations in elderly patients with rectal cancer treated with combined modality therapy and found that most patients required early termination of treatment, treatment interruptions, or dose reductions.42 While trimodality treatment is the standard of care in rectal cancer, there is conflicting data from retrospective studies regarding the tolerability and feasibility of this approach. It is important to proceed with caution but to still consider fit older patients with locally advanced rectal cancer for neoadjuvant chemotherapy and radiation followed by surgery.
In patients who have a complete response (CR) to neoadjuvant chemoradiation, watchful waiting rather than proceeding to surgery may be a reasonable strategy, especially in older patients. A systematic review of 867 patients with locally advanced rectal cancer showed no statistically significant difference in OS between patients who were observed with watchful waiting and those who underwent surgery.43 The International Watch and Wait Database includes 679 patients who were managed with a watch-and-wait regimen because they had a clinical CR after chemoradiation. An outcomes analysis of these patients showed that 25% had local regrowth, with 3-year OS of 91% overall and 87% in patients with local regrowth.44 In most patients (84%), regrowth of the tumor occurred within the first 2 years of follow up.
In frail older adults, for whom longer courses of treatment are not feasible or chemotherapy is contraindicated, short-course radiation therapy can be considered either in the neoadjuvant setting or alone for palliation.45 A randomized trial of short-course radiation versus long-course chemoradiation in patients with T3 rectal cancer found that the difference in 3-year local recurrence rates was not statistically significant.46
Chemotherapy
An expected natural decline in function occurs with age, but given the great variability that exists between patients, it is important to focus on physiologic age rather than chronologic age to determine ability to receive and tolerate anticancer treatment. Decreases in renal and hepatic function, cognitive impairment, changes in gastrointestinal motility, decrements in cardiac and bone marrow reserves, as well as comorbidities and polypharmacy affect a patient’s ability to tolerate chemotherapy.47,48 Toxicity tools such as CARG and CRASH can help to predict severity of toxicity with chemotherapy.13,23 The information provided by these tools can help guide conversations between the oncologist and patient regarding treatment plans.
Adjuvant Chemotherapy for Early-Stage Disease
Stage II Disease
Defining treatment guidelines for older patients with stage II colon cancer is difficult due to lack of data that shows benefit in this population. The QUASAR (Quick and Simple and Reliable) group’s prospective study of adjuvant single-agent 5-FU in stage II colon cancer patients showed an absolute improvement in survival of 3.6% when 5-FU was given after surgery (95% CI 1.0 to 6.0).49 The subgroup analysis of patients aged ≥ 70 years showed a limited benefit of adjuvant 5-FU (hazard ratio [HR] 1.13 [95% CI 0.74 to 1.75]). Given the limited benefit, adjuvant 5-FU for elderly patients with stage II colon cancer should be used judiciously as patients may have competing causes of morbidity or mortality.
The use of oxaliplatin-based therapy in the adjuvant setting for stage II disease was evaluated in a subgroup analysis of the MOSAIC study (Multicenter International Study of Oxaliplatin/5-FU/Leucovorin in the Adjuvant Treatment of Colon Cancer).50 Adjuvant oxaliplatin-based treatment may be offered to patients with stage II colon cancer that carries high-risk features (poorly differentiated histology, lymphovascular invasion, bowel obstruction and/or perforation, < 12 lymph nodes sampled, perineural invasion, or indeterminate or positive margins) due to a trend toward improved disease-free survival (DFS) at 5 years. Patients in this group who received adjuvant FOLFOX (leucovorin, oxaliplatin, 5-FU) versus 5-FU/leucovorin had a DFS of 82.3% versus 74.6%, respectively (HR 0.72 [95% CI 0.50 to 1.02]), a difference that was not statistically significant. A subgroup analysis of 315 patients aged 70 to 75 years with stage II colon cancer enrolled in the MOSAIC study found no statistically significant DFS or OS benefit with the addition of oxaliplatin to 5-FU/leucovorin.51 Therefore, use of this platinum/fluoropyrimidine combination for adjuvant therapy for high-risk stage II disease in older patients remains controversial given its associated risks and the lack of definitive data demonstrating a benefit in this patient group. Decisions regarding this therapy should be made through a shared discussion with patients about its risks and benefits.
Microsatellite status is an important biomarker in the evaluation of stage II CRC. Microsatellite stability is a marker of a functioning DNA mismatch repair system. In patients with colon cancer, tumor microsatellite stability is classified based on the percentage of abnormal microsatellite regions.52 Several studies have shown that patients with tumors that display high microsatellite instability (MSI-H) have an improved prognosis over patients with microsatellite stable tumors.53,54 While patients with stage II MSI-H colon cancer have better outcomes, MSI is associated with a reduced response to treatment with fluoropyrimidines, as demonstrated in a systematic review that found that patients with tumors with MSI obtained no benefit from adjuvant 5-FU (HR 1.24 [95% CI 0.72 to 2.14]).55 Aparicio and colleagues reported an increased prevalence of MSI-H tumors with increasing age.56 Therefore, mismatch repair phenotype should be considered when making adjuvant chemotherapy decisions in the older adult with colon cancer, as it may affect the decision to recommend single-agent 5-FU treatment.
Stage III Disease
The use of single-agent 5-FU for stage III resected CRC has been evaluated in multiple studies. Sargent et al performed a pooled analysis of 3351 patients from 7 randomized phase 3 trials comparing surgery and adjuvant 5-FU-based chemotherapy versus surgery alone in stage II or III colon cancer patients.57 Adjuvant chemotherapy was associated with improvement in both OS and time to tumor recurrence (HR 0.76 and 0.68, respectively). The 5-year OS was 71% for those who received adjuvant treatment and 64% for those who were treated with surgery alone. The benefit of adjuvant treatment was independent of age, and there was no difference in toxicity across age groups, except for 1 study which showed increased rates of leukopenia in the elderly. The oral fluoropyrimidine capecitabine was shown to be an effective alternative to 5-FU plus leucovorin as adjuvant treatment for those with resected stage III colon cancer.58 However, in the subgroup analysis of DFS in the intention-to-treat group, the improvement in DFS was not statistically significant in those aged ≥ 70 years. This study justified the phase 3 Xeloda in Adjuvant Colon Cancer Therapy (X-ACT) trial, which compared capecitabine and 5-FU/leucovorin as adjuvant therapy in patients with resected stage III colon cancer.59 The X-ACT trial showed no significant effect of age on DFS or OS.
