User login
Case Report
A 63-year-old woman was referred to our clinic for evaluation of multiple papules and nodules on the neck and trunk that had been present for 2 years. Three years prior to presentation she had been diagnosed with systemic sclerosis (SSc) after developing progressive diffuse cutaneous sclerosis, Raynaud phenomenon with digital pitted scarring, esophageal dysmotility, myositis, pericardial effusion, and interstitial lung disease. Serologic test results were positive for anti-Scl-70 antibodies. Antinuclear antibody test results were negative for anti–double-stranded DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 antibodies. The patient was treated with prednisolone 7.5 mg daily, nifedipine 15 mg daily, valsartan 80 mg daily, manidipine 20 mg daily, omeprazole 20 mg daily, and beraprost 80 mg daily. One year later, numerous asymptomatic flesh-colored papules and nodules developed on the neck, chest, abdomen, and back. There was no history of trauma or surgery at any of the affected sites.
On further investigation, anti–hepatitis C virus (HCV) antibodies were identified and confirmed by HCV ribonucleic acid polymerase chain reaction at the same time that the diagnosis of SSc was established. Hepatitis C virus genotype 3a was noted, and the patient’s viral load was 378,000 IU/mL. Therefore, a diagnosis of chronic HCV infection was established. The patient was initially unable to receive medical treatment due to lack of finances. A year and a half following the diagnosis of HCV infection, with worsening liver function tests and increasing viral load (1,369,113 IU/mL), the patient began therapy with peginterferon alfa-2b 80 mg weekly and ribavirin 800 mg daily. However, the medications were discontinued after 2 months when she developed severe hemolytic anemia related to ribavirin.
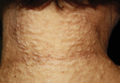
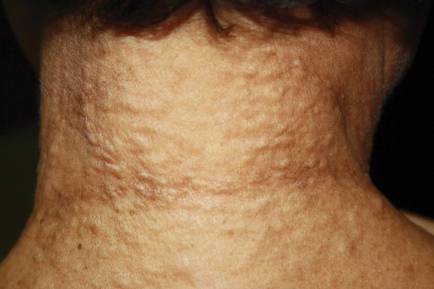
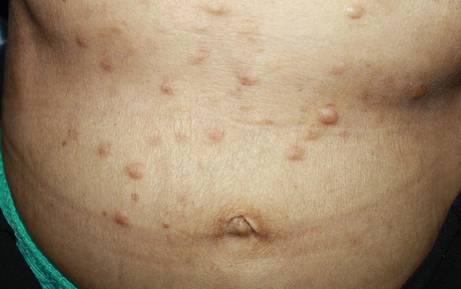
On physical examination, the patient was noted to have a masklike facies with a pinched nose and constricted opening of the mouth. Her skin was tightened and stiff extending from the fingers to the proximal extremities. Numerous well-circumscribed, flesh-colored, firm papules and nodules ranging from 2 to 20 mm in diameter were present on the neck (Figure 1), chest, abdomen (Figure 2), and back.
 |  |
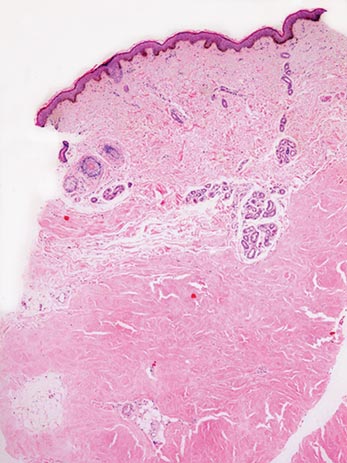
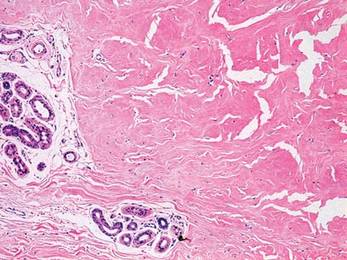
Two 4-mm punch biopsy samples obtained from a papule on the neck and a nodule on the abdomen revealed homogenized collagen bundles with scattered plump fibroblasts in the lower reticular dermis. Clinicopathologic correlation of the biopsy findings with the cutaneous examination resulted in a diagnosis of nodular scleroderma (Figures 3 and 4).
