User login
Prescribing antipsychotics in geriatric patients: Focus on dementia
According to the U.S. Department of Health and Human Services, in 2007, 88% of 1.4 million Medicare claims for
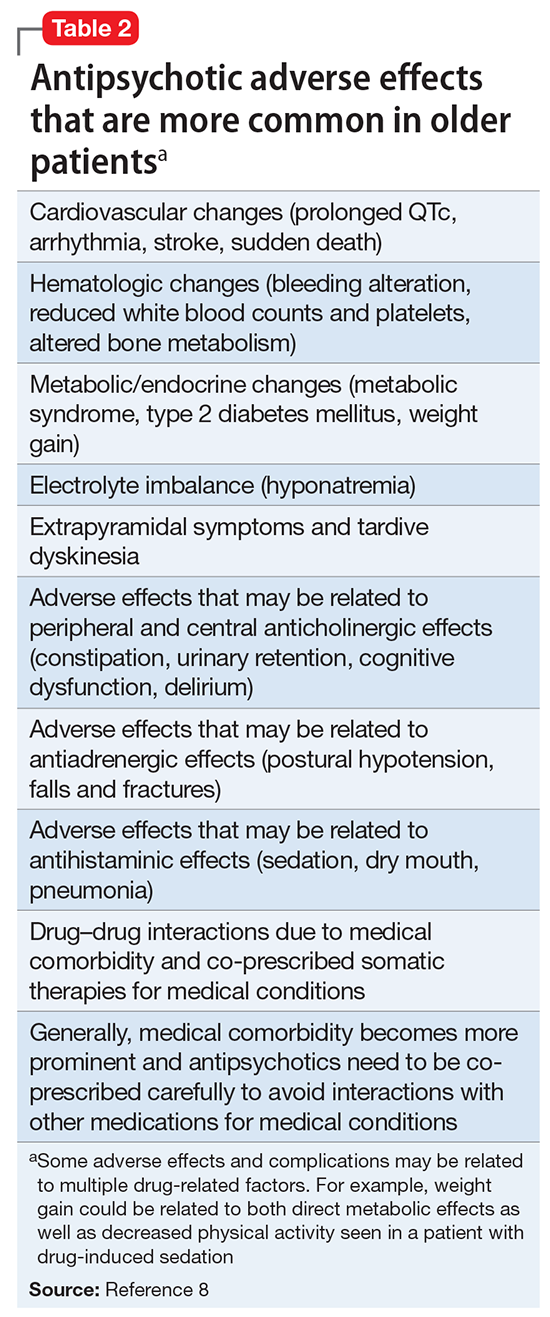
Because of the aging population and widespread prescription of antipsychotics to older patients, clinicians need information on the relative risks of using these medications in this population. In the United States, all antipsychotics carry a FDA “black-box” warning of the increased risk of death in older adults with dementia. In addition, the risk of death is increased when prescribing antipsychotics to older adults with other conditions, such as Parkinson’s disease,6 and other safety and tolerability concerns, including falls and fractures, sedation, metabolic abnormalities, and extrapyramidal effects, are highly relevant to geriatric patients.
This 3-part review summarizes findings and recommendations on prescribing antipsychotics to older individuals with schizophrenia, bipolar disorder, depression, and dementia. This third and final installment:
- briefly summarizes the major studies and analyses relevant to prescribing antipsychotics to older patients with dementia
- provides a summative opinion on safety and tolerability issues in these older patients
- highlights the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
Behavioral and psychological symptoms of dementia (BPSD) include agitation, delusional beliefs, repetitive questioning, hallucinations, aggression, wandering, and various socially inappropriate behaviors.7 These occur almost universally in all types and stages of dementia.7 BPSD are among the most complex, stressful, and costly aspects of dementia care, and lead to a myriad of poor health outcomes, including excess morbidity, mortality, hospital stays, and early nursing home placement.8-11 Because BPSD usually occur across all types and stages of dementia,7,12-16 the prevalence of BPSD mirrors the overall prevalence of dementia.
Although all expert organizations, including the American Psychiatric Association,17 recommend nonpharmacologic strategies as first-line treatment for BPSD, for the most part, these recommendations have not been translated into standard clinical management or routine care.18 Because of a perceived lack of other options, the current mainstay of treatment is the off-label use of psychotropics such as antipsychotics. Of all the agents currently used for BPSD, SGAs have the strongest evidence base, although benefits are modest at best (standardized effect size 0.13 to 0.16).19,20 In terms of individual SGAs, only risperidone is indicated for aggression in Canada and in Europe (not in the United States);
Clinical Trials
Adverse effects. A meta-analysis of RCTs of SGAs found that, compared with placebo, SGAs have increased rates of several adverse effects. These include somnolence (17% drug vs 7% placebo;
In the 42-site Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease RCT, 421 outpatients with Alzheimer’s disease and BPSD were randomized to an SGA (risperidone,
In the 2005 FDA black-box warning, pneumonia and cardiac adverse effects were cited as primary causes of death for patients with dementia taking SGAs. A subsequent observational study confirmed that use of either FGAs or SGAs in geriatric patients was associated with an increased risk of pneumonia, in a dose-dependent manner.27 Although there is limited data on cardiac adverse effects in older adults, especially those with dementia taking antipsychotics,28 1 observational study of nursing home residents29 found that those taking FGAs had a significantly higher risk of hospitalization for ventricular arrhythmia or cardiac arrest compared with those who were not taking FGAs. In contrast, there was no increased risk with SGAs.
Mortality.
In 2005, the FDA announced that based on a reanalysis of 17 placebo-controlled trials (many of which were unpublished) that SGAs were associated with a 1.7-fold increase in mortality compared with placebo.30 As a result, the FDA issued a black-box warning for using SGAs in patients with dementia. The overall OR in a published meta-analysis of mortality with SGAs was 1.54 (1.06 to 2.23; z = 2.28; P = .02), with pooled events of 3.5% mortality vs 2.3% (drug vs placebo).21 This meta-analysis21 also included ad hoc analyses of haloperidol; using combined data from 2 contrasts of haloperidol (with risperidone and quetiapine; 243 patients receiving haloperidol and 239 receiving placebo) they also found 15 deaths (6.2%) with haloperidol and 9 (3.8%) with placebo, resulting in an OR of 1.68.
Other clinical data
Observational studies. Most observational studies have confirmed concerns regarding increased mortality in patients with BPSD who take antipsychotics, with FGAs having a higher risk than SGAs18,31 and SGAs having a higher risk compared with most other psychotropics.32 Three studies that found no increase in mortality with antipsychotics in patients with dementia had methodological issues, including examining prevalence as opposed to new users,33,34 not controlling for exposure,10,33,34 power issues,10,34 not controlling for other psychiatric medications,10 and varying lengths of follow-up.10 An FDA black-box warning for FGAs was announced in 200830 based on 2 observational studies that showed an increased risk of mortality in older adults taking FGAs vs SGAs.35,36
In terms of specific SGAs, Kales et al37 examined the mortality risk associated with individual antipsychotics using various methods to control for confounding. Among a national sample of >33,000 older veterans with dementia newly started on haloperidol, risperidone, olanzapine, quetiapine, or
Most recently, a retrospective case-control study (90,786 patients age ≥65 with dementia) examined the number needed to harm (NNH; ie, number of patients needed to receive treatment that would result in 1 death) over 180 days following initiation of an FGA or SGA.38 This study found the following NNHs: haloperidol, 26 (95% CI, 15 to 99); risperidone, 27 (95% CI, 19 to 46); olanzapine, 40 (95% CI, 21 to 312); and quetiapine, 50 (95% CI, 30 to 150).38 These results are congruent with a review of observational studies that found the highest risk of mortality was associated with haloperidol and
Patterns of antipsychotic use in older dementia patients
There are high rates of antipsychotic use in patients with dementia. Before the FDA issued the black-box warning, the Aging Demographics and Memory study found that the rate of antipsychotic use in community (outpatient) older adults with dementia was approximately 19% between 2002 and 2004 in a representative sample of 307 older adults.39 Another study examining trends in community antipsychotic use in the U.S. Department of Veterans Affairs (VA) found that in the 1990s, SGA use was increasing; approximately 18% of outpatients with dementia were taking these agents.40 Use of SGAs began to decline in 2003, ahead of the 2005 black-box warning, in tandem with other advisories (eg, diabetes, metabolic syndrome,41 and stroke risk).42,43 Olanzapine and risperidone showed declining rates between 2003 and 2005, whereas quetiapine use significantly increased during this period. All 3 SGAs declined after the black-box warning. However, by the end of 2007, the use of SGAs had leveled off to approximately 12% of VA patients with dementia. A recent U.S. Government Accountability Office (GAO) report found that in 2012, 14% of older adult Medicare Part D enrollees with dementia living in the community were prescribed an antipsychotic.44
Use in nursing home residents. Because BPSD are one of the main reasons people with dementia are placed in nursing homes, it is not surprising that rates of antipsychotic use are higher in these settings than in the community. Prior to the black-box warning, studies found that 24% to 32% of nursing home residents were treated with antipsychotics.45-47 A study examining VA nursing homes (n = 133 facilities, n = 3,692 veterans) found that approximately 26% of residents were prescribed antipsychotics in 2004 to 2005.48 The Center for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care in Nursing Homes has appeared to lower antipsychotic medication use in nursing homes; the rate decreased from 24% in long-stay nursing home residents nationwide in 2011 to 19% by the end of 2014. Specific to dementia, a 2010 CMS report49 indicated that approximately 40% of nursing home residents with cognitive impairment and behavioral issues, without psychosis, received antipsychotics. The GAO data indicated that approximately 33% of older Medicare Part D enrollees with dementia who spent >100 days in a nursing home were prescribed an antipsychotic in 2012.44 A recent Canadian study using drug claims data found that overall psychotropic use in patients with dementia remains high, finding that three-fourths of all patients with dementia in long-term care are given at least 1 psychotropic, and up to one-third are prescribed SGAs.50 European data similarly show that antipsychotics continue to be prescribed to up to one-third of long-term care residents with dementia, with 7 out of 10 receiving an SGA.1

Conclusions

The Table provides a summary of the evidence regarding the use of antipsychotics in patients with dementia. Expert consensus is that among BPSD, aggression and psychosis are the primary indications for using antipsychotics.51 Based on multiple RCTs and meta-analyses, the evidence for using SGAs to treat these symptoms is moderate at best. However, in real-world practice settings, SGAs are widely used for symptoms, such as wandering, inappropriate behaviors, resistance to care, etc., for which there is no evidence for efficacy other than sedation. Furthermore, even when there is a potential for benefit, this must be balanced against the risk of adverse effects, including somnolence, worsened cognition, extrapyramidal symptoms, stroke, and mortality.