The addition of oxaliplatin to 5-FU in the adjuvant setting for stage III tumors has been studied and debated in the elderly population in multiple trials. The MOSAIC trial investigated FOLFOX versus 5-FU/leucovorin in the adjuvant setting.50 The addition of oxaliplatin was associated with a DFS and OS benefit, with a 20% reduction in risk of colon cancer recurrence and 16% reduction in risk of death in all patients. The National Surgical Adjuvant Breast and Bowel Project (NSABP) C-07 trial then studied 2409 patients with stage II or III colon cancer treated with weekly bolus 5-FU/leucovorin with or without oxaliplatin.60 In this study, OS was significantly improved with the addition of oxaliplatin in patients younger than 70 years, but OS at 5 years was 4.7% worse for patients aged ≥ 70 years treated with weekly 5-FU/leucovorin and oxaliplatin compared with those treated with weekly 5-FU/leucovorin (71.6% versus 76.3%, respectively). In contrast, the XELOXA trial (NO16968), which randomly assigned stage III colon cancer patients to capecitabine and oxaliplatin (XELOX) or bolus 5-FU/leucovorin (standard of care at study start), showed an efficacy benefit, albeit not statistically significant, in patients aged ≥ 70 years (HR 0.87 [95% CI 0.63 to 1.18]).61–63
The Adjuvant Colon Cancer Endpoints (ACCENT) database included 7 randomized trials totaling 14,528 patients with stage II or III colon cancer treated with adjuvant 5-FU with or without oxaliplatin or irinotecan.64 Subgroup analysis of patients aged ≥ 70 years (n = 2575) showed no benefit with an oxaliplatin-based regimen in DFS (HR 0.94 [95% CI 0.78 to 1.13]) or OS (HR 1.04 [95% CI, 0.85 to 1.27]). Based on these studies and the increased toxicity with oxaliplatin, oxaliplatin-based adjuvant chemotherapy is utilized less often than single-agent 5-FU in geriatric patients with early-stage colon cancer.65 Conversely, a recent pooled analysis of individual patient data from 4 randomized trials (NSABP C-08, XELOXA, X-ACT, and AVANT) showed improved DFS and OS with adjuvant XELOX or FOLFOX over single-agent 5-FU in patients aged ≥ 70 years (DFS HR 0.77 [95% CI 0.62 to 0.95], P = 0.014; OS HR 0.78 [95% CI 0.61 to 0.99], P = 0.045).66 This analysis also showed that grade 3 and 4 adverse events related to oxaliplatin were similar across age groups.
These data come from post-hoc analyses, and there is no prospective data to steer decision making in elderly patients with early-stage CRC (Table).
It is well established that patients with stage III colon cancer benefit from oxaliplatin-based adjuvant chemotherapy after curative surgical resection.68 However, older patients are less likely to be referred to oncology as compared with their younger counterparts, due to the conflicting data regarding the benefit of this approach in older adults. Studies have shown that when the referral is placed, the geriatric population is less likely to receive chemotherapy.69 Sanoff et al analyzed 4 data sets (SEER-Medicare, National Comprehensive Cancer Network, New York State Cancer Registry, and Cancer Care Outcomes Research and Surveillance Consortium) to assess the benefit of adjuvant chemotherapy for resected stage III CRC among patients aged ≥ 75 years. Their analysis showed that only 40% of patients evaluated received adjuvant chemotherapy for stage III CRC after surgical resection.70
Summary
Prospective data to guide the treatment of older patients with early-stage CRC in the adjuvant setting is lacking. For fit older patients with stage II disease, limited benefit will be derived from single-agent 5-FU. For those with stage III CRC, the benefit and toxicities of fluoropyrimidines as adjuvant therapy appear to be similar regardless of age. The addition of oxaliplatin to fluoropyrimidines in patients aged ≥ 70 years has not been proven to improve DFS or OS and could result in an incremental toxicity profile. Therefore, treatment plans must be individualized, and decisions should be made through an informed discussion evaluating the overall risk/benefit ratio of each approach.
Metastatic Disease
Palliative Chemotherapy
Approximately 20% of patients with CRC are diagnosed with metastatic disease at presentation, and 35% to 40% develop metastatic disease following surgery and adjuvant therapy.2 The mainstay of treatment in this population is systemic therapy in the form of chemotherapy with or without biologic agents. In this setting, several prospective studies specific to older adults have been completed, providing more evidence-based guidance to oncologists who see these patients. Folprecht et al retrospectively reviewed data from 22 clinical trials evaluating 5-FU-based palliative chemotherapy in 3825 patients with metastatic CRC, including 629 patients aged ≥ 70 years.71 OS in elderly patients (10.8 months [95% CI 9.7 to 11.8]) was equivalent to that in younger patients (11.3 months [95% CI 10.9 to 11.7], P = 0.31). Similarly, relative risk and progression-free survival (PFS) were comparable irrespective of age.
Standard of care for most patients with metastatic colon cancer consists of 5-FU/leucovorin in combination with either oxaliplatin (FOLFOX) or irinotecan (FOLFIRI) with a monoclonal antibody.72 A retrospective pooled analysis of patients with metastatic CRC compared the safety and efficacy of FOLFOX4 in patients aged < 70 years versus those aged ≥ 70 years.73 While age ≥ 70 years was associated with an increased rate of grade ≥ 3 hematologic toxicity, it was not associated with increased rates of severe neurologic events, diarrhea, nausea, vomiting, infection, 60-day mortality, or overall incidence of grade ≥ 3 toxicity. The benefit of treatment was consistent across both age groups; therefore, age alone should not exclude an otherwise healthy individual from receiving FOLFOX.