|
The patient began treatment with intralesional injections of triamcinolone 5 to 10 mg/mL for nodules as well as an ultrapotent corticosteroid cream, clobetasol propionate 0.05%, for small papules. Injections were performed at 4- to 8-week intervals and resulted in modest clinical improvement.
Comment
Scleroderma may be present only in the skin (morphea) or as a systemic disease (systemic scleroderma). Rarely, cutaneous involvement can exhibit a nodular or hypertrophic morphology, which has been described in the literature as nodular or keloidal scleroderma in a patient with known SSc1-10 and as nodular or keloidal morphea in localized cutaneous scleroderma.3,11-13
Histopathology
The distinction between the terms nodular scleroderma and keloidal scleroderma is not clear, and they are not necessarily interchangeable. To provide clarity, we find it useful to delineate specific histologic findings associated with the diagnoses of keloid, scleroderma, and the uncommon keloid/scleroderma overlap. The histopathologic findings of keloids include a fibrotic dermis and broad dispersed bundles of eosinophilic hyalinized collagen. The histopathologic findings of scleroderma include broad sclerotic bands of collagen throughout the dermis with loss of perieccrine fat. In the overlapping keloid/scleroderma condition, which is a variant of scleroderma, hyalinized collagen fibers and keloidal collagen appear in the same specimen.3,4
To distinguish these conditions, Barzilai et al5 proposed that only cases showing both clinical and histologic characteristics of a keloid should be referred to as keloidal morphea/scleroderma. They further stated that the terms nodular morphea or nodular scleroderma ought to be used only for cases that are indistinguishable histologically from scleroderma. The term morphea is appropriate when only a limited amount of skin disease is present, while scleroderma implies association with systemic disease.5 Likely, there is a histologic continuum in this variant of scleroderma, in which nodular morphea/scleroderma exists at one end and keloidal morphea/scleroderma exists at the other end.5,13
In the case of our patient, papulonodular lesions developed 1 year after the diagnosis of SSc was made, and the histopathologic examination revealed classic findings of scleroderma. As a result, our patient is most appropriately classified as having nodular scleroderma.
Clinical Features
Nodular scleroderma mostly affects young and middle-aged women and is clinically characterized by solitary or multiple firm, long-lasting papules or nodules on the upper trunk and chest, neck, and proximal extremities.1-4,6
Etiology and Pathogenesis
The triggers and cellular mechanisms of nodular scleroderma are unclear. Some authors have implicated matricellular protein and growth factors such as tenascin, connective tissue growth factor, and epidermal growth factor in nodule formation.7,8,11 Yamamoto et al9 cited chemical exposure to a silica-containing abrasive as the cause of nodular scleroderma in a worker.
Possible HCV Association
Some reports have indicated an association between nodular scleroderma and pathogens such as acid-fast bacteria10 and HCV.6 Of note, many extrahepatic conditions have been associated with HCV infection, such as membranoproliferative glomerulonephritis, cutaneous vasculitis, lichen planus, and porphyria cutanea tarda.14
The association of HCV infection with systemic autoimmune disease (SAD) has been described in a number of instances; cryoglobulinemia has most commonly been linked to HCV.15 Although the association between HCV and other SADs is less clear, there is growing interest in a possible relationship between them. To that end, physicians of the HISPAMEC (Hispanoamerican Study Group of Autoimmune Manifestations Associated With Hepatitis C Virus) study group described the clinical and immunologic characteristics of 1020 patients with SAD and associated chronic HCV infection. The 3 most frequent SADs (>90% of cases) were Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus.16 However, the strength of association differs for each SAD based on existing descriptions.16,17 Less commonly, there may be a causal relationship between HCV infection and SSc. It should be noted that most of these data are based on small series and case reports.6,16-19
The role of HCV in the pathogenesis of systemic scleroderma and other autoimmune diseases is unknown. It is also possible that the replication of HCV outside the liver, particularly in mononuclear cells, may suppress immune tolerance in genetically predisposed individuals.20
Conclusion
Nodular scleroderma associated with HCV infection is a rare entity. At present, it cannot be determined whether there is an etiopathologic association between HCV infection and SSc or whether the simultaneous diagnosis may be coincidental. Routine determination of HCV serology in scleroderma patients may help to clarify this issue.
