User login
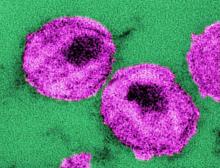
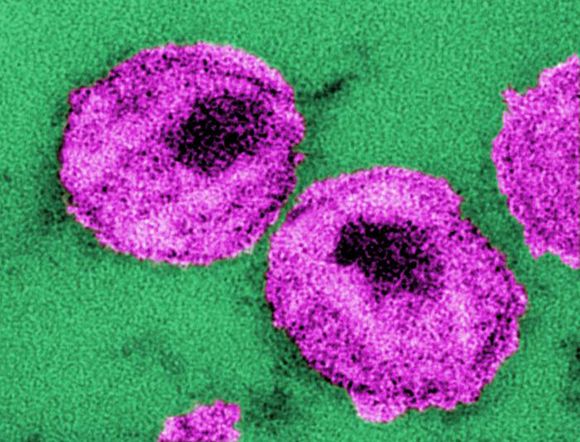
A mosaic adenovirus human immunodeficiency virus-1 (HIV-1) vaccine induced robust immune responses in humans and rhesus monkeys, and significantly protected the monkeys against repetitive simian/HIV (SHIV) mosaic challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa, according to a report published online in The Lancet.
Because a mosaic combination of antigens can induce an immunogenic response to a broad geographic range of viral subtypes, such a vaccine can offer the theoretical possibility of developing a global HIV-1 vaccine, according to Dan H. Barouch, MD, of Harvard Medical School, Boston, and his colleagues.
The researchers conducted a multicenter, randomized, double-blind, placebo-controlled, phase 1/2a trial (APPROACH) with 393 healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection and were recruited from 12 clinics in East Africa, South Africa, Thailand, and the United States. Participants were primed at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0.5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and given boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0.5 mL) vectors with or without adjuvanted Env gp140 protein. The placebo group received 0.9% saline.
Eligible participants were randomly assigned to one of eight study groups: Ad26/Ad26 plus high-dose gp140; Ad26/Ad26 plus low-dose gp140; Ad26/Ad26; Ad26/MVA plus high-dose gp140; Ad26/MVA plus low-dose gp140; Ad26/MVA; Ad26/high-dose gp140; and placebo. “Overall, no substantial differences in safety or tolerability of any of the seven active vaccine groups were observed,” according to Dr. Barouch and his colleagues.
In addition, the researchers vaccinated 72 Indian-origin rhesus monkeys (Macaca mulatta) using a study design that was similar to that of the human APPROACH clinical study.
The mosaic HIV-1 vaccine “induced robust humoral and cellular immune responses in both humans and rhesus monkeys,” which were similar in both species in “magnitude, durability, and phenotype,” the investigators reported. In addition, the vaccine “provided 67% protection against acquisition of six intrarectal SHIV challenges in rhesus monkeys.”
Based on these results, the mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine sufficiently met “pre-established safety and immunogenicity criteria to advance into a phase 2b clinical efficacy study in sub-Saharan Africa, which is now underway (NCT03060629),” according to the authors.
“Previous HIV-1 vaccine candidates have typically been limited to specific regions of the world. Optimized mosaic antigens offer the theoretical possibility of developing a global HIV-1 vaccine,” Dr. Barouch and his colleagues concluded.
This study was funded in part by Janssen Vaccines & Prevention BV and the National Institutes of Health. Dr. Barouch has received grant funding from Janssen Vaccines & Prevention BV and is a co-inventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention.
SOURCE: Barouch DH et al., The Lancet. 2018 July 6. doi: 10.1016/S0140-6736(18)31364-3.
A mosaic adenovirus human immunodeficiency virus-1 (HIV-1) vaccine induced robust immune responses in humans and rhesus monkeys, and significantly protected the monkeys against repetitive simian/HIV (SHIV) mosaic challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa, according to a report published online in The Lancet.
Because a mosaic combination of antigens can induce an immunogenic response to a broad geographic range of viral subtypes, such a vaccine can offer the theoretical possibility of developing a global HIV-1 vaccine, according to Dan H. Barouch, MD, of Harvard Medical School, Boston, and his colleagues.
The researchers conducted a multicenter, randomized, double-blind, placebo-controlled, phase 1/2a trial (APPROACH) with 393 healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection and were recruited from 12 clinics in East Africa, South Africa, Thailand, and the United States. Participants were primed at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0.5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and given boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0.5 mL) vectors with or without adjuvanted Env gp140 protein. The placebo group received 0.9% saline.
Eligible participants were randomly assigned to one of eight study groups: Ad26/Ad26 plus high-dose gp140; Ad26/Ad26 plus low-dose gp140; Ad26/Ad26; Ad26/MVA plus high-dose gp140; Ad26/MVA plus low-dose gp140; Ad26/MVA; Ad26/high-dose gp140; and placebo. “Overall, no substantial differences in safety or tolerability of any of the seven active vaccine groups were observed,” according to Dr. Barouch and his colleagues.