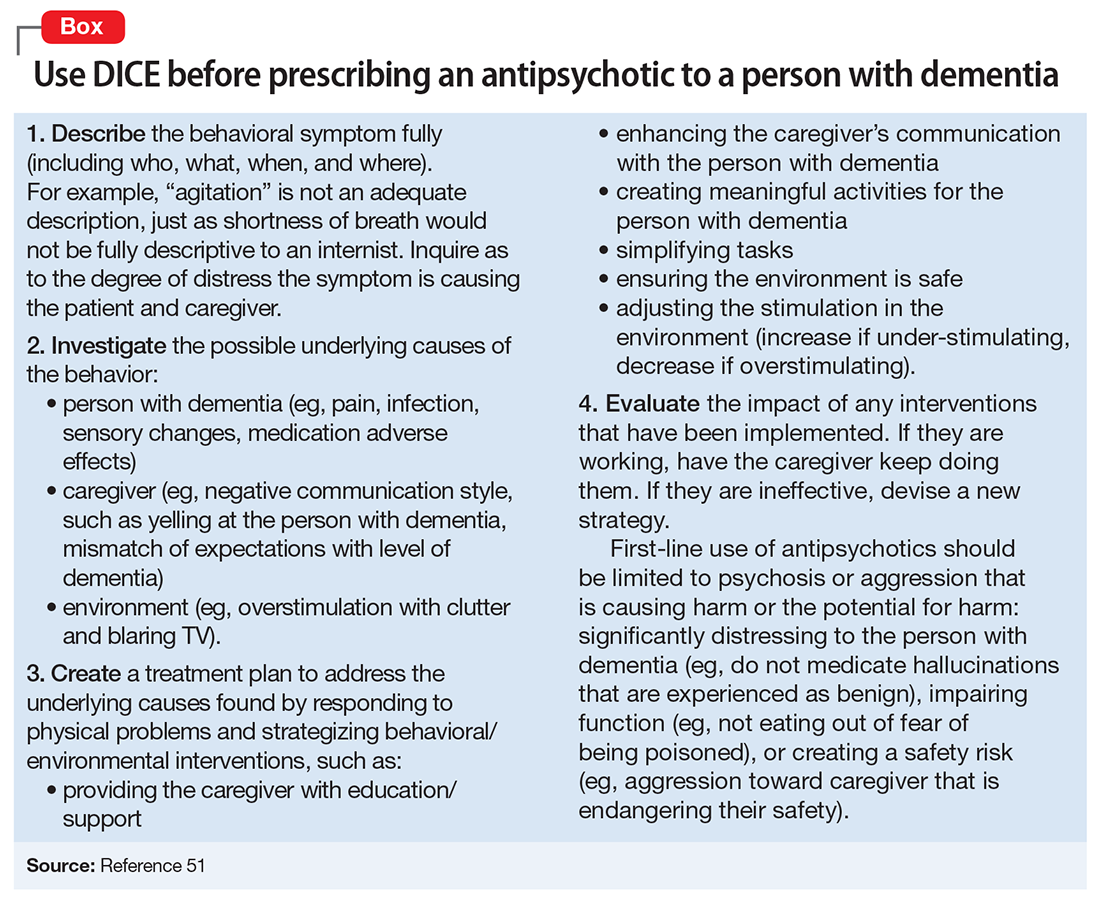
Clinicians who care for older adults with BPSD should strive to increase the use of first-line nonpharmacologic strategies, by using structured approaches such as DICE (Describe, Investigate, Create, Evaluate) described in the Box.51 Antipsychotics should be reserved for situations in which nonpharmacologic approaches are unsuccessful, or there is concern for serious or imminent risk to the patient or others.
In the future, observational studies using biomarkers, such as neuroimaging markers, of brain health in older patients taking antipsychotics for various durations may give us a better understanding of long-term antipsychotic safety and tolerability and the monitoring required to assess long-term burden of specific antipsychotics in real-world samples.52 However, because of various biases, observational data may not provide answers to all questions,53 and a major challenge is that the number of published RCTs specific to geriatric patients is not growing substantially. Pharmacotherapy evidence is not keeping up with demographic trends. Key developments in RCTs will be the inclusion of biomarkers via neuroimaging, drug serum or brain levels, and genetic profiling. Because of the modest findings of benefits of antipsychotics in dementia and safety concerns addressing brain health in preclinical or early stages, identification of effective non-drug interventions and identifying true disease-modifying agents will be the next challenges of dementia research.
1. Foebel AD, Liperoti R, Onder G, et al; SHELTER Study Investigators. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911-917.
2. Foebel A, Ballokova A, Wellens NI, et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128.
3. Parsons C, Johnston S, Mathie E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging. 2012;29(2):143-155.
4. Vidal X, Agustí A, Vallano A, et al; Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ project. Elderly patients treated with psychotropic medicines admitted to hospital: associated characteristics and inappropriate use. Eur J Clin Pharmacol. 2016;72(6):755-764.
5. Caron L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280-3297.
6. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with parkinson disease. JAMA Neurol. 2016;73(5):535-541.
7. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539.
8. Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441-449.
9. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
10. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
11. Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135-1143.
12. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026-1035.
13. Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
14. Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update-introduction. International Psychogeriatrics. 2000;12:9-12.
15. Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19(3):409-420.
16. Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145-1152.
17. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
18. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369.
19. Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553-563.
20. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom. 2007;76(4):213-218.
21. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210.
22. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:1:CD003476.
23. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
24. Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395.
25. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
26. Trifirò G, Sultana J, Spina E. Are the safety profiles of antipsychotic drugs used in dementia the same? An updated review of observational studies. Drug Saf. 2014;37(7):501-520.
27. Trifirò G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(7):418-425, W139-W140.
28. Sultana J, Trifirò G. Drug safety warnings: a message in a bottle. Analysis. 2008;179:438-446.
29. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090-1096.
30. U.S. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafety information forpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed October 20, 2017.
31. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341.
32. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
33. Simoni-Wastila L, Ryder PT, Qian J, et al. Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17(5):417-427.
34. Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15(5):416-424.
35. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
36. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
37. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
38. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
39. Rhee Y, Csernansky JG, Emanuel LL, et al. Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc. 2011;59(11):2100-2107.
40. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190-197.
41. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
42. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143.
43. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269-1270.
44. United States Government Accountability Office. Antipsychotic drug use: HHS has initiatives to reduce use among older adults in nursing homes, but should expand efforts to other settings. http://www.gao.gov/assets/670/668221.pdf. Published January 2015. Accessed October 20, 2017.
45. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89-95.
46. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24(10):1110-1118.
47. Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6(4):187-197.
48. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
49. Bonner A. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. Center for Medicare and Medicaid Services. http://ltcombudsman.org/uploads/files/support/alice-bonner-slides.pdf. Published April 28, 2013. Accessed October 20, 2017.
50. Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269.
51. Kales HC, Gitlin LN, Lyketsos CG, et al; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
52. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615.
53. Mulsant BH. Challenges of the treatment of neuropsychiatric symptoms associated with dementia. Am J Geriatr Psychiatry. 2014;22(4):317-320.
According to the U.S. Department of Health and Human Services, in 2007, 88% of 1.4 million Medicare claims for
Because of the aging population and widespread prescription of antipsychotics to older patients, clinicians need information on the relative risks of using these medications in this population. In the United States, all antipsychotics carry a FDA “black-box” warning of the increased risk of death in older adults with dementia. In addition, the risk of death is increased when prescribing antipsychotics to older adults with other conditions, such as Parkinson’s disease,6 and other safety and tolerability concerns, including falls and fractures, sedation, metabolic abnormalities, and extrapyramidal effects, are highly relevant to geriatric patients.
This 3-part review summarizes findings and recommendations on prescribing antipsychotics to older individuals with schizophrenia, bipolar disorder, depression, and dementia. This third and final installment:
- briefly summarizes the major studies and analyses relevant to prescribing antipsychotics to older patients with dementia
- provides a summative opinion on safety and tolerability issues in these older patients
- highlights the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
Behavioral and psychological symptoms of dementia (BPSD) include agitation, delusional beliefs, repetitive questioning, hallucinations, aggression, wandering, and various socially inappropriate behaviors.7 These occur almost universally in all types and stages of dementia.7 BPSD are among the most complex, stressful, and costly aspects of dementia care, and lead to a myriad of poor health outcomes, including excess morbidity, mortality, hospital stays, and early nursing home placement.8-11 Because BPSD usually occur across all types and stages of dementia,7,12-16 the prevalence of BPSD mirrors the overall prevalence of dementia.
Although all expert organizations, including the American Psychiatric Association,17 recommend nonpharmacologic strategies as first-line treatment for BPSD, for the most part, these recommendations have not been translated into standard clinical management or routine care.18 Because of a perceived lack of other options, the current mainstay of treatment is the off-label use of psychotropics such as antipsychotics. Of all the agents currently used for BPSD, SGAs have the strongest evidence base, although benefits are modest at best (standardized effect size 0.13 to 0.16).19,20 In terms of individual SGAs, only risperidone is indicated for aggression in Canada and in Europe (not in the United States);
Clinical Trials
Adverse effects. A meta-analysis of RCTs of SGAs found that, compared with placebo, SGAs have increased rates of several adverse effects. These include somnolence (17% drug vs 7% placebo;
In the 42-site Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease RCT, 421 outpatients with Alzheimer’s disease and BPSD were randomized to an SGA (risperidone,
In the 2005 FDA black-box warning, pneumonia and cardiac adverse effects were cited as primary causes of death for patients with dementia taking SGAs. A subsequent observational study confirmed that use of either FGAs or SGAs in geriatric patients was associated with an increased risk of pneumonia, in a dose-dependent manner.27 Although there is limited data on cardiac adverse effects in older adults, especially those with dementia taking antipsychotics,28 1 observational study of nursing home residents29 found that those taking FGAs had a significantly higher risk of hospitalization for ventricular arrhythmia or cardiac arrest compared with those who were not taking FGAs. In contrast, there was no increased risk with SGAs.
Mortality.
In 2005, the FDA announced that based on a reanalysis of 17 placebo-controlled trials (many of which were unpublished) that SGAs were associated with a 1.7-fold increase in mortality compared with placebo.30 As a result, the FDA issued a black-box warning for using SGAs in patients with dementia. The overall OR in a published meta-analysis of mortality with SGAs was 1.54 (1.06 to 2.23; z = 2.28; P = .02), with pooled events of 3.5% mortality vs 2.3% (drug vs placebo).21 This meta-analysis21 also included ad hoc analyses of haloperidol; using combined data from 2 contrasts of haloperidol (with risperidone and quetiapine; 243 patients receiving haloperidol and 239 receiving placebo) they also found 15 deaths (6.2%) with haloperidol and 9 (3.8%) with placebo, resulting in an OR of 1.68.