1. Krell JM, Solomon AR, Glavey CM, et al. Nodular scleroderma. J Am Acad Dermatol. 1995;32:343-345.
2. Cannick L 3rd, Douglas G, Crater S, et al. Nodular scleroderma: case report and literature review. J Rheumatol. 2003;30:2500-2502.
3. Rencic A, Brinster NK, Nousari CH. Keloid morphea and nodular scleroderma: two distinct clinical variants of scleroderma? J Cutan Med Surg. 2003;7:20-24.
4. Wriston CC, Rubin AI, Elenitsas R, et al. Nodular scleroderma: a report of 2 cases. Am J Dermatopathol. 2008;30:385-388.
5. Barzilai A, Lyakhovitsky A, Horowitz A, et al. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327-330.
6. Melani L, Caproni M, Cardinali C, et al. A case of nodular scleroderma. J Dermatol. 2005;32:1028-1031.
7. Mizutani H, Taniguchi H, Sakakura T, et al. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;22:267-271.
8. Yamamoto T, Sawada Y, Katayama I, et al. Nodular scleroderma: increased expression of connective tissue growth factor. Dermatology. 2005;211:218-223.
9. Yamamoto T, Furuse Y, Katayama I, et al. Nodular scleroderma in a worker using a silica-containing abrasive. J Dermatol. 1994;21:751-754.
10. Cantwell AR Jr, Rowe L, Kelso DW. Nodular scleroderma and pleomorphic acid-fast bacteria. Arch Dermatol. 1980;116:1283-1290.
11. Yamamoto T, Sakashita S, Sawada Y, et al. Possible role of epidermal growth factor in the lesional skin of nodular morphea. Acta Derm Venereol. 1998;78:312-313.
12. Jain K, Dayal S, Jain VK, et al. Blaschko linear nodular morphea with dermal mucinosis. Arch Dermatol. 2007;143:953-955.
13. Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218:63-66.
14. Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620.
15. Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606-1610.
16. Ramos-Casals M, Munoz S, Medina F, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448.
17. Ramos-Casals M, Jara LJ, Medina F, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257:549-557.
18. Abu-Shakra M, Sukenik S, Buskila D. Systemic sclerosis: another rheumatic disease associated with hepatitis C virus infection. Clin Rheumatol. 2000;19:378-380.
19. Yamamoto M, Yamamoto T, Tsuboi R. Discoid lupus erythematosus in a patient with scleroderma and hepatitis C virus infection. Rheumatol Int. 2010;30:969-971.
20. Abu-Shakra M, Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1992;55:285-313.
Case Report
A 63-year-old woman was referred to our clinic for evaluation of multiple papules and nodules on the neck and trunk that had been present for 2 years. Three years prior to presentation she had been diagnosed with systemic sclerosis (SSc) after developing progressive diffuse cutaneous sclerosis, Raynaud phenomenon with digital pitted scarring, esophageal dysmotility, myositis, pericardial effusion, and interstitial lung disease. Serologic test results were positive for anti-Scl-70 antibodies. Antinuclear antibody test results were negative for anti–double-stranded DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 antibodies. The patient was treated with prednisolone 7.5 mg daily, nifedipine 15 mg daily, valsartan 80 mg daily, manidipine 20 mg daily, omeprazole 20 mg daily, and beraprost 80 mg daily. One year later, numerous asymptomatic flesh-colored papules and nodules developed on the neck, chest, abdomen, and back. There was no history of trauma or surgery at any of the affected sites.