These post-hoc analyses show that fit older patients who were candidates for trial participation tolerated these treatments well; however, these treatments may be more challenging for less fit older adults. The UK Medical Research Council FOCUS2 (Fluorouracil, Oxaliplatin, CPT11 [irinotecan]: Use and Sequencing) study was a prospective phase 3 trial that included 459 patients with metastatic CRC who were deemed too frail or not fit enough for standard-dose chemotherapy by their oncologists.74 In this group, 43% of patients were older than 75 years and 13% were older than 80 years. Patients were randomly assigned to receive infusional 5-FU with levofolinate; oxaliplatin and 5-FU; capecitabine; or oxaliplatin and capecitabine; all regimens were initiated with an empiric 20% dose reduction. The addition of oxaliplatin suggested some improvement in PFS, but this was not significant (5.8 months versus 4.5 months, HR 0.84 [95% CI 0.69 to 1.01], P = 0.07). Oxaliplatin was not associated with increased grade 3 or 4 toxicities. Capecitabine is often viewed as less toxic because it is taken by mouth, but this study found that replacement of 5-FU with capecitabine did not improve quality of life. Grade 3 or 4 toxicities were seen more frequently in those receiving capecitabine than in those receiving 5-FU (40% versus 30%, P = 0.03) in this older and frailer group of patients. As the patients on this study were frail and treatment dose was reduced, this data may not apply to fit older adults who are candidates for standard therapy.
When managing an older patient with metastatic CRC, it is important to tailor therapy based on goals of care, toxicity of proposed treatment, other comorbidities, and the patient’s functional status. One approach to minimizing toxicity in the older population is the stop-and-go strategy. The OPTIMOX1 study showed that stopping oxaliplatin after 6 cycles of FOLFOX7 and continuing maintenance therapy with infusional 5-FU/leucovorin alone for 12 cycles prior to reintroducing FOLFOX7 achieved efficacy similar to continuous FOLFOX4 with decreased toxicity.75 Figer et al studied an exploratory cohort of 37 patients aged 76 to 80 years who were included in the OPTIMOX1 study.76 The overall relative risk, median PFS, and median OS did not differ between the older patients in this cohort and younger patients studied in the original study. Older patients did experience more neutropenia, neurotoxicity, and overall grade 3 to 4 toxicity, but there were no toxic deaths in patients older than 75 years. The approach of giving treatment breaks, as in OPTIMOX2, may also provide patients with better quality of life, but perhaps at the expense of cancer-related survival.77
The combination of irinotecan and 5-FU has also been studied as treatment for patients with metastatic CRC. A pooled analysis of 2691 patients aged ≥ 70 years with metastatic CRC across 4 phase 3 randomized trials investigating irinotecan and 5-FU demonstrated that irinotecan-containing chemotherapy provided similar benefits to both older and younger patients with similar risk of toxicity.78 A phase 2 trial studying FOLFIRI as first-line treatment in older metastatic CRC patients showed this to be a safe and active regimen with manageable toxicity.79 Another randomized phase 3 trial for older patients compared 5-FU/leucovorin with or without irinotecan for first-line treatment of metastatic CRC (FFCD 2001-02).80 The study accrued 282 patients aged ≥ 75 years (median age 80 years), and found that the addition of irinotecan to infusional 5-FU–based chemotherapy did not significantly increase either PFS or OS. Aparicio et al performed a substudy of baseline geriatric evaluation prior to treatment in the FFCD 2001-02 study and assessed the value of geriatric parameters for predicting outcomes (objective response rate [ORR], PFS, and OS).81 Multivariate analysis showed that none of the geriatric parameters were predictive of ORR or PFS but that normal IADL was associated with better OS. This combination may still be appropriate for some older patients with metastatic disease, while single- agent 5-FU may be more appropriate in frail patients.
Biologic Agents
VEGF Inhibitors
Targeted biologic agents have been studied in the treatment of metastatic CRC. Bevacizumab is a recombinant, humanized monoclonal antibody against vascular endothelial growth factor (VEGF) that is approved in the first-line setting for treatment of metastatic CRC. A pooled analysis examined 439 patients 65 years of age and older with metastatic CRC who received bevacizumab plus chemotherapy versus placebo plus chemotherapy.82 In this analysis, the addition of bevacizumab was associated with an improvement in OS (19.3 months versus 14.3 months, HR 0.7 [95% CI 0.55 to 0.90], P = 0.006) and in PFS (9.2 months versus 6.2 months, HR 0.52 [95% CI 0.40 to 0.67], P < 0.0001). Known adverse events associated with bevacizumab were seen in the bevacizumab plus chemotherapy group but not at increased rates in the older population compared to their younger counterparts. Conversely, another pooled analysis found that while there was a PFS and OS benefit in older patients receiving bevacizumab, there was an increased incidence of thrombotic events in patients older than 65 years.83 The BEAT (Bevacizumab Expanded Access Trial) and BRiTE (Bevacizumab Regimens Investigation of Treatment Effects) studies showed similar clinical outcomes across all age groups.84,85 While older patients experienced more arterial thromboembolic events with the addition of bevacizumab, other factors such as ECOG PS, prior anticoagulation, and history of arterial disease were more predictive of these adverse events than age.
The randomized phase 3 AVEX study explored the efficacy and tolerability of capecitabine plus bevacizumab versus capecitabine alone in 280 frail patients aged ≥ 70 years.86 PFS in the capecitabine/bevacizumab arm was 9.1 months versus 5.1 months in the capecitabine alone arm. While the OS difference was not statistically significant, patients in the capecitabine/bevacizumab arm had an OS of 20.7 months versus 16.8 months in the capecitabine alone group. As reported in prior studies, patients in the capecitabine/bevacizumab arm had increased rates of toxic events (40%) compared with those who received capecitabine alone (22%), with reports of hypertension, hand-foot syndrome, bleeding, and thrombotic events. More recently, the phase 2 PRODIGE 20 trial studied the addition of bevacizumab to chemotherapy (5-FU, FOLFOX, or FOLFIRI) based on physician choice in untreated metastatic CRC patients aged ≥ 75 years (median age 80 years).87 They found that the addition of bevacizumab to standard of care chemotherapy was both safe and effective. The adverse events seen with bevacizumab, such as hypertension and thrombotic events, were consistent with prior studies.