Other clinical data
Observational studies. Most observational studies have confirmed concerns regarding increased mortality in patients with BPSD who take antipsychotics, with FGAs having a higher risk than SGAs18,31 and SGAs having a higher risk compared with most other psychotropics.32 Three studies that found no increase in mortality with antipsychotics in patients with dementia had methodological issues, including examining prevalence as opposed to new users,33,34 not controlling for exposure,10,33,34 power issues,10,34 not controlling for other psychiatric medications,10 and varying lengths of follow-up.10 An FDA black-box warning for FGAs was announced in 200830 based on 2 observational studies that showed an increased risk of mortality in older adults taking FGAs vs SGAs.35,36
In terms of specific SGAs, Kales et al37 examined the mortality risk associated with individual antipsychotics using various methods to control for confounding. Among a national sample of >33,000 older veterans with dementia newly started on haloperidol, risperidone, olanzapine, quetiapine, or
Most recently, a retrospective case-control study (90,786 patients age ≥65 with dementia) examined the number needed to harm (NNH; ie, number of patients needed to receive treatment that would result in 1 death) over 180 days following initiation of an FGA or SGA.38 This study found the following NNHs: haloperidol, 26 (95% CI, 15 to 99); risperidone, 27 (95% CI, 19 to 46); olanzapine, 40 (95% CI, 21 to 312); and quetiapine, 50 (95% CI, 30 to 150).38 These results are congruent with a review of observational studies that found the highest risk of mortality was associated with haloperidol and
Patterns of antipsychotic use in older dementia patients
There are high rates of antipsychotic use in patients with dementia. Before the FDA issued the black-box warning, the Aging Demographics and Memory study found that the rate of antipsychotic use in community (outpatient) older adults with dementia was approximately 19% between 2002 and 2004 in a representative sample of 307 older adults.39 Another study examining trends in community antipsychotic use in the U.S. Department of Veterans Affairs (VA) found that in the 1990s, SGA use was increasing; approximately 18% of outpatients with dementia were taking these agents.40 Use of SGAs began to decline in 2003, ahead of the 2005 black-box warning, in tandem with other advisories (eg, diabetes, metabolic syndrome,41 and stroke risk).42,43 Olanzapine and risperidone showed declining rates between 2003 and 2005, whereas quetiapine use significantly increased during this period. All 3 SGAs declined after the black-box warning. However, by the end of 2007, the use of SGAs had leveled off to approximately 12% of VA patients with dementia. A recent U.S. Government Accountability Office (GAO) report found that in 2012, 14% of older adult Medicare Part D enrollees with dementia living in the community were prescribed an antipsychotic.44
Use in nursing home residents. Because BPSD are one of the main reasons people with dementia are placed in nursing homes, it is not surprising that rates of antipsychotic use are higher in these settings than in the community. Prior to the black-box warning, studies found that 24% to 32% of nursing home residents were treated with antipsychotics.45-47 A study examining VA nursing homes (n = 133 facilities, n = 3,692 veterans) found that approximately 26% of residents were prescribed antipsychotics in 2004 to 2005.48 The Center for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care in Nursing Homes has appeared to lower antipsychotic medication use in nursing homes; the rate decreased from 24% in long-stay nursing home residents nationwide in 2011 to 19% by the end of 2014. Specific to dementia, a 2010 CMS report49 indicated that approximately 40% of nursing home residents with cognitive impairment and behavioral issues, without psychosis, received antipsychotics. The GAO data indicated that approximately 33% of older Medicare Part D enrollees with dementia who spent >100 days in a nursing home were prescribed an antipsychotic in 2012.44 A recent Canadian study using drug claims data found that overall psychotropic use in patients with dementia remains high, finding that three-fourths of all patients with dementia in long-term care are given at least 1 psychotropic, and up to one-third are prescribed SGAs.50 European data similarly show that antipsychotics continue to be prescribed to up to one-third of long-term care residents with dementia, with 7 out of 10 receiving an SGA.1

Conclusions

The Table provides a summary of the evidence regarding the use of antipsychotics in patients with dementia. Expert consensus is that among BPSD, aggression and psychosis are the primary indications for using antipsychotics.51 Based on multiple RCTs and meta-analyses, the evidence for using SGAs to treat these symptoms is moderate at best. However, in real-world practice settings, SGAs are widely used for symptoms, such as wandering, inappropriate behaviors, resistance to care, etc., for which there is no evidence for efficacy other than sedation. Furthermore, even when there is a potential for benefit, this must be balanced against the risk of adverse effects, including somnolence, worsened cognition, extrapyramidal symptoms, stroke, and mortality.
Clinicians who care for older adults with BPSD should strive to increase the use of first-line nonpharmacologic strategies, by using structured approaches such as DICE (Describe, Investigate, Create, Evaluate) described in the Box.51 Antipsychotics should be reserved for situations in which nonpharmacologic approaches are unsuccessful, or there is concern for serious or imminent risk to the patient or others.
In the future, observational studies using biomarkers, such as neuroimaging markers, of brain health in older patients taking antipsychotics for various durations may give us a better understanding of long-term antipsychotic safety and tolerability and the monitoring required to assess long-term burden of specific antipsychotics in real-world samples.52 However, because of various biases, observational data may not provide answers to all questions,53 and a major challenge is that the number of published RCTs specific to geriatric patients is not growing substantially. Pharmacotherapy evidence is not keeping up with demographic trends. Key developments in RCTs will be the inclusion of biomarkers via neuroimaging, drug serum or brain levels, and genetic profiling. Because of the modest findings of benefits of antipsychotics in dementia and safety concerns addressing brain health in preclinical or early stages, identification of effective non-drug interventions and identifying true disease-modifying agents will be the next challenges of dementia research.
According to the U.S. Department of Health and Human Services, in 2007, 88% of 1.4 million Medicare claims for
Because of the aging population and widespread prescription of antipsychotics to older patients, clinicians need information on the relative risks of using these medications in this population. In the United States, all antipsychotics carry a FDA “black-box” warning of the increased risk of death in older adults with dementia. In addition, the risk of death is increased when prescribing antipsychotics to older adults with other conditions, such as Parkinson’s disease,6 and other safety and tolerability concerns, including falls and fractures, sedation, metabolic abnormalities, and extrapyramidal effects, are highly relevant to geriatric patients.
This 3-part review summarizes findings and recommendations on prescribing antipsychotics to older individuals with schizophrenia, bipolar disorder, depression, and dementia. This third and final installment:
- briefly summarizes the major studies and analyses relevant to prescribing antipsychotics to older patients with dementia
- provides a summative opinion on safety and tolerability issues in these older patients
- highlights the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
Behavioral and psychological symptoms of dementia (BPSD) include agitation, delusional beliefs, repetitive questioning, hallucinations, aggression, wandering, and various socially inappropriate behaviors.7 These occur almost universally in all types and stages of dementia.7 BPSD are among the most complex, stressful, and costly aspects of dementia care, and lead to a myriad of poor health outcomes, including excess morbidity, mortality, hospital stays, and early nursing home placement.8-11 Because BPSD usually occur across all types and stages of dementia,7,12-16 the prevalence of BPSD mirrors the overall prevalence of dementia.
Although all expert organizations, including the American Psychiatric Association,17 recommend nonpharmacologic strategies as first-line treatment for BPSD, for the most part, these recommendations have not been translated into standard clinical management or routine care.18 Because of a perceived lack of other options, the current mainstay of treatment is the off-label use of psychotropics such as antipsychotics. Of all the agents currently used for BPSD, SGAs have the strongest evidence base, although benefits are modest at best (standardized effect size 0.13 to 0.16).19,20 In terms of individual SGAs, only risperidone is indicated for aggression in Canada and in Europe (not in the United States);
Clinical Trials
Adverse effects. A meta-analysis of RCTs of SGAs found that, compared with placebo, SGAs have increased rates of several adverse effects. These include somnolence (17% drug vs 7% placebo;
In the 42-site Clinical Antipsychotic Trials of Intervention Effectiveness Alzheimer’s disease RCT, 421 outpatients with Alzheimer’s disease and BPSD were randomized to an SGA (risperidone,
In the 2005 FDA black-box warning, pneumonia and cardiac adverse effects were cited as primary causes of death for patients with dementia taking SGAs. A subsequent observational study confirmed that use of either FGAs or SGAs in geriatric patients was associated with an increased risk of pneumonia, in a dose-dependent manner.27 Although there is limited data on cardiac adverse effects in older adults, especially those with dementia taking antipsychotics,28 1 observational study of nursing home residents29 found that those taking FGAs had a significantly higher risk of hospitalization for ventricular arrhythmia or cardiac arrest compared with those who were not taking FGAs. In contrast, there was no increased risk with SGAs.
Mortality.
In 2005, the FDA announced that based on a reanalysis of 17 placebo-controlled trials (many of which were unpublished) that SGAs were associated with a 1.7-fold increase in mortality compared with placebo.30 As a result, the FDA issued a black-box warning for using SGAs in patients with dementia. The overall OR in a published meta-analysis of mortality with SGAs was 1.54 (1.06 to 2.23; z = 2.28; P = .02), with pooled events of 3.5% mortality vs 2.3% (drug vs placebo).21 This meta-analysis21 also included ad hoc analyses of haloperidol; using combined data from 2 contrasts of haloperidol (with risperidone and quetiapine; 243 patients receiving haloperidol and 239 receiving placebo) they also found 15 deaths (6.2%) with haloperidol and 9 (3.8%) with placebo, resulting in an OR of 1.68.