On further investigation, anti–hepatitis C virus (HCV) antibodies were identified and confirmed by HCV ribonucleic acid polymerase chain reaction at the same time that the diagnosis of SSc was established. Hepatitis C virus genotype 3a was noted, and the patient’s viral load was 378,000 IU/mL. Therefore, a diagnosis of chronic HCV infection was established. The patient was initially unable to receive medical treatment due to lack of finances. A year and a half following the diagnosis of HCV infection, with worsening liver function tests and increasing viral load (1,369,113 IU/mL), the patient began therapy with peginterferon alfa-2b 80 mg weekly and ribavirin 800 mg daily. However, the medications were discontinued after 2 months when she developed severe hemolytic anemia related to ribavirin.
On physical examination, the patient was noted to have a masklike facies with a pinched nose and constricted opening of the mouth. Her skin was tightened and stiff extending from the fingers to the proximal extremities. Numerous well-circumscribed, flesh-colored, firm papules and nodules ranging from 2 to 20 mm in diameter were present on the neck (Figure 1), chest, abdomen (Figure 2), and back.
 |  |
Two 4-mm punch biopsy samples obtained from a papule on the neck and a nodule on the abdomen revealed homogenized collagen bundles with scattered plump fibroblasts in the lower reticular dermis. Clinicopathologic correlation of the biopsy findings with the cutaneous examination resulted in a diagnosis of nodular scleroderma (Figures 3 and 4).
|
The patient began treatment with intralesional injections of triamcinolone 5 to 10 mg/mL for nodules as well as an ultrapotent corticosteroid cream, clobetasol propionate 0.05%, for small papules. Injections were performed at 4- to 8-week intervals and resulted in modest clinical improvement.
Comment
Scleroderma may be present only in the skin (morphea) or as a systemic disease (systemic scleroderma). Rarely, cutaneous involvement can exhibit a nodular or hypertrophic morphology, which has been described in the literature as nodular or keloidal scleroderma in a patient with known SSc1-10 and as nodular or keloidal morphea in localized cutaneous scleroderma.3,11-13
Histopathology
The distinction between the terms nodular scleroderma and keloidal scleroderma is not clear, and they are not necessarily interchangeable. To provide clarity, we find it useful to delineate specific histologic findings associated with the diagnoses of keloid, scleroderma, and the uncommon keloid/scleroderma overlap. The histopathologic findings of keloids include a fibrotic dermis and broad dispersed bundles of eosinophilic hyalinized collagen. The histopathologic findings of scleroderma include broad sclerotic bands of collagen throughout the dermis with loss of perieccrine fat. In the overlapping keloid/scleroderma condition, which is a variant of scleroderma, hyalinized collagen fibers and keloidal collagen appear in the same specimen.3,4
To distinguish these conditions, Barzilai et al5 proposed that only cases showing both clinical and histologic characteristics of a keloid should be referred to as keloidal morphea/scleroderma. They further stated that the terms nodular morphea or nodular scleroderma ought to be used only for cases that are indistinguishable histologically from scleroderma. The term morphea is appropriate when only a limited amount of skin disease is present, while scleroderma implies association with systemic disease.5 Likely, there is a histologic continuum in this variant of scleroderma, in which nodular morphea/scleroderma exists at one end and keloidal morphea/scleroderma exists at the other end.5,13
In the case of our patient, papulonodular lesions developed 1 year after the diagnosis of SSc was made, and the histopathologic examination revealed classic findings of scleroderma. As a result, our patient is most appropriately classified as having nodular scleroderma.