A newer antiangiogenic agent, ziv-aflibercept, has been approved for the second-line treatment of metastatic CRC. The VELOUR trial demonstrated that the addition of ziv-aflibercept to FOLFIRI benefited patients across all age groups compared with FOLFIRI plus placebo in patients who had failed prior oxaliplatin-based chemotherapy.88,89 Ramucirumab is a human IgG-1 monoclonal antibody approved in second-line treatment in combination with FOLFIRI. A subgroup analysis of the RAISE study showed that the survival benefit was similar in patients aged ≥ 65 years versus those < 65 years.90 Based on the above data, the use of a VEGF inhibitor in combination with chemotherapy should be considered in older patients with metastatic CRC. Furthermore, based on the conflicting data regarding the benefit of FOLFOX/FOLFIRI over single-agent 5-FU discussed above, the combination of capecitabine plus bevacizumab may be considered a front-line treatment option in older patients based on the AVEX study.
EGFR Inhibitors
Cetuximab and panitumumab are anti-epidermal growth factor receptor (EGFR) antibodies approved for the treatment of RAS wild-type metastatic CRC. Data regarding the use of EGFR inhibitors in the geriatric population is scarce and the data that does exist is conflicting.91,92 The PRIME study demonstrated that panitumumab plus FOLFOX had a PFS benefit compared to FOLFOX alone in KRAS wild-type metastatic CRC patients.92 While the study met its primary endpoint, the benefit did not translate to patients aged ≥ 65 years in subgroup analysis. Conversely, a retrospective study of the efficacy and safety of cetuximab in elderly patients with heavily pretreated metastatic CRC found similar efficacy in older and younger patients as well as no increased adverse events in the older population.91 A phase 2 trial investigating cetuximab as single-agent first-line treatment of metastatic CRC in fit older patients found cetuximab to be safe with moderate activity in this population, but did not support the use of cetuximab as first-line single-agent treatment in fit geriatric patients who may be candidates for combination therapy.93 Our group studied the patterns of use and tolerance of anti-EGFR antibodies in 117 older adults with metastatic CRC with a median age of 73 years.94 The study showed that older age at the time of treatment was associated with administration of anti-EGFR antibody as monotherapy rather than in combination with chemotherapy (P = 0.0009). We found no association between age and presence of grade 3 or higher toxicity. In addition, the toxicity profile seen in older patients was similar to what has been demonstrated in prior studies involving a younger patient population. Given the discordance seen between studies, additional prospective trials are needed to elucidate the efficacy and safety of EGFR inhibitors in the geriatric population.
Other Agents
Two newer agents approved in the treatment of metastatic CRC are regorafenib, a multikinase inhibitor, and trifluridine/tipiracil (TFD/TPI), a nucleoside analog combined with an inhibitor of thymidine phosphorylase. The phase 3 CORRECT trial studied regorafenib as monotherapy in previously treated metastatic CRC and found an OS benefit of 1.4 months and minimal PFS benefit.95 Van Cutsem et al performed a subgroup analysis by age and found similar OS benefit in patients < 65 years of age and ≥ 65 years.96 The most frequent adverse events grade 3 or higher were hand-foot syndrome, fatigue, diarrhea, hypertension, and desquamation/rash, which were seen at similar rates in both age groups. More recently, the phase 2 Regorafenib Dose Optimization Study (ReDOS) found that weekly dose escalation of regorafenib from 80 mg to 160 mg daily over 3 weeks was superior to the standard 160 mg daily dosing in patients with metastatic CRC.97 The dose escalation group had a longer median OS, although this difference was not statistically significant, as well as a more favorable toxicity profile. Therefore, this new dosing strategy may be a reasonable option for older patients with pretreated metastatic CRC. A study of TFD/TPI versus placebo in refractory metastatic CRC found an OS benefit of 7.1 months versus 5.3 months.98 In subgroup analyses, the OS benefit extended to both patients < 65 years and ≥ 65 years. Given the sparse data on these newer agents in the geriatric population and the modest benefit they provide to those with refractory metastatic CRC, more data is needed to determine their utility in elderly patients. The decision to use these agents in the older patients warrants a thorough discussion with the patient regarding risks, benefit, and treatment goals.
Immunotherapy
Between 3.5% and 6.5% of stage IV colorectal cancers are MSI-H and have deficient mismatch repair (dMMR).99–101 A recent phase 2 trial studied the use of pembrolizumab, an IgG4 monoclonal antibody against PD-1 (programmed cell death-1), in heavily pretreated patients with dMMR metastatic CRC, MMR-proficient (pMMR) metastatic CRC, and noncolorectal dMMR metastatic cancer.102 Patients with dMMR metastatic CRC had a 50% ORR and 89% disease control rate (DCR), as compared with an ORR of 0% and DCR of 16% in patients with pMMR metastatic CRC. There was also an OS and PFS benefit seen in the dMMR CRC group as compared with the pMMR CRC group. Another phase 2 study, CheckMate 142, studied the anti-PD-1 monoclonal antibody nivolumab with or without ipilimumab (a monoclonal antibody against cytotoxic T-lymphocyte antigen 4) in patients with dMMR and pMMR metastatic CRC.103 In the interim analysis, nivolumab was found to provide both disease control and durable response in patients with dMMR metastatic CRC.
While these studies led to the FDA approval of pembrolizumab and nivolumab for management of previously treated MSI-H or dMMR metastatic CRC, data on the use of immunotherapy in older adults is scarce. Immunosenescence, or the gradual deterioration of the immune system that comes with aging, may impact the efficacy of immune checkpoint inhibitors (ICI) in older patients with advanced cancer.104 There is conflicting data on the efficacy of PD-1 and programmed death ligand-1) PD-L1 inhibitors in older patients across different cancers. A meta-analysis of immunotherapy in older adults with a variety of malignancies showed overall efficacy comparable to that seen in adults younger than 65 years.105 However, another review found ICIs to be less effective in older patients with head and neck, non-small cell lung cancer, and renal cell carcinoma compared with their younger counterparts.104 Regarding the toxicity profile of ICIs in the elderly, similar rates of grade 3 or higher adverse events in patients younger than 65 years and older than 65 years have been reported.106 However, patients aged ≥ 70 years had increased rates of grade 3 to 5 adverse events as compared to patients younger than 65 years (71.7% versus 58.4%, respectively). Given the scant data on ICIs in older patients with MSI-H or dMMR metastatic CRC, more clinical trials inclusive of this population are needed in order to determine the efficacy and safety of immunotherapy.