Other clinical data
Observational studies. Most observational studies have confirmed concerns regarding increased mortality in patients with BPSD who take antipsychotics, with FGAs having a higher risk than SGAs18,31 and SGAs having a higher risk compared with most other psychotropics.32 Three studies that found no increase in mortality with antipsychotics in patients with dementia had methodological issues, including examining prevalence as opposed to new users,33,34 not controlling for exposure,10,33,34 power issues,10,34 not controlling for other psychiatric medications,10 and varying lengths of follow-up.10 An FDA black-box warning for FGAs was announced in 200830 based on 2 observational studies that showed an increased risk of mortality in older adults taking FGAs vs SGAs.35,36
In terms of specific SGAs, Kales et al37 examined the mortality risk associated with individual antipsychotics using various methods to control for confounding. Among a national sample of >33,000 older veterans with dementia newly started on haloperidol, risperidone, olanzapine, quetiapine, or
Most recently, a retrospective case-control study (90,786 patients age ≥65 with dementia) examined the number needed to harm (NNH; ie, number of patients needed to receive treatment that would result in 1 death) over 180 days following initiation of an FGA or SGA.38 This study found the following NNHs: haloperidol, 26 (95% CI, 15 to 99); risperidone, 27 (95% CI, 19 to 46); olanzapine, 40 (95% CI, 21 to 312); and quetiapine, 50 (95% CI, 30 to 150).38 These results are congruent with a review of observational studies that found the highest risk of mortality was associated with haloperidol and
Patterns of antipsychotic use in older dementia patients
There are high rates of antipsychotic use in patients with dementia. Before the FDA issued the black-box warning, the Aging Demographics and Memory study found that the rate of antipsychotic use in community (outpatient) older adults with dementia was approximately 19% between 2002 and 2004 in a representative sample of 307 older adults.39 Another study examining trends in community antipsychotic use in the U.S. Department of Veterans Affairs (VA) found that in the 1990s, SGA use was increasing; approximately 18% of outpatients with dementia were taking these agents.40 Use of SGAs began to decline in 2003, ahead of the 2005 black-box warning, in tandem with other advisories (eg, diabetes, metabolic syndrome,41 and stroke risk).42,43 Olanzapine and risperidone showed declining rates between 2003 and 2005, whereas quetiapine use significantly increased during this period. All 3 SGAs declined after the black-box warning. However, by the end of 2007, the use of SGAs had leveled off to approximately 12% of VA patients with dementia. A recent U.S. Government Accountability Office (GAO) report found that in 2012, 14% of older adult Medicare Part D enrollees with dementia living in the community were prescribed an antipsychotic.44
Use in nursing home residents. Because BPSD are one of the main reasons people with dementia are placed in nursing homes, it is not surprising that rates of antipsychotic use are higher in these settings than in the community. Prior to the black-box warning, studies found that 24% to 32% of nursing home residents were treated with antipsychotics.45-47 A study examining VA nursing homes (n = 133 facilities, n = 3,692 veterans) found that approximately 26% of residents were prescribed antipsychotics in 2004 to 2005.48 The Center for Medicare and Medicaid Services (CMS) National Partnership to Improve Dementia Care in Nursing Homes has appeared to lower antipsychotic medication use in nursing homes; the rate decreased from 24% in long-stay nursing home residents nationwide in 2011 to 19% by the end of 2014. Specific to dementia, a 2010 CMS report49 indicated that approximately 40% of nursing home residents with cognitive impairment and behavioral issues, without psychosis, received antipsychotics. The GAO data indicated that approximately 33% of older Medicare Part D enrollees with dementia who spent >100 days in a nursing home were prescribed an antipsychotic in 2012.44 A recent Canadian study using drug claims data found that overall psychotropic use in patients with dementia remains high, finding that three-fourths of all patients with dementia in long-term care are given at least 1 psychotropic, and up to one-third are prescribed SGAs.50 European data similarly show that antipsychotics continue to be prescribed to up to one-third of long-term care residents with dementia, with 7 out of 10 receiving an SGA.1

Conclusions

The Table provides a summary of the evidence regarding the use of antipsychotics in patients with dementia. Expert consensus is that among BPSD, aggression and psychosis are the primary indications for using antipsychotics.51 Based on multiple RCTs and meta-analyses, the evidence for using SGAs to treat these symptoms is moderate at best. However, in real-world practice settings, SGAs are widely used for symptoms, such as wandering, inappropriate behaviors, resistance to care, etc., for which there is no evidence for efficacy other than sedation. Furthermore, even when there is a potential for benefit, this must be balanced against the risk of adverse effects, including somnolence, worsened cognition, extrapyramidal symptoms, stroke, and mortality.
Clinicians who care for older adults with BPSD should strive to increase the use of first-line nonpharmacologic strategies, by using structured approaches such as DICE (Describe, Investigate, Create, Evaluate) described in the Box.51 Antipsychotics should be reserved for situations in which nonpharmacologic approaches are unsuccessful, or there is concern for serious or imminent risk to the patient or others.
In the future, observational studies using biomarkers, such as neuroimaging markers, of brain health in older patients taking antipsychotics for various durations may give us a better understanding of long-term antipsychotic safety and tolerability and the monitoring required to assess long-term burden of specific antipsychotics in real-world samples.52 However, because of various biases, observational data may not provide answers to all questions,53 and a major challenge is that the number of published RCTs specific to geriatric patients is not growing substantially. Pharmacotherapy evidence is not keeping up with demographic trends. Key developments in RCTs will be the inclusion of biomarkers via neuroimaging, drug serum or brain levels, and genetic profiling. Because of the modest findings of benefits of antipsychotics in dementia and safety concerns addressing brain health in preclinical or early stages, identification of effective non-drug interventions and identifying true disease-modifying agents will be the next challenges of dementia research.
1. Foebel AD, Liperoti R, Onder G, et al; SHELTER Study Investigators. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911-917.
2. Foebel A, Ballokova A, Wellens NI, et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128.
3. Parsons C, Johnston S, Mathie E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging. 2012;29(2):143-155.
4. Vidal X, Agustí A, Vallano A, et al; Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ project. Elderly patients treated with psychotropic medicines admitted to hospital: associated characteristics and inappropriate use. Eur J Clin Pharmacol. 2016;72(6):755-764.
5. Caron L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280-3297.
6. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with parkinson disease. JAMA Neurol. 2016;73(5):535-541.
7. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539.
8. Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441-449.
9. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
10. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
11. Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135-1143.
12. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026-1035.
13. Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
14. Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update-introduction. International Psychogeriatrics. 2000;12:9-12.
15. Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19(3):409-420.
16. Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145-1152.
17. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
18. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369.
19. Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553-563.
20. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom. 2007;76(4):213-218.
21. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210.
22. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:1:CD003476.
23. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
24. Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395.
25. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
26. Trifirò G, Sultana J, Spina E. Are the safety profiles of antipsychotic drugs used in dementia the same? An updated review of observational studies. Drug Saf. 2014;37(7):501-520.
27. Trifirò G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(7):418-425, W139-W140.
28. Sultana J, Trifirò G. Drug safety warnings: a message in a bottle. Analysis. 2008;179:438-446.
29. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090-1096.
30. U.S. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafety information forpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed October 20, 2017.
31. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341.
32. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
33. Simoni-Wastila L, Ryder PT, Qian J, et al. Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17(5):417-427.
34. Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15(5):416-424.
35. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
36. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
37. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
38. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
39. Rhee Y, Csernansky JG, Emanuel LL, et al. Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc. 2011;59(11):2100-2107.
40. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190-197.
41. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
42. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143.
43. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269-1270.
44. United States Government Accountability Office. Antipsychotic drug use: HHS has initiatives to reduce use among older adults in nursing homes, but should expand efforts to other settings. http://www.gao.gov/assets/670/668221.pdf. Published January 2015. Accessed October 20, 2017.
45. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89-95.
46. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24(10):1110-1118.
47. Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6(4):187-197.
48. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
49. Bonner A. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. Center for Medicare and Medicaid Services. http://ltcombudsman.org/uploads/files/support/alice-bonner-slides.pdf. Published April 28, 2013. Accessed October 20, 2017.
50. Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269.
51. Kales HC, Gitlin LN, Lyketsos CG, et al; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
52. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615.
53. Mulsant BH. Challenges of the treatment of neuropsychiatric symptoms associated with dementia. Am J Geriatr Psychiatry. 2014;22(4):317-320.
1. Foebel AD, Liperoti R, Onder G, et al; SHELTER Study Investigators. Use of antipsychotic drugs among residents with dementia in European long-term care facilities: results from the SHELTER study. J Am Med Dir Assoc. 2014;15(12):911-917.
2. Foebel A, Ballokova A, Wellens NI, et al. A retrospective, longitudinal study of factors associated with new antipsychotic medication use among recently admitted long-term care residents. BMC Geriatr. 2015;15:128.
3. Parsons C, Johnston S, Mathie E, et al. Potentially inappropriate prescribing in older people with dementia in care homes: a retrospective analysis. Drugs Aging. 2012;29(2):143-155.
4. Vidal X, Agustí A, Vallano A, et al; Potentially Inappropriate Prescription in Older Patients in Spain (PIPOPS) Investigators’ project. Elderly patients treated with psychotropic medicines admitted to hospital: associated characteristics and inappropriate use. Eur J Clin Pharmacol. 2016;72(6):755-764.
5. Caron L, Cottencin O, Lapeyre-Mestre M, et al. Off-label prescribing of antipsychotics in adults, children and elderly individuals: a systematic review of recent prescription trends. Curr Pharm Des. 2015;21(23):3280-3297.
6. Weintraub D, Chiang C, Kim HM, et al. Association of antipsychotic use with mortality risk in patients with parkinson disease. JAMA Neurol. 2016;73(5):535-541.
7. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement. 2011;7(5):532-539.
8. Kales HC, Chen P, Blow FC, et al. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. Am J Geriatr Psychiatry. 2005;13(6):441-449.
9. Yaffe K, Fox P, Newcomer R, et al. Patient and caregiver characteristics and nursing home placement in patients with dementia. JAMA. 2002;287(16):2090-2097.
10. Lopez OL, Becker JT, Chang YF, et al. The long-term effects of conventional and atypical antipsychotics in patients with probable Alzheimer’s disease. Am J Psychiatry. 2013;170(9):1051-1058.
11. Vilalta-Franch J, López-Pousa S, Calvó-Perxas L, et al. Psychosis of Alzheimer disease: prevalence, incidence, persistence, risk factors, and mortality. Am J Geriatr Psychiatry. 2013;21(11):1135-1143.
12. Spalletta G, Musicco M, Padovani A, et al. Neuropsychiatric symptoms and syndromes in a large cohort of newly diagnosed, untreated patients with Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):1026-1035.
13. Steinberg M, Shao H, Zandi P, et al; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
14. Finkel SI, Burns A. Behavioral and psychological symptoms of dementia (BPSD): a clinical and research update-introduction. International Psychogeriatrics. 2000;12:9-12.
15. Lyketsos CG. Neuropsychiatric symptoms (behavioral and psychological symptoms of dementia) and the development of dementia treatments. Int Psychogeriatr. 2007;19(3):409-420.
16. Kunik ME, Snow AL, Davila JA, et al. Causes of aggressive behavior in patients with dementia. J Clin Psychiatry. 2010;71(9):1145-1152.
17. Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am J Psychiatry. 2016;173(5):543-546.
18. Kales HC, Gitlin LN, Lyketsos CG. Assessment and management of behavioral and psychological symptoms of dementia. BMJ. 2015;350:h369. doi: 10.1136/bmj.h369.
19. Schneider LS, Pollock VE, Lyness SA. A metaanalysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc. 1990;38(5):553-563.