Clinical Features
Nodular scleroderma mostly affects young and middle-aged women and is clinically characterized by solitary or multiple firm, long-lasting papules or nodules on the upper trunk and chest, neck, and proximal extremities.1-4,6
Etiology and Pathogenesis
The triggers and cellular mechanisms of nodular scleroderma are unclear. Some authors have implicated matricellular protein and growth factors such as tenascin, connective tissue growth factor, and epidermal growth factor in nodule formation.7,8,11 Yamamoto et al9 cited chemical exposure to a silica-containing abrasive as the cause of nodular scleroderma in a worker.
Possible HCV Association
Some reports have indicated an association between nodular scleroderma and pathogens such as acid-fast bacteria10 and HCV.6 Of note, many extrahepatic conditions have been associated with HCV infection, such as membranoproliferative glomerulonephritis, cutaneous vasculitis, lichen planus, and porphyria cutanea tarda.14
The association of HCV infection with systemic autoimmune disease (SAD) has been described in a number of instances; cryoglobulinemia has most commonly been linked to HCV.15 Although the association between HCV and other SADs is less clear, there is growing interest in a possible relationship between them. To that end, physicians of the HISPAMEC (Hispanoamerican Study Group of Autoimmune Manifestations Associated With Hepatitis C Virus) study group described the clinical and immunologic characteristics of 1020 patients with SAD and associated chronic HCV infection. The 3 most frequent SADs (>90% of cases) were Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus.16 However, the strength of association differs for each SAD based on existing descriptions.16,17 Less commonly, there may be a causal relationship between HCV infection and SSc. It should be noted that most of these data are based on small series and case reports.6,16-19
The role of HCV in the pathogenesis of systemic scleroderma and other autoimmune diseases is unknown. It is also possible that the replication of HCV outside the liver, particularly in mononuclear cells, may suppress immune tolerance in genetically predisposed individuals.20
Conclusion
Nodular scleroderma associated with HCV infection is a rare entity. At present, it cannot be determined whether there is an etiopathologic association between HCV infection and SSc or whether the simultaneous diagnosis may be coincidental. Routine determination of HCV serology in scleroderma patients may help to clarify this issue.
Case Report
A 63-year-old woman was referred to our clinic for evaluation of multiple papules and nodules on the neck and trunk that had been present for 2 years. Three years prior to presentation she had been diagnosed with systemic sclerosis (SSc) after developing progressive diffuse cutaneous sclerosis, Raynaud phenomenon with digital pitted scarring, esophageal dysmotility, myositis, pericardial effusion, and interstitial lung disease. Serologic test results were positive for anti-Scl-70 antibodies. Antinuclear antibody test results were negative for anti–double-stranded DNA, anti-nRNP, anti-Ro/La, anti-Sm, and anti-Jo-1 antibodies. The patient was treated with prednisolone 7.5 mg daily, nifedipine 15 mg daily, valsartan 80 mg daily, manidipine 20 mg daily, omeprazole 20 mg daily, and beraprost 80 mg daily. One year later, numerous asymptomatic flesh-colored papules and nodules developed on the neck, chest, abdomen, and back. There was no history of trauma or surgery at any of the affected sites.
On further investigation, anti–hepatitis C virus (HCV) antibodies were identified and confirmed by HCV ribonucleic acid polymerase chain reaction at the same time that the diagnosis of SSc was established. Hepatitis C virus genotype 3a was noted, and the patient’s viral load was 378,000 IU/mL. Therefore, a diagnosis of chronic HCV infection was established. The patient was initially unable to receive medical treatment due to lack of finances. A year and a half following the diagnosis of HCV infection, with worsening liver function tests and increasing viral load (1,369,113 IU/mL), the patient began therapy with peginterferon alfa-2b 80 mg weekly and ribavirin 800 mg daily. However, the medications were discontinued after 2 months when she developed severe hemolytic anemia related to ribavirin.