In addition, the researchers vaccinated 72 Indian-origin rhesus monkeys (Macaca mulatta) using a study design that was similar to that of the human APPROACH clinical study.
The mosaic HIV-1 vaccine “induced robust humoral and cellular immune responses in both humans and rhesus monkeys,” which were similar in both species in “magnitude, durability, and phenotype,” the investigators reported. In addition, the vaccine “provided 67% protection against acquisition of six intrarectal SHIV challenges in rhesus monkeys.”
Based on these results, the mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine sufficiently met “pre-established safety and immunogenicity criteria to advance into a phase 2b clinical efficacy study in sub-Saharan Africa, which is now underway (NCT03060629),” according to the authors.
“Previous HIV-1 vaccine candidates have typically been limited to specific regions of the world. Optimized mosaic antigens offer the theoretical possibility of developing a global HIV-1 vaccine,” Dr. Barouch and his colleagues concluded.
This study was funded in part by Janssen Vaccines & Prevention BV and the National Institutes of Health. Dr. Barouch has received grant funding from Janssen Vaccines & Prevention BV and is a co-inventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention.
SOURCE: Barouch DH et al., The Lancet. 2018 July 6. doi: 10.1016/S0140-6736(18)31364-3.
A mosaic adenovirus human immunodeficiency virus-1 (HIV-1) vaccine induced robust immune responses in humans and rhesus monkeys, and significantly protected the monkeys against repetitive simian/HIV (SHIV) mosaic challenges in rhesus monkeys. This vaccine concept is currently being evaluated in a phase 2b clinical efficacy study in sub-Saharan Africa, according to a report published online in The Lancet.
Because a mosaic combination of antigens can induce an immunogenic response to a broad geographic range of viral subtypes, such a vaccine can offer the theoretical possibility of developing a global HIV-1 vaccine, according to Dan H. Barouch, MD, of Harvard Medical School, Boston, and his colleagues.
The researchers conducted a multicenter, randomized, double-blind, placebo-controlled, phase 1/2a trial (APPROACH) with 393 healthy, HIV-1-uninfected participants (aged 18-50 years) who were considered at low risk for HIV-1 infection and were recruited from 12 clinics in East Africa, South Africa, Thailand, and the United States. Participants were primed at weeks 0 and 12 with Ad26.Mos.HIV (5 × 1010 viral particles per 0.5 mL) expressing mosaic HIV-1 envelope (Env)/Gag/Pol antigens and given boosters at weeks 24 and 48 with Ad26.Mos.HIV or modified vaccinia Ankara (MVA; 108 plaque-forming units per 0.5 mL) vectors with or without adjuvanted Env gp140 protein. The placebo group received 0.9% saline.
Eligible participants were randomly assigned to one of eight study groups: Ad26/Ad26 plus high-dose gp140; Ad26/Ad26 plus low-dose gp140; Ad26/Ad26; Ad26/MVA plus high-dose gp140; Ad26/MVA plus low-dose gp140; Ad26/MVA; Ad26/high-dose gp140; and placebo. “Overall, no substantial differences in safety or tolerability of any of the seven active vaccine groups were observed,” according to Dr. Barouch and his colleagues.
In addition, the researchers vaccinated 72 Indian-origin rhesus monkeys (Macaca mulatta) using a study design that was similar to that of the human APPROACH clinical study.
The mosaic HIV-1 vaccine “induced robust humoral and cellular immune responses in both humans and rhesus monkeys,” which were similar in both species in “magnitude, durability, and phenotype,” the investigators reported. In addition, the vaccine “provided 67% protection against acquisition of six intrarectal SHIV challenges in rhesus monkeys.”
Based on these results, the mosaic Ad26/Ad26 plus gp140 HIV-1 vaccine sufficiently met “pre-established safety and immunogenicity criteria to advance into a phase 2b clinical efficacy study in sub-Saharan Africa, which is now underway (NCT03060629),” according to the authors.
“Previous HIV-1 vaccine candidates have typically been limited to specific regions of the world. Optimized mosaic antigens offer the theoretical possibility of developing a global HIV-1 vaccine,” Dr. Barouch and his colleagues concluded.
This study was funded in part by Janssen Vaccines & Prevention BV and the National Institutes of Health. Dr. Barouch has received grant funding from Janssen Vaccines & Prevention BV and is a co-inventor on HIV-1 vaccine antigen patents that have been licensed to Janssen Vaccines & Prevention.
SOURCE: Barouch DH et al., The Lancet. 2018 July 6. doi: 10.1016/S0140-6736(18)31364-3.
FROM THE LANCET