Palliative Care
The incorporation of palliative care early following the diagnosis of cancer has been shown to improve quality of life, decrease depression, and help with symptom management.107 The triggers for geriatric patients to initiate palliative care may be different from those of younger patients, as older patients may have different goals of care.108 Older patients will often choose quality over quantity of life when making treatment decisions.109 The ideal medical treatment for the frail patient with colorectal cancer would focus on treating disease while providing palliative measures to help support the patient and improve quality of life. It is paramount that patients maintain functional independence as loss of independence is recognized as a major threat to an older patient’s quality of life.110 The optimal way to achieve these goals is through the efforts of a multidisciplinary care team including not only physicians and nurses, but also social workers, nutritionists, physical therapists, and family who can provide support for the patient’s psychosocial, cognitive, and medical needs.111 Although cancer and noncancer–related death occur more frequently in the geriatric population, data to guide a specific palliative care approach to the elderly population is lacking.108
Conclusion
Colorectal cancer is a disease of older adults with a median age at diagnosis of 67 years.1 With the aging population, oncologists will be faced with treating increasing numbers of older patients, and must adjust their practice to accommodate this population of patients. Treating geriatric patients is challenging given the lack of available data to guide the treatment approach. Although several prospective elderly-specific studies have been conducted evaluating treatments for metastatic CRC, most treatment decisions are made based on the available retrospective studies and pooled analyses. Oncologists must carefully consider and evaluate each patient based on physiologic age rather than chronologic age.112 Overall, older patients should be given the opportunity to receive standard of care treatments in the appropriate setting. The decision to modify treatment plans should be made after a thorough evaluation by a multidisciplinary team and a discussion with the patient regarding their goals and the risks and benefits of the treatment. Geriatric assessment tools can help the care team identify patients with various geriatric syndromes that may not be detected on routine oncology evaluation. This type of evaluation is time consuming and is rarely done in a busy oncology practice. Ongoing studies are aiming to develop a method to incorporate geriatric assessments into the care of older adults.Additional prospective trials targeting older, more frail patients are essential to improve upon our knowledge so we can provide best care for this growing elderly population.
1. National Cancer Institute. SEER cancer stat facts: colorectal cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed March 1, 2018.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
3. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017:1-8.
4. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States, current population reports, P25-1140. Washington, DC: U.S. Census Bureau; 2014.
5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7.
6. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4.
7. Vijayvergia N, Li T, Wong YN, et al. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer 2016;122:3191–8.
8. Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435–44.
9. National Comprehensive Cancer Network. Older adult oncology (Version 2.2017). Accessed March 1, 2018,
10. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72.
11. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J 2014;20:358–66.
12. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
13. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
14. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
15. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
16. Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
17. Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 2004;52:1681–7.
18. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7.
19. Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218–26.
20. Martucci RB, Barbosa MV, D’Almeida CA, et al. Undernutrition as independent predictor of early mortality in elderly cancer patients. Nutrition 2017;34:65–70.
21. Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. J Clin Oncol 2014;32:2627–34.
22. Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore 2005;34:250–6.
23. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.
24. Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013;11:1494-502.
25. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300.
26. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014; 9:e115060.
27. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg 2009;13:100–4.
28. Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: surgical treatment and long-term survival. Int J Colorectal Dis 2008;23:601–10.
29. Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg 2008;195:344–8.
30. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–74.
31. Shalaby M, Di Lorenzo N, Franceschilli L, et al. Outcome of colorectal surgery in elderly populations. Ann Coloproctol 2016;32:139–43.
32. Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300.
33. PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63.
34. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76.
35. de Liguori Carino N, van Leeuwen BL, Ghaneh P, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol 2008;67:273–8.
36. Tamandl D, Gruenberger B, Herberger B, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol 2009;100:364–71.
37. Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015–21.
38. Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215–21.
39. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
40. Martling A, Holm T, Johansson H, et al, Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902.
41. Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–23.
42. Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735–41.
43. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–13.
44. van der Valk M. The International Watch & Wait database (IWWD) for rectal cancer: An update. J Clin Oncol 2017;35 suppl:521.
45. Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol 2003;45:305–11.
46. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–33.
47. McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 2014;32:2570–80.
48. Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204–20.
49. Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.
50. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
51. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
52. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010;36:550–6.
53. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
54. Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
55. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
56. Aparicio T, Schischmanoff O, Poupardin C, et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis 2013;45:245–50.
57. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7.
58. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
59. Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
60. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
61. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
62. Haller DG, Cassidy J, Tabernero J, et al. Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): impact of age on disease-free survival (DFS) [abstract]. J Clin Oncol 2010;28:3521.
63. Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
64. McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
65. Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
66. Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24.
67. Aparicio T, Francois E, Cristol-Dalstein L, et al. PRODIGE 34-FFCD 1402-ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig Liver Dis 2016;48:206–7.
68. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
69. Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000;135:182–5.
70. Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
71. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol 2004;15:1330–8.
72. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1–9.
73. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
74. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749–59.
75. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400.
76. Figer A, Perez-Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007;110:2666–71.
77. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33.
78. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008;26:1443–51.
79. Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology 2005;69:384–90.
80. Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol 2016;27:121–7.
81. Aparicio T, Gargot D, Teillet L, et al. Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108.
82. Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
83. Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 2010;136:737–43.
84. Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
85. Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology 2010;78:329–39.
86. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85.
87. Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol 2018;29:133–8.
88. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506.
89. Ruff P, Van Cutsem E, Lakomy R, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–9.
90. Obermannova R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 2016;27:2082–90.
91. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol 2008;67:255-62.
92. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55.
93. Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339–45.
94. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer 2014;13:192–8.
95. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12.
96. Van Cutsem E, Sobrero A, Siena S, et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial [abstract]. J Clin Oncol 2013;31(15 suppl):3636-3636.
97. Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): an ACCRU Network study [abstract]. J Clin Oncol 2018;36(4 suppl):611-611.
98. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19.
99. Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73.
100. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30.
101. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
102. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20.
103. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.
104. Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155–66.
105. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26.
106. Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers [abstract]. J Clin Oncol 2016;34(15 suppl):10010-10010.
107. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41.
108. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014;5:197–203.
109. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer 2008;113:3459–66.
110. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82.
111. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center. Clin J Oncol Nurs 2007;11:929–33.