On physical examination, the patient was noted to have a masklike facies with a pinched nose and constricted opening of the mouth. Her skin was tightened and stiff extending from the fingers to the proximal extremities. Numerous well-circumscribed, flesh-colored, firm papules and nodules ranging from 2 to 20 mm in diameter were present on the neck (Figure 1), chest, abdomen (Figure 2), and back.
 |  |
Two 4-mm punch biopsy samples obtained from a papule on the neck and a nodule on the abdomen revealed homogenized collagen bundles with scattered plump fibroblasts in the lower reticular dermis. Clinicopathologic correlation of the biopsy findings with the cutaneous examination resulted in a diagnosis of nodular scleroderma (Figures 3 and 4).
|
The patient began treatment with intralesional injections of triamcinolone 5 to 10 mg/mL for nodules as well as an ultrapotent corticosteroid cream, clobetasol propionate 0.05%, for small papules. Injections were performed at 4- to 8-week intervals and resulted in modest clinical improvement.
Comment
Scleroderma may be present only in the skin (morphea) or as a systemic disease (systemic scleroderma). Rarely, cutaneous involvement can exhibit a nodular or hypertrophic morphology, which has been described in the literature as nodular or keloidal scleroderma in a patient with known SSc1-10 and as nodular or keloidal morphea in localized cutaneous scleroderma.3,11-13
Histopathology
The distinction between the terms nodular scleroderma and keloidal scleroderma is not clear, and they are not necessarily interchangeable. To provide clarity, we find it useful to delineate specific histologic findings associated with the diagnoses of keloid, scleroderma, and the uncommon keloid/scleroderma overlap. The histopathologic findings of keloids include a fibrotic dermis and broad dispersed bundles of eosinophilic hyalinized collagen. The histopathologic findings of scleroderma include broad sclerotic bands of collagen throughout the dermis with loss of perieccrine fat. In the overlapping keloid/scleroderma condition, which is a variant of scleroderma, hyalinized collagen fibers and keloidal collagen appear in the same specimen.3,4
To distinguish these conditions, Barzilai et al5 proposed that only cases showing both clinical and histologic characteristics of a keloid should be referred to as keloidal morphea/scleroderma. They further stated that the terms nodular morphea or nodular scleroderma ought to be used only for cases that are indistinguishable histologically from scleroderma. The term morphea is appropriate when only a limited amount of skin disease is present, while scleroderma implies association with systemic disease.5 Likely, there is a histologic continuum in this variant of scleroderma, in which nodular morphea/scleroderma exists at one end and keloidal morphea/scleroderma exists at the other end.5,13
In the case of our patient, papulonodular lesions developed 1 year after the diagnosis of SSc was made, and the histopathologic examination revealed classic findings of scleroderma. As a result, our patient is most appropriately classified as having nodular scleroderma.
Clinical Features
Nodular scleroderma mostly affects young and middle-aged women and is clinically characterized by solitary or multiple firm, long-lasting papules or nodules on the upper trunk and chest, neck, and proximal extremities.1-4,6
Etiology and Pathogenesis
The triggers and cellular mechanisms of nodular scleroderma are unclear. Some authors have implicated matricellular protein and growth factors such as tenascin, connective tissue growth factor, and epidermal growth factor in nodule formation.7,8,11 Yamamoto et al9 cited chemical exposure to a silica-containing abrasive as the cause of nodular scleroderma in a worker.