20. Yury CA, Fisher JE. Meta-analysis of the effectiveness of atypical antipsychotics for the treatment of behavioural problems in persons with dementia. Psychother Psychosom. 2007;76(4):213-218.
21. Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14(3):191-210.
22. Ballard CG, Waite J. The effectiveness of atypical antipsychotics for aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006:1:CD003476.
23. Sink KM, Holden KF, Yaffe K. Pharmacological treatment of neuropsychiatric symptoms of dementia: a review of the evidence. JAMA. 2005;293(5):596-608.
24. Aisen PS, Cummings J, Schneider LS. Symptomatic and nonamyloid/tau based pharmacologic treatment for Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006395. doi: 10.1101/cshperspect.a006395.
25. Schneider LS, Tariot PN, Dagerman KS, et al; CATIE-AD Study Group. Effectiveness of atypical antipsychotic drugs in patients with Alzheimer’s disease. N Engl J Med. 2006;355(15):1525-1538.
26. Trifirò G, Sultana J, Spina E. Are the safety profiles of antipsychotic drugs used in dementia the same? An updated review of observational studies. Drug Saf. 2014;37(7):501-520.
27. Trifirò G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152(7):418-425, W139-W140.
28. Sultana J, Trifirò G. Drug safety warnings: a message in a bottle. Analysis. 2008;179:438-446.
29. Liperoti R, Gambassi G, Lapane KL, et al. Cerebrovascular events among elderly nursing home patients treated with conventional or atypical antipsychotics. J Clin Psychiatry. 2005;66(9):1090-1096.
30. U.S. Food and Drug Administration. Public health advisory: deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafety information forpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed October 20, 2017.
31. Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353(22):2335-2341.
32. Kales HC, Valenstein M, Kim HM, et al. Mortality risk in patients with dementia treated with antipsychotics versus other psychiatric medications. Am J Psychiatry. 2007;164(10):1568-1576; quiz 1623.
33. Simoni-Wastila L, Ryder PT, Qian J, et al. Association of antipsychotic use with hospital events and mortality among medicare beneficiaries residing in long-term care facilities. Am J Geriatr Psychiatry. 2009;17(5):417-427.
34. Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15(5):416-424.
35. Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775-786.
36. Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176(5):627-632.
37. Kales HC, Kim HM, Zivin K, et al. Risk of mortality among individual antipsychotics in patients with dementia. Am J Psychiatry. 2012;169(1):71-79.
38. Maust DT, Kim HM, Seyfried LS, et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: number needed to harm. JAMA Psychiatry. 2015;72(5):438-445.
39. Rhee Y, Csernansky JG, Emanuel LL, et al. Psychotropic medication burden and factors associated with antipsychotic use: an analysis of a population-based sample of community-dwelling older persons with dementia. J Am Geriatr Soc. 2011;59(11):2100-2107.
40. Kales HC, Zivin K, Kim HM, et al. Trends in antipsychotic use in dementia 1999-2007. Arch Gen Psychiatry. 2011;68(2):190-197.
41. American Diabetes Association; American Psychiatric Association; American Association of Clinical Endocrinologists; North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
42. Brodaty H, Ames D, Snowdon J, et al. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry. 2003;64(2):134-143.
43. Wooltorton E. Risperidone (Risperdal): increased rate of cerebrovascular events in dementia trials. CMAJ. 2002;167(11):1269-1270.
44. United States Government Accountability Office. Antipsychotic drug use: HHS has initiatives to reduce use among older adults in nursing homes, but should expand efforts to other settings. http://www.gao.gov/assets/670/668221.pdf. Published January 2015. Accessed October 20, 2017.
45. Chen Y, Briesacher BA, Field TS, et al. Unexplained variation across US nursing homes in antipsychotic prescribing rates. Arch Intern Med. 2010;170(1):89-95.
46. Feng Z, Hirdes JP, Smith TF, et al. Use of physical restraints and antipsychotic medications in nursing homes: a cross-national study. Int J Geriatr Psychiatry. 2009;24(10):1110-1118.
47. Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6(4):187-197.
48. Gellad WF, Aspinall SL, Handler SM, et al. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50(11):954-960.
49. Bonner A. Improving dementia care and reducing unnecessary use of antipsychotic medications in nursing homes. Center for Medicare and Medicaid Services. http://ltcombudsman.org/uploads/files/support/alice-bonner-slides.pdf. Published April 28, 2013. Accessed October 20, 2017.
50. Vasudev A, Shariff SZ, Liu K, et al. Trends in psychotropic dispensing among older adults with dementia living in long-term care facilities: 2004-2013. Am J Geriatr Psychiatry. 2015;23(12):1259-1269.
51. Kales HC, Gitlin LN, Lyketsos CG, et al; Detroit Expert Panel on Assessment and Management of Neuropsychiatric Symptoms of Dementia. Management of neuropsychiatric symptoms of dementia in clinical settings: recommendations from a multidisciplinary expert panel. J Am Geriatr Soc. 2014;62(4):762-769.
52. Andreasen NC, Liu D, Ziebell S, et al. Relapse duration, treatment intensity, and brain tissue loss in schizophrenia: a prospective longitudinal MRI study. Am J Psychiatry. 2013;170(6):609-615.
53. Mulsant BH. Challenges of the treatment of neuropsychiatric symptoms associated with dementia. Am J Geriatr Psychiatry. 2014;22(4):317-320.
Prescribing antipsychotics in geriatric patients: Focus on major depressive disorder
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
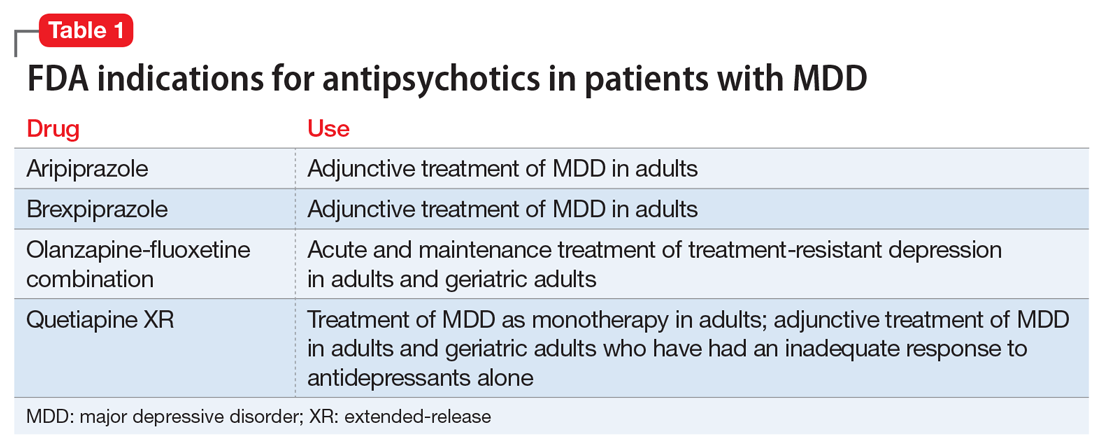
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
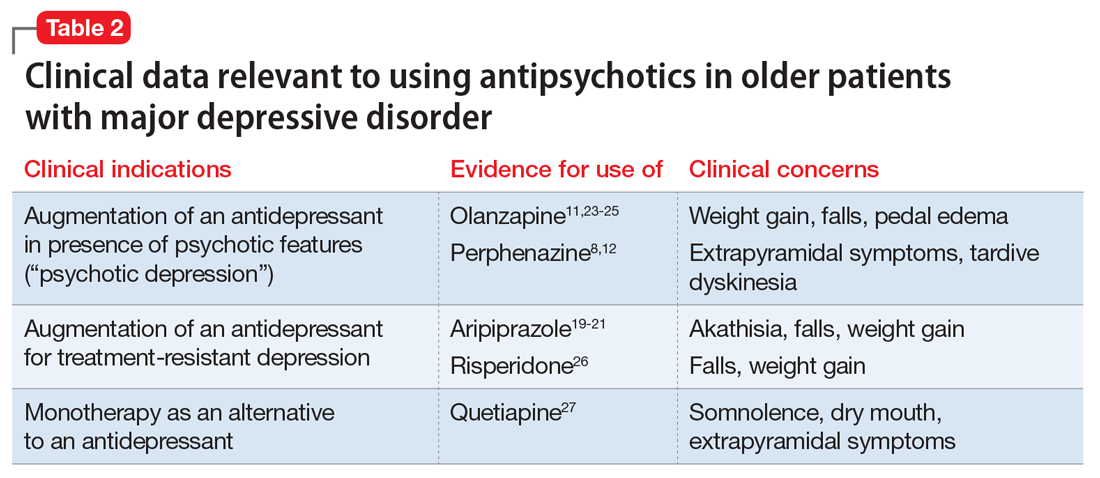
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
The proportion of older adults in the world population is growing rapidly. In the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.1 As a result, there is an increased urgency in examining benefits vs risks of antipsychotics in older individuals. In a 2010 U.S. nationally representative observational study, antipsychotic use was observed to rise slowly during early and middle adulthood, peaking at approximately age 55, declining slightly between ages 55 and 65, and then rising again after age 65, with >2% of individuals ages 80 to 84 receiving an antipsychotic.2 This is likely due to the chronology of psychotic, mood, and neurocognitive disorders across the life span. In this large national study, long-term antipsychotic treatment was common, and older patients were more likely to receive their prescriptions from non-psychiatrist physicians than from psychiatrists.2 Among patients receiving an antipsychotic, the proportion of those receiving it for >120 days was 54% for individuals ages 70 to 74; 49% for individuals ages 75 to 79; and 46% for individuals ages 80 to 84.
This 3-part review summarizes findings and risk–benefit considerations when prescribing antipsychotics to older individuals. Part 1 focused on those with chronic psychotic disorders, such as schizophrenia or bipolar disorder,3 and part 3 will cover patients with dementia. This review (part 2) aims to:
- briefly summarize the results of randomized controlled trials (RCTs) of second-generation antipsychotics (SGAs) and other major studies and analyses in older patients with major depressive disorder (MDD)
- provide a summative opinion on the relative risks and benefits associated with using antipsychotics in older adults with MDD
- highlight the gaps in the evidence base and areas that need additional research.