112. Sheridan J, Walsh P, Kevans D, et al. Determinants of short- and long-term survival from colorectal cancer in very elderly patients. J Geriatr Oncol 2014;5:376–83.
1. National Cancer Institute. SEER cancer stat facts: colorectal cancer. http://seer.cancer.gov/statfacts/html/colorect.html. Accessed March 1, 2018.
2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7–30.
3. Kochanek KD, Murphy S, Xu J, Arias E. Mortality in the United States, 2016. NCHS Data Brief 2017:1-8.
4. Ortman JM, Velkoff VA, Hogan H. An aging nation: the older population in the United States, current population reports, P25-1140. Washington, DC: U.S. Census Bureau; 2014.
5. Hutchins LF, Unger JM, Crowley JJ, et al. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med 1999;341:2061–7.
6. Unger JM, Coltman CA Jr, Crowley JJ, et al. Impact of the year 2000 Medicare policy change on older patient enrollment to cancer clinical trials. J Clin Oncol 2006;24:141–4.
7. Vijayvergia N, Li T, Wong YN, et al. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer 2016;122:3191–8.
8. Kalsi T, Babic-Illman G, Ross PJ, et al. The impact of comprehensive geriatric assessment interventions on tolerance to chemotherapy in older people. Br J Cancer 2015;112:1435–44.
9. National Comprehensive Cancer Network. Older adult oncology (Version 2.2017). Accessed March 1, 2018,
10. Balducci L. Frailty: a common pathway in aging and cancer. Interdiscip Top Gerontol 2013;38:61–72.
11. Baijal P, Periyakoil V. Understanding frailty in cancer patients. Cancer J 2014;20:358–66.
12. Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55.
13. Hurria A, Togawa K, Mohile SG, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 2011;29:3457–65.
14. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969;9:179–86.
15. Katz S, Downs TD, Cash HR, Grotz RC. Progress in development of the index of ADL. Gerontologist 1970;10:20–30.
16. Klepin HD, Geiger AM, Tooze JA, et al. Physical performance and subsequent disability and survival in older adults with malignancy: results from the health, aging and body composition study. J Am Geriatr Soc 2010;58:76–82.
17. Gupta SK, Lamont EB. Patterns of presentation, diagnosis, and treatment in older patients with colon cancer and comorbid dementia. J Am Geriatr Soc 2004;52:1681–7.
18. Dewys WD, Begg C, Lavin PT, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med 1980;69:491–7.
19. Aaldriks AA, van der Geest LG, Giltay EJ, et al. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. J Geriatr Oncol 2013;4:218–26.
20. Martucci RB, Barbosa MV, D’Almeida CA, et al. Undernutrition as independent predictor of early mortality in elderly cancer patients. Nutrition 2017;34:65–70.
21. Naeim A, Aapro M, Subbarao R, Balducci L. Supportive care considerations for older adults with cancer. J Clin Oncol 2014;32:2627–34.
22. Kua J. The prevalence of psychological and psychiatric sequelae of cancer in the elderly - how much do we know? Ann Acad Med Singapore 2005;34:250–6.
23. Extermann M, Boler I, Reich RR, et al. Predicting the risk of chemotherapy toxicity in older patients: the Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 2012;118:3377–86.
24. Kim J, Hurria A. Determining chemotherapy tolerance in older patients with cancer. J Natl Compr Canc Netw 2013;11:1494-502.
25. Decoster L, Van Puyvelde K, Mohile S, et al. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol 2015;26:288–300.
26. Soubeyran P, Bellera C, Goyard J, et al. Screening for vulnerability in older cancer patients: the ONCODAGE Prospective Multicenter Cohort Study. PLoS One 2014; 9:e115060.
27. Gurevitch AJ, Davidovitch B, Kashtan H. Outcome of right colectomy for cancer in octogenarians. J Gastrointest Surg 2009;13:100–4.
28. Schiffmann L, Ozcan S, Schwarz F, et al. Colorectal cancer in the elderly: surgical treatment and long-term survival. Int J Colorectal Dis 2008;23:601–10.
29. Ong ES, Alassas M, Dunn KB, Rajput A. Colorectal cancer surgery in the elderly: acceptable morbidity? Am J Surg 2008;195:344–8.
30. Surgery for colorectal cancer in elderly patients: a systematic review. Colorectal Cancer Collaborative Group. Lancet 2000;356:968–74.
31. Shalaby M, Di Lorenzo N, Franceschilli L, et al. Outcome of colorectal surgery in elderly populations. Ann Coloproctol 2016;32:139–43.
32. Frasson M, Braga M, Vignali A, et al. Benefits of laparoscopic colorectal resection are more pronounced in elderly patients. Dis Colon Rectum 2008;51:296–300.
33. PACE participants, Audisio RA, Pope D, et al. Shall we operate? Preoperative assessment in elderly cancer patients (PACE) can help. A SIOG surgical task force prospective study. Crit Rev Oncol Hematol 2008;65:156–63.
34. Adam R, Frilling A, Elias D, et al. Liver resection of colorectal metastases in elderly patients. Br J Surg 2010;97:366–76.
35. de Liguori Carino N, van Leeuwen BL, Ghaneh P, et al. Liver resection for colorectal liver metastases in older patients. Crit Rev Oncol Hematol 2008;67:273–8.
36. Tamandl D, Gruenberger B, Herberger B, et al. Surgery after neoadjuvant chemotherapy for colorectal liver metastases is safe and feasible in elderly patients. J Surg Oncol 2009;100:364–71.
37. Shahir MA, Lemmens VE, van de Poll-Franse LV, et al. Elderly patients with rectal cancer have a higher risk of treatment-related complications and a poorer prognosis than younger patients: a population-based study. Eur J Cancer 2006;42:3015–21.
38. Chang GJ, Skibber JM, Feig BW, Rodriguez-Bigas M. Are we undertreating rectal cancer in the elderly? An epidemiologic study. Ann Surg 2007;246:215–21.
39. Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8,507 patients from 22 randomised trials. Lancet 2001;358:1291–304.
40. Martling A, Holm T, Johansson H, et al, Stockholm Colorectal Cancer Study Group. The Stockholm II trial on preoperative radiotherapy in rectal carcinoma: long-term follow-up of a population-based study. Cancer 2001;92:896–902.
