The antipsychotic lurasidone (Latuda, Sunovion Pharmaceuticals) has long-term efficacy in the treatment of bipolar depression (BD) in children and adolescents, new research suggests.
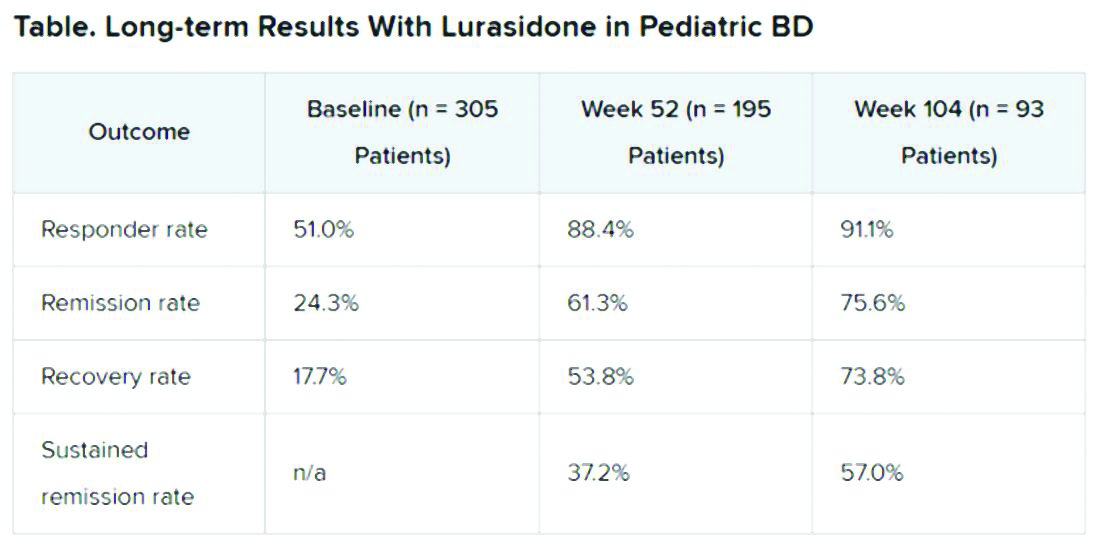
In an open-label extension study involving patients aged 10-17 years, up to 2 years of treatment with lurasidone was associated with continued improvement in depressive symptoms. There were progressively higher rates of remission, recovery, and sustained remission.
Coinvestigator Manpreet K. Singh, MD, director of the Stanford Pediatric Mood Disorders Program, Stanford (Calif.) University, noted that early onset of BD is common. Although in pediatric populations, prevalence has been fairly stable at around 1.8%, these patients have “a very limited number of treatment options available for the depressed phases of BD,” which is often predominant and can be difficult to identify.
“A lot of youths who are experiencing depressive symptoms in the context of having had a manic episode will often have a relapsing and remitting course, even after the acute phase of treatment, so because kids can be on medications for long periods of time, a better understanding of what works ... is very important,” Dr. Singh said in an interview.
The findings were presented at the virtual American Society of Clinical Psychopharmacology (ASCP) 2020 annual meeting.
Long-term Efficacy
The Food and Drug Administration approved lurasidone as monotherapy for BD in children and adolescents in 2018. The aim of the current study was to evaluate the drug’s long-term efficacy in achieving response or remission in this population.
A total of 305 children who completed an initial 6-week double-blind study of lurasidone versus placebo entered the 2-year, open-label extension study. In the extension, they either continued taking lurasidone or were switched from placebo to lurasidone 20-80 mg/day. Of this group, 195 children completed 52 weeks of treatment, and 93 completed 104 weeks of treatment.
Efficacy was measured with the Children’s Depression Rating Scale, Revised (CDRS-R) and the Clinical Global Impression, Bipolar Depression Severity scale (CGI-BP-S). Functioning was evaluated with the clinician-rated Children’s Global Assessment Scale (CGAS); on that scale, a score of 70 or higher indicates no clinically meaningful functional impairment.
Remission criteria were met if a patient achieved a CDRS-R total score of 28 or less, a Young Mania Rating Scale (YMRS) total score of 8 or less, and a CGI-BP-S depression score of 3 or less.
Recovery criteria were met if a patient achieved remission and had a CGAS score of at least 70.
Sustained remission, a more stringent outcome, required that the patient meet remission criteria for at least 24 consecutive weeks.
In addition, there was a strong inverse correlation (r = –0.71) between depression severity, as measured by CDRS-R total score, and functioning, as measured by the CGAS.
“That’s the cool thing: As the depression symptoms and severity came down, the overall functioning in these kids improved,” Dr. Singh noted.
“This improvement in functioning ends up being much more clinically relevant and useful to clinicians than just showing an improvement in a set of symptoms because what brings a kid – or even an adult, for that matter – to see a clinician to get treatment is because something about their symptoms is causing significant functional impairment,” she said.
“So this is the take-home message: You can see that lurasidone ... demonstrates not just recovery from depressive symptoms but that this reduction in depressive symptoms corresponds to an improvement in functioning for these youths,” she added.