Summary of benefits, place in treatment armamentarium
The prevalence of MDD and clinically significant depressive symptoms in communitydwelling older adults is 3% to 4% and 15%, respectively, and as high as 16% and 50%, respectively, in nursing home residents.4 Because late-life depression is associated with suffering, disability, and excessive mortality, it needs to be recognized and treated aggressively.5 Antidepressants are the mainstay of pharmacotherapy for late-life depression. Guidelines and expert opinion informed by the current evidence recommend using selective serotonin reuptake inhibitors, such as
Over the past decade, several antipsychotics have been FDA-approved for treating MDD:
Data from several rigorously conducted RCTs support using an antidepressant plus an FGA or SGA as first-line pharmacotherapy in younger and older patients with “psychotic depression.”8-12 SGAs also can be used as augmenting agents when there is only a partial response to antidepressants.13-15 In this situation, guidelines and experts favor an augmentation strategy over switching to another antidepressant.5,9,10,16 Until recently, most published pharmacologic trials for late-life treatment-resistant depression supported using lithium to augment antidepressants.14,17 However, because several antipsychotics are now FDA-approved for treating MDD, and in light of positive findings from several studies relevant to older patients,18-21 many experts now support using SGAs to augment antidepressants in older patients with nonpsychotic depression.5,15
Clinical trials
Olanzapine plus sertraline as first-line pharmacotherapy for MDD with psychotic features. Meyers et al11 reported on a double-blind randomized comparison of olanzapine plus placebo vs olanzapine plus sertraline in 259 patients with MDD with psychotic features. An unusual feature of this trial is that it included a similar number of younger and older participants (ages 18 to 93): 117 participants were age <60 (mean age [standard deviation (SD)]: 41.3 [10.8]) and 142 were age ≥60 (mean age [SD]: 71.7 [7.8]). The same dose titration schedules based on efficacy and tolerability were used in both younger and older participants. At the end of the study, the mean dose (SD) of sertraline (or placebo) did not differ significantly in younger (174.3 mg/d [34.1]) and older participants (165.7 mg/d [43.4]). However, the mean dose (SD) of olanzapine was significantly higher in younger patients (15.7 mg/d [4.7]) than in older participants (13.4 mg/d [5.1]).
In both age groups, olanzapine plus sertraline was more efficacious than olanzapine plus placebo, and there was no statistical interaction between age, time, and treatment group (ie, the trajectories of improvement were similar in older and younger patients receiving either olanzapine or olanzapine plus sertraline). Similarly, drop-out rates because of poor tolerability did not differ significantly in younger (4.3%) and older participants (5.6%). However, in a multinomial regression, older participants were more likely to discontinue treatment because of poor tolerability.22 Older participants were significantly less likely to experience weight gain (mean [SD]: +3.3 [4.9] vs +6.5 [6.6] kg) or an increase in fasting glucose and more likely to experience a fall, pedal edema, or extrapyramidal symptoms.11,22-24 Cholesterol and triglyceride increased significantly and similarly in both age groups. The incidence of symptoms of tardive dyskinesia (TD) over the 12-week trial was low (<5%) in both younger and older participants, and clinically diagnosed TD was reported in only 1 (older) participant.25
Venlafaxine plus aripiprazole for treatment-resistant MDD. In the largest double-blind randomized study of augmentation pharmacotherapy for late-life treatment-resistant depression published to date, Lenze et al21 compared venlafaxine plus aripiprazole vs venlafaxine plus placebo in 181 patients age >60 (mean age 66, with 49 participants age >70) with MDD who did not remit after 12 weeks of treatment with venlafaxine (up to 300 mg/d). After 12 weeks of augmentation, remission rates were significantly higher with aripiprazole than with placebo: 40 (44%) vs 26 (29%); odds ratio (95% confidence interval [CI]): 2.0 (1.1 to 3.7). The median final aripiprazole dose was 7 mg/d (range 2 to 15 mg/d) in remitters and 10 mg/d (range 2 to 15 mg/d) in nonremitters.
Five of 90 participants (5%) discontinued aripiprazole (1 each: suicide, jitteriness/akathisia, worsening parkinsonism; and 2 withdrew consent); 8 of 90 (9%) discontinued placebo (2 each: lack of efficacy, headache; 1: worsening parkinsonism; and 3 withdrew consent). The completed suicide occurred after 5 weeks of treatment with aripiprazole and was judged to be “neither due to emergent suicidal ideation nor to aripiprazole side-effects, but was concluded by investigators to be a result of the individual’s persisting and long-standing suicidal ideation.”21 Including the suicide, there were 4 serious adverse events (5%) in those receiving aripiprazole (1 each: suicide, congestive heart failure, mild stroke, and diverticulitis) and 2 (2%) in those receiving placebo (1 each: myocardial infarction, hospitalized for vomiting due to accidentally taking extra venlafaxine). In 86 participants receiving aripiprazole and 87 receiving placebo, the most frequently reported adverse effects were increased dream activity (aripiprazole: 23 [27%] vs placebo: 12 [14%]), weight gain (17 [20%] vs 8 [9%]), and tremor (5 [6%] vs 0). Akathisia and parkinsonism were observed more frequently with aripiprazole than with placebo (akathisia: 24 [26%] of 91 vs 11 [12%] of 90; parkinsonism: 15 [17%] of 86 vs 2 [2%] of 81). Akathisia was generally mild and resolved with dose adjustment; however, it was associated with a transient increase in suicidality in 3 (3%) participants receiving aripiprazole vs 0 receiving placebo and persisted at the end of the trial in 5 (5%) participants receiving aripiprazole vs 2 (2%) receiving placebo. Participants receiving aripiprazole had a significantly larger increase in weight (mean [SD]: +1.93 [3.00] vs +0.01 [3.15] kg), but there were no differences between aripiprazole and placebo in changes in body fat, total cholesterol, high-density lipoprotein, low-density lipoprotein, triglycerides, glucose, insulin concentration, or QTc.
Citalopram plus risperidone for treatment-resistant MDD. Alexopoulos et al26 reported an analysis of data from 110 patients age ≥55 years (mean age [SD]: 63.4 [4.8]), among 489 mixed-age patients with MDD. Participants (n = 110) who did not respond to 1 to 3 antidepressants (venlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, or bupropion in >90%) during their current depressive episode completed 4 to 6 weeks of treatment with citalopram up to 40 mg/d; 93 did not respond and were treated with open-label risperidone (0.25 to 1 mg/d) augmentation for 4 to 6 weeks. Sixty-three (68%) of these 93 patients remitted and were randomized to 24 weeks of double-blind continuation treatment with citalopram plus risperidone vs citalopram plus placebo. Neither the median times to relapse (105 vs 57 days) nor the relapse rates (risperidone: 18 of 32 [56%] vs placebo: 20 of 31 [65%]) differed significantly. During the open-label risperidone augmentation, the most common adverse events were dizziness and dry mouth (n = 9 each, 9.7% of 93). During the continuation phase, headache (n = 3; 9.1% of 32) was observed with risperidone but not with placebo (n = 0). There was no incident parkinsonism or abnormal movements noted, but risperidone was associated with weight gain during both the open-label risperidone augmentation phase (mean [SD]: +0.9 [2.1] kg) and the continuation phase (risperidone: +0.8 [3.5] vs placebo: −0.3 [2.8] kg).
Quetiapine XR monotherapy for MDD. Katila et al27 reported on a placebo-controlled RCT of quetiapine XR (median dose, 158.7 mg/d; range, 50 to 300 mg/d) in 338 patients age ≥66 years (mean age [SD], 71.3 [7.5]) presenting with MDD and a major depressive episode with a duration <1 year and no history of failed antidepressants trials from 2 classes (more than two-thirds of participants had not received treatment). After 9 weeks, the reduction in depressive symptoms on the Montgomery-Åsberg Depression Rating Scale was significantly larger with quetiapine XR than with placebo (mean [SD]: −16.0 [9.3] vs −9.0 [9.9]). There were congruent, significant differences between quetiapine and placebo in terms of response rate (quetiapine XR: 105 of 164 [64%] vs placebo: 52 of 171 [30.4%]) and remission rate (92 of 164 [56.1%] vs placebo: 40 of 171 [23.4%]). The drop-out rates for all causes were similar, but the drop-out rate attributed to adverse events was higher with quetiapine than placebo (16 of 166 [9.6%] vs 7 of 172 [4.1%]). Most quetiapine drop-outs were attributable to dizziness, headache, and somnolence (n = 4 each), and placebo drop-outs were because of headache (n = 2). Consistent with the profile of quetiapine, adverse events with a rate that was at least 5% higher with quetiapine than with placebo included somnolence (64 of 166 [38.6%] vs 16 of 172 [9.3%]), dry mouth (34 [20.5%] vs 18 [10.5%]), and extrapyramidal symptoms (12 [7.2%] vs 4 [2.3%]). Changes in weight and laboratory test results (eg, glucose, lipid profile) were minimal and not clinically meaningful.
Other clinical data. The efficacy and relatively good tolerability of aripiprazole in older patients with treatment-resistant depression observed in the RCT by Lenze et al21 is congruent with the earlier results of 2 small (N = 20 and 24) pilot studies.18,19 In both studies, the remission rate was 50%, and the most prevalent adverse effects were agitation/restlessness/akathisia or drowsiness/sedation. Similarly, in a post hoc pooled analysis of 409 participants ages 50 to 67 from 3 placebo-controlled randomized trials, the remission rate was significantly higher with aripiprazole than with placebo (32.5% vs 17.1%), and the most common adverse effects were akathisia or restlessness (64 of 210 [30.4%]), somnolence (18 [8.6%]), and insomnia (17 [8.1%]).20
Clinical considerations
When assessing the relative benefits and risks of antipsychotics in older patients, it is important to remember that conclusions and summative opinions are necessarily influenced by the source of the data. Because much of what we know about the use of antipsychotics in geriatric adults is from clinical trials, we know more about their acute efficacy and tolerability than their long-term effectiveness and safety.28 There are similar issues regarding the role of antipsychotics in treating MDD in late life. Based on the results of several RCTs,8,11 a combination of an antidepressant plus an antipsychotic is the recommended pharmacotherapy for the acute treatment of MDD with psychotic features (Table 2).8,11,12,19-21,23-27 However, there are no published data to guide how long the antipsychotic should be continued.29
In older patients with MDD without psychotic features, 1 relatively large placebo-controlled RCT,21 2 smaller open studies,18,19 and a post hoc analysis of a large placebo-controlled RCT in mixed-age adults20 support the efficacy and relatively good tolerability of aripiprazole augmentation of an antidepressant for treatment-resistant MDD. Similarly, 1 large placebo-controlled RCT supports the efficacy and relatively good tolerability of quetiapine for non–treatment-resistant MDD. However, there are no comparative data assessing the relative merits of using these antipsychotics vs other pharmacologic strategies (eg, switching to another antidepressant, lithium augmentation, or combination of 2 antidepressants). Because older patients are more likely to experience adverse effects that may have more serious consequences (Table 3), many prudent clinicians reserve using antipsychotics as a third-line treatment in older patients with MDD without psychotic features and limit the duration of their use to a few months.30
Although some data have accumulated in recent years, there are significant gaps in knowledge on the safety and tolerability of antipsychotics in older adults. The era of “big data” may provide important answers to questions such as the relative place of antipsychotics vs lithium in preserving brain health among people with bipolar disorder or treatment-resistant MDD31; whether there are true ethnic differences in terms of drugs response and adverse effect prevalence in antipsychotics32,33; or the role of pharmacogenetic evaluation in establishing individual risk–benefit ratios of antipsychotics.34
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
1. United Nations. Department of Economic and Social Affairs Population Division. World Population Ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Published 2001. Accessed September 27, 2017.