41. Pasetto LM, Friso ML, Pucciarelli S, et al. Rectal cancer neoadjuvant treatment in elderly patients. Anticancer Res 2006;26:3913–23.
42. Margalit DN, Mamon HJ, Ancukiewicz M, et al. Tolerability of combined modality therapy for rectal cancer in elderly patients aged 75 years and older. Int J Radiat Oncol Biol Phys 2011;81:e735–41.
43. Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501–13.
44. van der Valk M. The International Watch & Wait database (IWWD) for rectal cancer: An update. J Clin Oncol 2017;35 suppl:521.
45. Donato V, Valeriani M, Zurlo A. Short course radiation therapy for elderly cancer patients. Evidences from the literature review. Crit Rev Oncol Hematol 2003;45:305–11.
46. Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827–33.
47. McCleary NJ, Dotan E, Browner I. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol 2014;32:2570–80.
48. Millan M, Merino S, Caro A, et al. Treatment of colorectal cancer in the elderly. World J Gastrointest Oncol 2015;7:204–20.
49. Quasar Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020–9.
50. Andre T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109–16.
51. Tournigand C, Andre T, Bonnetain F, et al. Adjuvant therapy with fluorouracil and oxaliplatin in stage II and elderly patients (between ages 70 and 75 years) with colon cancer: subgroup analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer trial. J Clin Oncol 2012;30:3353–60.
52. Winder T, Lenz HJ. Molecular predictive and prognostic markers in colon cancer. Cancer Treat Rev 2010;36:550–6.
53. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med 2003;349:247–57.
54. Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med 2000;342:69–77.
55. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609–18.
56. Aparicio T, Schischmanoff O, Poupardin C, et al. Deficient mismatch repair phenotype is a prognostic factor for colorectal cancer in elderly patients. Dig Liver Dis 2013;45:245–50.
57. Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 2001;345:1091–7.
58. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med 2005;352:2696–704.
59. Twelves C, Scheithauer W, McKendrick J, et al. Capecitabine versus 5-fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results from the X-ACT trial with analysis by age and preliminary evidence of a pharmacodynamic marker of efficacy. Ann Oncol 2012;23:1190–7.
60. Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. J Clin Oncol 2011;29:3768–74.
61. Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465–71.
62. Haller DG, Cassidy J, Tabernero J, et al. Efficacy findings from a randomized phase III trial of capecitabine plus oxaliplatin versus bolus 5-FU/LV for stage III colon cancer (NO16968): impact of age on disease-free survival (DFS) [abstract]. J Clin Oncol 2010;28:3521.
63. Schmoll HJ, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil/folinic acid as adjuvant therapy for stage III colon cancer: final results of the NO16968 randomized controlled phase III trial. J Clin Oncol 2015;33:3733–40.
64. McCleary NJ, Meyerhardt JA, Green E, et al. Impact of age on the efficacy of newer adjuvant therapies in patients with stage II/III colon cancer: findings from the ACCENT database. J Clin Oncol 2013;31:2600–6.
65. Kahn KL, Adams JL, Weeks JC, et al. Adjuvant chemotherapy use and adverse events among older patients with stage III colon cancer. JAMA 2010;303:1037–45.
66. Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24.
67. Aparicio T, Francois E, Cristol-Dalstein L, et al. PRODIGE 34-FFCD 1402-ADAGE: Adjuvant chemotherapy in elderly patients with resected stage III colon cancer: A randomized phase 3 trial. Dig Liver Dis 2016;48:206–7.
68. Gill S, Loprinzi CL, Sargent DJ, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol 2004;22:1797–806.
69. Mahoney T, Kuo YH, Topilow A, Davis JM. Stage III colon cancers: why adjuvant chemotherapy is not offered to elderly patients. Arch Surg 2000;135:182–5.
70. Sanoff HK, Carpenter WR, Sturmer T, et al. Effect of adjuvant chemotherapy on survival of patients with stage III colon cancer diagnosed after age 75 years. J Clin Oncol 2012;30:2624–34.
71. Folprecht G, Cunningham D, Ross P, et al. Efficacy of 5-fluorouracil-based chemotherapy in elderly patients with metastatic colorectal cancer: a pooled analysis of clinical trials. Ann Oncol 2004;15:1330–8.
72. Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii1–9.
73. Goldberg RM, Tabah-Fisch I, Bleiberg H, et al. Pooled analysis of safety and efficacy of oxaliplatin plus fluorouracil/leucovorin administered bimonthly in elderly patients with colorectal cancer. J Clin Oncol 2006;24:4085–91.
74. Seymour MT, Thompson LC, Wasan HS, et al. Chemotherapy options in elderly and frail patients with metastatic colorectal cancer (MRC FOCUS2): an open-label, randomised factorial trial. Lancet 2011;377:1749–59.
75. Tournigand C, Cervantes A, Figer A, et al. OPTIMOX1: a randomized study of FOLFOX4 or FOLFOX7 with oxaliplatin in a stop-and-Go fashion in advanced colorectal cancer--a GERCOR study. J Clin Oncol 2006;24:394–400.
76. Figer A, Perez-Staub N, Carola E, et al. FOLFOX in patients aged between 76 and 80 years with metastatic colorectal cancer: an exploratory cohort of the OPTIMOX1 study. Cancer 2007;110:2666–71.
77. Chibaudel B, Maindrault-Goebel F, Lledo G, et al. Can chemotherapy be discontinued in unresectable metastatic colorectal cancer? The GERCOR OPTIMOX2 Study. J Clin Oncol 2009;27:5727–33.
78. Folprecht G, Seymour MT, Saltz L, et al. Irinotecan/fluorouracil combination in first-line therapy of older and younger patients with metastatic colorectal cancer: combined analysis of 2,691 patients in randomized controlled trials. J Clin Oncol 2008;26:1443–51.
79. Souglakos J, Pallis A, Kakolyris S, et al. Combination of irinotecan (CPT-11) plus 5-fluorouracil and leucovorin (FOLFIRI regimen) as first line treatment for elderly patients with metastatic colorectal cancer: a phase II trial. Oncology 2005;69:384–90.
80. Aparicio T, Lavau-Denes S, Phelip JM, et al. Randomized phase III trial in elderly patients comparing LV5FU2 with or without irinotecan for first-line treatment of metastatic colorectal cancer (FFCD 2001-02). Ann Oncol 2016;27:121–7.
