User login
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
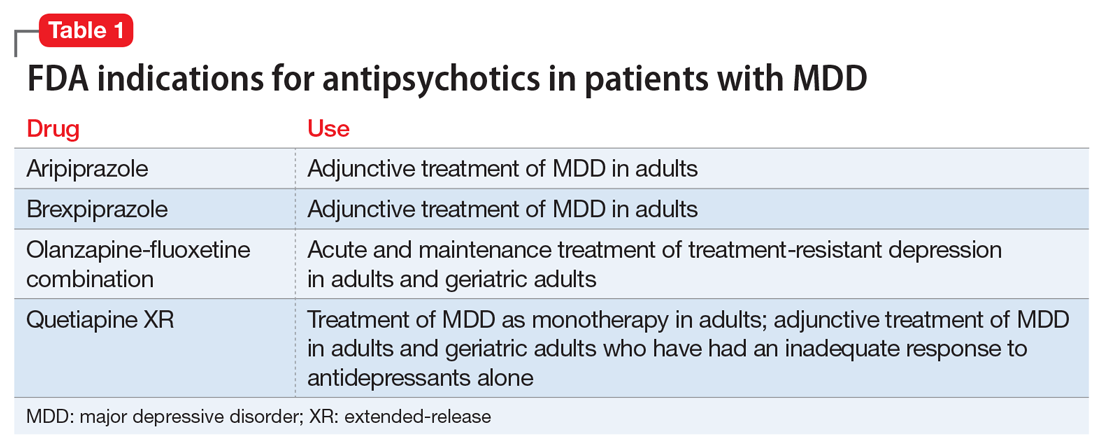
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
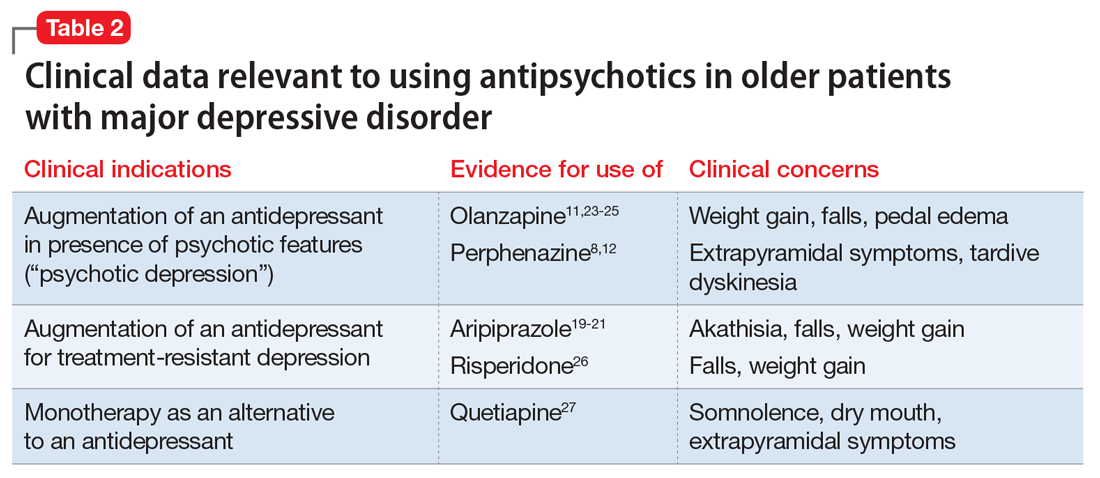
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
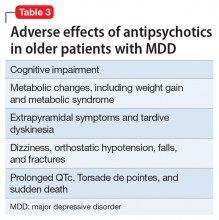
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.