2. Olfson M, King M, Schoenbaum M. Antipsychotic treatment of adults in the United States. J Clin Psychiatry. 2015;76(10):1346-1353.
3. Sajatovic M, Kales HC, Mulsant BH. Prescribing antipsychotics in geriatric patients: focus on schizophrenia and bipolar disorder. Current Psychiatry. 2017;16(10):20-26,28.
4. Hybels CF, Blazer DG. Epidemiology of late-life mental disorders. Clin Geriatr Med. 2003;19(4):663-696,v.
5. Mulsant BH, Blumberger DM, Ismail Z, et al. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30(3):517-534.
6. Mulsant BH, Pollock BG. Psychopharmacology. In: Steffens DC, Blazer DG, Thakur ME, eds. The American Psychiatric Publishing textbook of geriatric psychiatry. 5th ed. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
7. U.S. Food and Drug Administration. Public health advisory. deaths with antipsychotics in elderly patients with behavioral disturbances. https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm053171. Updated August 16, 2013. Accessed September 27, 2017.
8. Andreescu C, Mulsant BH, Rothschild AJ, et al. Pharmacotherapy of major depression with psychotic features: what is the evidence? Psychiatric Annals. 2006;35(1):31-38.
9. Buchanan D, Tourigny-Rivard MF, Cappeliez P, et al. National guidelines for seniors’ mental health: the assessment and treatment of depression. Canadian Journal of Geriatrics. 2006;9(suppl 2):S52-S58.
10. Canadian Coalition for Senior’s Mental Health. National guidelines for senior’s mental health. The assessment and treatment of depression 2006. http://www.ccsmh.ca/projects/depression. Accessed February 28, 2016.
11. Meyers BS, Flint AJ, Rothschild AJ, et al. A double-blind randomized controlled trial of olanzapine plus sertraline vs olanzapine plus placebo for psychotic depression: the study of pharmacotherapy of psychotic depression (STOP-PD). Arch Gen Psychiatry. 2009;66(8):838-847.
12. Mulsant BH, Sweet RA, Rosen J, et al. A double-blind randomized comparison of nortriptyline plus perphenazine versus nortriptyline plus placebo in the treatment of psychotic depression in late life. J Clin Psychiatry. 2001;62(8):597-604.
13. Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193-1198.
14. Maust DT, Oslin DW, Thase ME. Going beyond antidepressant monotherapy for incomplete response in nonpsychotic late-life depression: a critical review. Am J Geriatr Psychiatry. 2013;21(10):973-986.
15. Patel K, Abdool PS, Rajji TK, et al. Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opin Pharmacother. 2017;18(6):599-609.
16. Alexopoulos GS, Katz IR, Reynolds CF 3rd, et al. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7(6):361-376.
17. Cooper C, Katona C, Lyketsos K, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry. 2011;168(7):681-688.
18. Rutherford B, Sneed J, Miyazaki M, et al. An open trial of aripiprazole augmentation for SSRI non-remitters with late-life depression. Int J Geriatr Psychiatry. 2007;22(10):986-991.
19. Sheffrin M, Driscoll HC, Lenze EJ, et al. Pilot study of augmentation with aripiprazole for incomplete response in late-life depression: getting to remission. J Clin Psychiatry. 2009;70(2):208-213.
20. Steffens DC, Nelson JC, Eudicone JM, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26(6):564-572.
21. Lenze EJ, Mulsant BH, Blumberger DM, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386(10011):2404-2412.
22. Deligiannidis KM, Rothschild AJ, Barton BA, et al. A gender analysis of the study of pharmacotherapy of psychotic depression (STOP-PD): gender and age as predictors of response and treatment-associated changes in body mass index and metabolic measures. J Clin Psychiatry. 2013;74(10):1003-1009.
23. Flint AJ, Iaboni A, Mulsant BH, et al. Effect of sertraline on risk of falling in older adults with psychotic depression on olanzapine: results of a randomized placebo-controlled trial. Am J Geriatr Psychiatry. 2014;22(4):332-336.
24. Smith E, Rothschild AJ, Heo M, et al. Weight gain during olanzapine treatment for psychotic depression: effects of dose and age. Int Clin Psychopharmacol. 2008;23(3):130-137.
25. Blumberger DM, Mulsant BH, Kanellopoulos D, et al. The incidence of tardive dyskinesia in the study of pharmacotherapy for psychotic depression. J Clin Psychopharmacol. 2013;33(3):391-397.
26. Alexopoulos GS, Canuso CM, Gharabawi GM, et al. Placebo-controlled study of relapse prevention with risperidone augmentation in older patients with resistant depression. Am J Geriatr Psychiatry. 2008;16(1):21-30.
27. Katila H, Mezhebovsky I, Mulroy A, et al. Randomized, double-blind study of the efficacy and tolerability of extended release quetiapine fumarate (quetiapine XR) monotherapy in elderly patients with major depressive disorder. Am J Geriatr Psychiatry. 2013;21(8):769-784.
28. Sultana J, Trifiro G. Drug safety warnings: a message in a bottle. J Drug Des Res. 2014;1(1):1004.
29. Flint A, Meyers BS, Rothschild AR, et al; STOP-PD II Study Group. Sustaining remission of psychotic depression: rationale, design and methodology of STOP-PD II. BMC Psychiatry. 2013;13:38.
30. Alexopoulos GS; PROSPECT Group. Interventions for depressed elderly primary care patients. Int J Geriatr Psychiatry. 2001;16(6):553-559.
31. Sajatovic M, Forester BP, Gildengers A, et al. Aging changes and medical complexity in late-life bipolar disorder: emerging research findings that may help advance care. Neuropsychiatry (London). 2013;3(6):621-633.
32. Bigos KL, Bies RR, Pollock BG, et al. Genetic variation in CYP3A43 explains racial difference in olanzapine clearance. Mol Psychiatry. 2011;16(6):620-625.
33. Jin Y, Pollock BG, Coley K, et al. Population pharmacokinetics of perphenazine in schizophrenia patients from CATIE: impact of race and smoking. J Clin Pharmacol. 2010;50(1):73-80.
34. Mulsant BH. Is there a role for antidepressant and antipsychotic pharmacogenetics in clinical practice in 2014? Can J Psychiatry. 2014;59(2):59-61.
Prescribing antipsychotics in geriatric patients: Focus on schizophrenia and bipolar disorder
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
Antipsychotics are FDA-approved as a primary treatment for schizophrenia and bipolar disorder and as adjunctive therapy for major depressive disorder. In the United States, approximately 26% of antipsychotic prescriptions written for these indications are for individuals age >65.1 Additionally, antipsychotics are widely used to treat behavioral symptoms associated with dementia.1 The rapid expansion of the use of second-generation antipsychotics (SGAs), in particular, has been driven in part by their lower risk for extrapyramidal symptoms (EPS) compared with first-generation antipsychotics (FGAs).1 However, a growing body of data indicates that all antipsychotics have a range of adverse effects in older patients. This focus is critical in light of demographic trends—in the next 10 to 15 years, the population age >60 will grow 3.5 times more rapidly than the general population.2
In this context, psychiatrists need information on the relative risks of antipsychotics for older patients. This 3-part series summarizes findings and recommendations on safety and tolerability when prescribing antipsychotics in older individuals with chronic psychotic disorders, such as schizophrenia, bipolar disorder, depression, and dementia. This review aims to:
- briefly summarize the major studies and analyses relevant to older patients with these diagnoses
- provide a summative opinion on safety and tolerability issues in these older adults
- highlight the gaps in the evidence base and areas that need additional research.
Part 1 focuses on older adults with schizophrenia or bipolar disorder. Subsequent articles will focus on prescribing antipsychotics to older adults with depression and those with dementia.
Schizophrenia
Summary of benefits, place in treatment armamentarium. Individuals with schizophrenia have a shorter life expectancy than that of the general population mostly as a result of suicide and comorbid physical illnesses,3 but the number of patients with schizophrenia age >55 will double over the next 2 decades.4 With aging, both positive and negative symptoms may be a focus of treatment (Table 1).5,6 Antipsychotics are a first-line treatment for older patients with schizophrenia with few medication alternatives.7 Safety risks associated with antipsychotics in older people span a broad spectrum (Table 2).8
A 6-week prospective RCT evaluated paliperidone extended-release vs placebo in 114 older adults (age ≥65 years; mean age, 70 years) with schizophrenia.14 There was an optional 24-week extension of open-label treatment with paliperidone. Mean daily dose of paliperidone was 8.4 mg. Efficacy measures did not show consistent statistically significant differences between treatment groups. Discontinuation rates were similar between paliperidone (7%) vs placebo (8%). Serious adverse events occurred in 3% of paliperidone-treated vs 8% of placebo-treated patients. Elevated prolactin levels occurred in one-half of paliperidone-treated patients. There were no prolactin or glucose treatment-related adverse events or significant mean changes in body weight for either paliperidone-treated or placebo-treated patients. Safety findings in the 24-week, open-label extension group were consistent with the RCT results.
Howanitz et al15 conducted a 12-week, prospective RCT that compared clozapine (mean dose, 300 mg/d) with chlorpromazine (mean dose, 600 mg/d) in 42 older adults (mean age, 67 years) with schizophrenia. Drop-out rate prior to 5 weeks was 19% and similar between groups. Common adverse effects included sialorrhea, hematologic abnormalities, sedation, tachycardia, EPS, and weight gain. Although both drugs were effective, more patients taking clozapine had tachycardia and weight gain, while more chlorpromazine patients reported sedation.
There have been other, less rigorous studies.7,8 Most of these studies evaluated risperidone and olanzapine, and most were conducted in “younger” geriatric patients (age <75 years). Although patients who participate in clinical trials may be healthier than “typical” patients, adverse effects such as EPS, sedation, and weight gain were still relatively common in these studies.