81. Aparicio T, Gargot D, Teillet L, et al. Geriatric factors analyses from FFCD 2001-02 phase III study of first-line chemotherapy for elderly metastatic colorectal cancer patients. Eur J Cancer 2017;74:98–108.
82. Kabbinavar FF, Hurwitz HI, Yi J, et al. Addition of bevacizumab to fluorouracil-based first-line treatment of metastatic colorectal cancer: pooled analysis of cohorts of older patients from two randomized clinical trials. J Clin Oncol 2009;27:199–205.
83. Cassidy J, Saltz LB, Giantonio BJ, et al. Effect of bevacizumab in older patients with metastatic colorectal cancer: pooled analysis of four randomized studies. J Cancer Res Clin Oncol 2010;136:737–43.
84. Van Cutsem E, Rivera F, Berry S, et al. Safety and efficacy of first-line bevacizumab with FOLFOX, XELOX, FOLFIRI and fluoropyrimidines in metastatic colorectal cancer: the BEAT study. Ann Oncol 2009;20:1842–7.
85. Kozloff MF, Berlin J, Flynn PJ, et al. Clinical outcomes in elderly patients with metastatic colorectal cancer receiving bevacizumab and chemotherapy: results from the BRiTE observational cohort study. Oncology 2010;78:329–39.
86. Cunningham D, Lang I, Marcuello E, et al. Bevacizumab plus capecitabine versus capecitabine alone in elderly patients with previously untreated metastatic colorectal cancer (AVEX): an open-label, randomised phase 3 trial. Lancet Oncol 2013;14:1077–85.
87. Aparicio T, Bouche O, Taieb J, et al. Bevacizumab+chemotherapy versus chemotherapy alone in elderly patients with untreated metastatic colorectal cancer: a randomized phase II trial-PRODIGE 20 study results. Ann Oncol 2018;29:133–8.
88. Van Cutsem E, Tabernero J, Lakomy R, et al. Addition of aflibercept to fluorouracil, leucovorin, and irinotecan improves survival in a phase III randomized trial in patients with metastatic colorectal cancer previously treated with an oxaliplatin-based regimen. J Clin Oncol 2012;30:3499–506.
89. Ruff P, Van Cutsem E, Lakomy R, et al. Observed benefit and safety of aflibercept in elderly patients with metastatic colorectal cancer: An age-based analysis from the randomized placebo-controlled phase III VELOUR trial. J Geriatr Oncol 2018;9:32–9.
90. Obermannova R, Van Cutsem E, Yoshino T, et al. Subgroup analysis in RAISE: a randomized, double-blind phase III study of irinotecan, folinic acid, and 5-fluorouracil (FOLFIRI) plus ramucirumab or placebo in patients with metastatic colorectal carcinoma progression. Ann Oncol 2016;27:2082–90.
91. Bouchahda M, Macarulla T, Spano JP, et al. Cetuximab efficacy and safety in a retrospective cohort of elderly patients with heavily pretreated metastatic colorectal cancer. Crit Rev Oncol Hematol 2008;67:255-62.
92. Douillard JY, Siena S, Cassidy J, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol 2014;25:1346–55.
93. Sastre J, Gravalos C, Rivera F, et al. First-line cetuximab plus capecitabine in elderly patients with advanced colorectal cancer: clinical outcome and subgroup analysis according to KRAS status from a Spanish TTD Group Study. Oncologist 2012;17:339–45.
94. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer 2014;13:192–8.
95. Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303–12.
96. Van Cutsem E, Sobrero A, Siena S, et al. Regorafenib (REG) in progressive metastatic colorectal cancer (mCRC): Analysis of age subgroups in the phase III CORRECT trial [abstract]. J Clin Oncol 2013;31(15 suppl):3636-3636.
97. Bekaii-Saab TS, Ou FS, Anderson DM, et al. Regorafenib dose optimization study (ReDOS): Randomized phase II trial to evaluate dosing strategies for regorafenib in refractory metastatic colorectal cancer (mCRC): an ACCRU Network study [abstract]. J Clin Oncol 2018;36(4 suppl):611-611.
98. Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19.
99. Koopman M, Kortman GA, Mekenkamp L, et al. Deficient mismatch repair system in patients with sporadic advanced colorectal cancer. Br J Cancer 2009;100:266–73.
100. Venderbosch S, Nagtegaal ID, Maughan TS, et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–30.
101. Lochhead P, Kuchiba A, Imamura Y, et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst 2013;105:1151–6.
102. Le DT, Uram JN, Wang H, et al. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–20.
103. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–91.
104. Daste A, Domblides C, Gross-Goupil M, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer 2017;82:155–66.
105. Elias R, Giobbie-Hurder A, McCleary NJ, et al. Efficacy of PD-1 & PD-L1 inhibitors in older adults: a meta-analysis. J Immunother Cancer 2018;6:26.
106. Singh H, Kim G, Maher VE, et al. FDA subset analysis of the safety of nivolumab in elderly patients with advanced cancers [abstract]. J Clin Oncol 2016;34(15 suppl):10010-10010.
107. Temel JS, Greer JA, El-Jawahri A, et al. Effects of early integrated palliative care in patients with lung and GI cancer: a randomized clinical trial. J Clin Oncol 2017;35:834–41.
108. Brighi N, Balducci L, Biasco G. Cancer in the elderly: is it time for palliative care in geriatric oncology? J Geriatr Oncol 2014;5:197–203.
109. Meropol NJ, Egleston BL, Buzaglo JS, et al. Cancer patient preferences for quality and length of life. Cancer 2008;113:3459–66.
110. Bagshaw SM, Stelfox HT, Johnson JA, et al. Long-term association between frailty and health-related quality of life among survivors of critical illness: a prospective multicenter cohort study. Crit Care Med 2015;43:973–82.
111. Lynch MP, Marcone D, Kagan SH. Developing a multidisciplinary geriatric oncology program in a community cancer center. Clin J Oncol Nurs 2007;11:929–33.
112. Sheridan J, Walsh P, Kevans D, et al. Determinants of short- and long-term survival from colorectal cancer in very elderly patients. J Geriatr Oncol 2014;5:376–83.