Possible HCV Association
Some reports have indicated an association between nodular scleroderma and pathogens such as acid-fast bacteria10 and HCV.6 Of note, many extrahepatic conditions have been associated with HCV infection, such as membranoproliferative glomerulonephritis, cutaneous vasculitis, lichen planus, and porphyria cutanea tarda.14
The association of HCV infection with systemic autoimmune disease (SAD) has been described in a number of instances; cryoglobulinemia has most commonly been linked to HCV.15 Although the association between HCV and other SADs is less clear, there is growing interest in a possible relationship between them. To that end, physicians of the HISPAMEC (Hispanoamerican Study Group of Autoimmune Manifestations Associated With Hepatitis C Virus) study group described the clinical and immunologic characteristics of 1020 patients with SAD and associated chronic HCV infection. The 3 most frequent SADs (>90% of cases) were Sjögren syndrome, rheumatoid arthritis, and systemic lupus erythematosus.16 However, the strength of association differs for each SAD based on existing descriptions.16,17 Less commonly, there may be a causal relationship between HCV infection and SSc. It should be noted that most of these data are based on small series and case reports.6,16-19
The role of HCV in the pathogenesis of systemic scleroderma and other autoimmune diseases is unknown. It is also possible that the replication of HCV outside the liver, particularly in mononuclear cells, may suppress immune tolerance in genetically predisposed individuals.20
Conclusion
Nodular scleroderma associated with HCV infection is a rare entity. At present, it cannot be determined whether there is an etiopathologic association between HCV infection and SSc or whether the simultaneous diagnosis may be coincidental. Routine determination of HCV serology in scleroderma patients may help to clarify this issue.
1. Krell JM, Solomon AR, Glavey CM, et al. Nodular scleroderma. J Am Acad Dermatol. 1995;32:343-345.
2. Cannick L 3rd, Douglas G, Crater S, et al. Nodular scleroderma: case report and literature review. J Rheumatol. 2003;30:2500-2502.
3. Rencic A, Brinster NK, Nousari CH. Keloid morphea and nodular scleroderma: two distinct clinical variants of scleroderma? J Cutan Med Surg. 2003;7:20-24.
4. Wriston CC, Rubin AI, Elenitsas R, et al. Nodular scleroderma: a report of 2 cases. Am J Dermatopathol. 2008;30:385-388.
5. Barzilai A, Lyakhovitsky A, Horowitz A, et al. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327-330.
6. Melani L, Caproni M, Cardinali C, et al. A case of nodular scleroderma. J Dermatol. 2005;32:1028-1031.
7. Mizutani H, Taniguchi H, Sakakura T, et al. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;22:267-271.
8. Yamamoto T, Sawada Y, Katayama I, et al. Nodular scleroderma: increased expression of connective tissue growth factor. Dermatology. 2005;211:218-223.
9. Yamamoto T, Furuse Y, Katayama I, et al. Nodular scleroderma in a worker using a silica-containing abrasive. J Dermatol. 1994;21:751-754.
10. Cantwell AR Jr, Rowe L, Kelso DW. Nodular scleroderma and pleomorphic acid-fast bacteria. Arch Dermatol. 1980;116:1283-1290.
11. Yamamoto T, Sakashita S, Sawada Y, et al. Possible role of epidermal growth factor in the lesional skin of nodular morphea. Acta Derm Venereol. 1998;78:312-313.
12. Jain K, Dayal S, Jain VK, et al. Blaschko linear nodular morphea with dermal mucinosis. Arch Dermatol. 2007;143:953-955.
13. Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218:63-66.
14. Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620.
15. Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606-1610.
16. Ramos-Casals M, Munoz S, Medina F, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448.
17. Ramos-Casals M, Jara LJ, Medina F, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257:549-557.
18. Abu-Shakra M, Sukenik S, Buskila D. Systemic sclerosis: another rheumatic disease associated with hepatitis C virus infection. Clin Rheumatol. 2000;19:378-380.
19. Yamamoto M, Yamamoto T, Tsuboi R. Discoid lupus erythematosus in a patient with scleroderma and hepatitis C virus infection. Rheumatol Int. 2010;30:969-971.
20. Abu-Shakra M, Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1992;55:285-313.
1. Krell JM, Solomon AR, Glavey CM, et al. Nodular scleroderma. J Am Acad Dermatol. 1995;32:343-345.
2. Cannick L 3rd, Douglas G, Crater S, et al. Nodular scleroderma: case report and literature review. J Rheumatol. 2003;30:2500-2502.
3. Rencic A, Brinster NK, Nousari CH. Keloid morphea and nodular scleroderma: two distinct clinical variants of scleroderma? J Cutan Med Surg. 2003;7:20-24.
4. Wriston CC, Rubin AI, Elenitsas R, et al. Nodular scleroderma: a report of 2 cases. Am J Dermatopathol. 2008;30:385-388.
5. Barzilai A, Lyakhovitsky A, Horowitz A, et al. Keloid-like scleroderma. Am J Dermatopathol. 2003;25:327-330.
6. Melani L, Caproni M, Cardinali C, et al. A case of nodular scleroderma. J Dermatol. 2005;32:1028-1031.
7. Mizutani H, Taniguchi H, Sakakura T, et al. Nodular scleroderma: focally increased tenascin expression differing from that in the surrounding scleroderma skin. J Dermatol. 1995;22:267-271.
8. Yamamoto T, Sawada Y, Katayama I, et al. Nodular scleroderma: increased expression of connective tissue growth factor. Dermatology. 2005;211:218-223.
9. Yamamoto T, Furuse Y, Katayama I, et al. Nodular scleroderma in a worker using a silica-containing abrasive. J Dermatol. 1994;21:751-754.
10. Cantwell AR Jr, Rowe L, Kelso DW. Nodular scleroderma and pleomorphic acid-fast bacteria. Arch Dermatol. 1980;116:1283-1290.
11. Yamamoto T, Sakashita S, Sawada Y, et al. Possible role of epidermal growth factor in the lesional skin of nodular morphea. Acta Derm Venereol. 1998;78:312-313.
12. Jain K, Dayal S, Jain VK, et al. Blaschko linear nodular morphea with dermal mucinosis. Arch Dermatol. 2007;143:953-955.
13. Kauer F, Simon JC, Sticherling M. Nodular morphea. Dermatology. 2009;218:63-66.
14. Gumber SC, Chopra S. Hepatitis C: a multifaceted disease. review of extrahepatic manifestations. Ann Intern Med. 1995;123:615-620.
15. Ferri C, Greco F, Longombardo G, et al. Antibodies to hepatitis C virus in patients with mixed cryoglobulinemia. Arthritis Rheum. 1991;34:1606-1610.
16. Ramos-Casals M, Munoz S, Medina F, et al. Systemic autoimmune diseases in patients with hepatitis C virus infection: characterization of 1020 cases (The HISPAMEC Registry). J Rheumatol. 2009;36:1442-1448.
17. Ramos-Casals M, Jara LJ, Medina F, et al. Systemic autoimmune diseases co-existing with chronic hepatitis C virus infection (the HISPAMEC Registry): patterns of clinical and immunological expression in 180 cases. J Intern Med. 2005;257:549-557.
18. Abu-Shakra M, Sukenik S, Buskila D. Systemic sclerosis: another rheumatic disease associated with hepatitis C virus infection. Clin Rheumatol. 2000;19:378-380.
19. Yamamoto M, Yamamoto T, Tsuboi R. Discoid lupus erythematosus in a patient with scleroderma and hepatitis C virus infection. Rheumatol Int. 2010;30:969-971.
20. Abu-Shakra M, Shoenfeld Y. Chronic infections and autoimmunity. Immunol Ser. 1992;55:285-313.
Practice Points
- Nodular scleroderma is a rare form of cutaneous scleroderma that can occur in association with systemic scleroderma or localized morphea.
- The clinical features are characterized by solitary or multiple, firm, long-lasting papules or nodules on the neck, upper trunk, and proximal extremities.
- The pathogenesis is still unclear. Some reports have suggested that matricellular protein and growth factor, acid-fast bacteria, organic solvents, or the hepatitis C virus may be involved.