Other clinical data. A major consideration in treating older adults with schizophrenia is balancing the need to administer an antipsychotic dose high enough to alleviate psychotic symptoms while minimizing dose-dependent adverse effects. There is a U-shaped relationship between age and vulnerability to antipsychotic adverse effects,16,17 wherein adverse effects are highest at younger and older ages. Evidence supports using the lowest effective antipsychotic dose for geriatric patients with schizophrenia. Positive emission tomography (PET) studies suggest that older patients develop EPS with lower doses despite lower receptor occupancy.17,18 A recent study of 35 older patients (mean age, 60.1 years) with schizophrenia obtained PET, clinical measures, and blood pharmacokinetic measures before and after reduction of risperidone or olanzapine doses.18 A ≥40% reduction in dose was associated with reduced adverse effects, particularly EPS and elevation of prolactin levels. Moreover, the therapeutic window of striatal D2/D3 receptor occupancy appeared to be 50% to 60% in these older patients, compared with 65% to 80% in younger patients.
Long-term risks of antipsychotic treatment across the lifespan are less clear, with evidence suggesting both lower and higher mortality risk.19,20 It is difficult to fully disentangle the long-term risks of antipsychotics from the cumulative effects of lifestyle and comorbidity among individuals who have lived with schizophrenia for decades. Large naturalistic studies that include substantial numbers of older people with schizophrenia might be a way to elicit more information on long-term safety. The Schizophrenia Outpatient Health Outcome (SOHO) study was a large naturalistic trial that recruited >10,000 individuals with schizophrenia in 10 European countries.21 Although the SOHO study found differences between antipsychotics and adverse effects, such as EPS, weight gain, and sexual dysfunction, because the mean age of these patients was approximately 40 years and the follow-up period was only 3 years, it is difficult to draw conclusions that could be relevant to older individuals who have had schizophrenia for decades.
Bipolar Disorder
Clinical trials: Bipolar depression. A post hoc, secondary analysis of two 8-week, double-blind, randomized, placebo-controlled studies in bipolar depression compared 2 dosages of quetiapine (300 mg/d and 600 mg/d) with placebo in mixed-age patients.31 In a subgroup of 72 patients, ages 55 to 65, remission occurred more often with quetiapine than with placebo. Study discontinuation rates were similar between older people and younger people (age <55 years): quetiapine, 300 mg/d, 29.2%; quetiapine, 600 mg/d, 48.1%; and placebo, 29.6% in older adults, compared with 37.1%, 45.8%, and 38.1%, respectively, in younger adults. In all patients, the most common reason for discontinuation was adverse events with quetiapine and lack of efficacy for placebo. Adverse event rates were similar in older and younger adults. Dry mouth and dizziness were more common in older adults. Proportions of adults experiencing clinically significant weight gain (≥7% of body weight) were 5.3%, 8.3%, and 0% in older adults receiving quetiapine, 300 mg/d, quetiapine, 600 mg/d, and placebo, respectively, compared with 7.2%, 10.1%, and 2.6% in younger adults. EPS and treatment-emergent mania were minimal.
A secondary analysis of mixed-age, RCTs examined response in older adults (age ≥55 years) with bipolar I depression who received lurasidone as monotherapy or adjunctive therapy.32 In the monotherapy study, these patients were randomized to 6 weeks of lurasidone 20 to 60 mg/d, lurasidone 80 to 120 mg/d, or placebo. In the adjunctive therapy study, they were randomized to lurasidone 20 to 120 mg/d or placebo with either lithium or valproate. There were 83 older adults (17.1% of the sample) in the monotherapy study and 53 (15.6%) in the adjunctive therapy study. Mean improvement in depression was significantly higher for both doses of lurasidone monotherapy than placebo. Adjunctive lurasidone was not associated with statistically significant improvement vs placebo. The most frequent adverse events in older patients on lurasidone monotherapy 20 to 60 mg/d or 80 to 120 mg/d were nausea (18.5% and 9.7%, respectively) and somnolence (11.1% and 0%, respectively). Akathisia (9.7%) and insomnia (9.7%) were the most common adverse events in the group receiving 80 to 120 mg/d, with the rate of akathisia exhibiting a dose-related increase. Weight change with lurasidone was similar to placebo, and there were no clinically meaningful group changes in vital signs, electrocardiography, or laboratory parameters.
A small (N = 20) open study found improvement in older adults with bipolar depression with aripiprazole (mean dose, 10.3 mg/d).33 Adverse effects included restlessness and weight gain (n = 3, 9% each), sedation (n = 2, 10%), and drooling and diarrhea/loose stools (n = 1, 5% each). In another small study (N = 15) using asenapine (mean dose, 11.2 mg/d) in mainly older bipolar patients with depression, the most common adverse effects were gastrointestinal (GI) discomfort (n = 5, 33%) and restlessness, tremors, cognitive difficulties, and sluggishness (n = 2, 13% each).34
Clinical trials: Bipolar mania. Researchers conducted a pooled analysis of two 12-week randomized trials comparing quetiapine with placebo in a mixed-age sample with bipolar mania.35 In a subgroup of 59 older patients (mean age, 62.9 years), manic symptoms improved significantly more with quetiapine (modal dose, 550 mg/d) than with placebo. Adverse effects reported by >10% of older patients were dry mouth, somnolence, postural hypotension, insomnia, weight gain, and dizziness. Insomnia was reported by >10% of patients receiving placebo.
In a case series of 11 elderly patients with mania receiving asenapine, Baruch et al36 reported a 63% remission rate. One patient discontinued the study because of a new rash, 1 discontinued after developing peripheral edema, and 3 patients reported mild sedation.
Beyer et al37 reported on a post hoc analysis of 94 older adults (mean age, 57.1 years; range, 50.1 to 74.8 years) with acute bipolar mania receiving olanzapine (n = 47), divalproex (n = 31), or placebo (n = 16) in a pooled olanzapine clinical trials database. Patients receiving olanzapine or divalproex had improvement in mania; those receiving placebo did not improve. Safety findings were comparable with reports in younger patients with mania.
Other clinical data. Adverse effects found in mixed-age samples using secondary analyses of clinical trials need to be interpreted with caution because these types of studies usually exclude individuals with significant medical comorbidity. Medical burden, cognitive impairment, or concomitant medications generally necessitate slower drug titration and lower total daily dosing. For example, a secondary analysis of the U.S. National Institute of Health-funded Systematic Treatment Enhancement Program for Bipolar Disorder study, which had broader inclusion criteria than most clinical trials, reported that, although recovery rates in older adults with bipolar disorder were fairly good (78.5%), lower doses of risperidone were used in older vs younger patients.38
Clinical considerations
Interpretation of the relative risks of antipsychotics in older people must be tempered by the caveat that there is limited high-quality data (Table 4). Antipsychotics are the first-line therapy for older patients with schizophrenia, although their use is supported by a small number of prospective RCTs. SGAs are preferred because of their lower propensity to cause EPS and other motor adverse effects. Older persons with schizophrenia have an EPS threshold lower than younger patients and determining the lowest effective dosage may minimize EPS and cognitive adverse effects. As individuals with long-standing schizophrenia get older, their antipsychotic dosages may need to be reduced, and clinicians need to monitor for adverse effects that are more common among older people, such as tardive dyskinesia and metabolic abnormalities. In healthy, “younger” geriatric patients, monitoring for adverse effects may be similar to monitoring of younger patients. Patients who are older or frail may need more frequent assessment.
Like older adults with schizophrenia, geriatric patients with bipolar disorder have reduced drug tolerability and experience more adverse effects than younger patients. There are no prospective controlled studies that evaluated using antipsychotics in older patients with bipolar disorder. In older bipolar patients, the most problematic adverse effects of antipsychotics are akathisia, parkinsonism, other EPS, sedation and dizziness (which may increase fall risk), and GI discomfort. A key tolerability and safety consideration when treating older adults with bipolar disorder is the role of antipsychotics in relation to the use of lithium and mood stabilizers. Some studies have suggested that lithium has neuroprotective effects when used long-term; however, at least 1 report suggested that long-term antipsychotic treatment may be associated with neurodegeneration.39
The literature does not provide strong evidence on the many clinical variations that we see in routine practice settings, such as combinations of drug treatments or drugs prescribed to patients with specific comorbid conditions. There is a need for large cohort studies that monitor treatment course, medical comorbidity, and prognosis. Additionally, well-designed clinical trials such as the DART-AD, which investigated longer-term trajectories of people with dementia taking antipsychotics, should serve as a model for the type of research that is needed to better understand outcome variability among older people with chronic psychotic or bipolar disorders.40
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.
1. Alexander GC, Gallagher SA, Mascola A, et al. Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf. 2011;20(2):177-184.
2. United Nations, Department of Economic and Social Affairs, Population Division. World population ageing: 1950-2050. http://www.un.org/esa/population/publications/worldageing19502050. Accessed September 1, 2017.
3. Lawrence D, Kisely S, Pais J. The epidemiology of excess mortality in people with mental illness. Can J Psychiatry. 2010;55(12):752-760.
4. Cohen CI, Vahia I, Reyes P, et al. Focus on geriatric psychiatry: schizophrenia in later life: clinical symptoms and social well-being. Psychiatr Serv. 2008;59(3):232-234.
5. Jeste DV, Barak Y, Madhusoodanan S, et al. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. Am J Geriatr Psychiatry. 2003;11(6):638-647.
6. Kalache SM, Mulsant BH, Davies SJ, et al. The impact of aging, cognition, and symptoms on functional competence in individuals with schizophrenia across the lifespan. Schizophr Bull. 2015;41(2):374-381.
7. Suzuki T, Remington G, Uchida H, et al. Management of schizophrenia in late life with antipsychotic medications: a qualitative review. Drugs Aging. 2011;28(12):961-980.
8. Mulsant BH, Pollock BG. Psychopharmacology. In: David C. Steffens DC, Blazer DG, Thakur ME (eds). The American Psychiatric Publishing Textbook of Geriatric Psychiatry, 5th Edition. Arlington, VA: American Psychiatric Publishing; 2015:527-587.
9. Cohen CI, Meesters PD, Zhao J. New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry. 2015;2(4):340-350.
10. Marriott RG, Neil W, Waddingham S. Antipsychotic medication for elderly people with schizophrenia. Cochrane Database Syst Rev. 2006;(1):CD005580.
11. Essali A, Ali G. Antipsychotic drug treatment for elderly people with late-onset schizophrenia. Cochrane Database Syst Rev. 2012(2):CD004162.
12. Scott J, Greenwald BS, Kramer E, et al. Atypical (second generation) antipsychotic treatment response in very late-onset schizophrenia-like psychosis. Int Psychogeriatr. 2011;23(5):742-748.
13. Rado J, Janicak PG. Pharmacological and clinical profile of recently approved second-generation antipsychotics: implications for treatment of schizophrenia in older patients. Drugs Aging. 2012;29(10):783-791.
14. Tzimos A, Samokhvalov V, Kramer M, et al. Safety and tolerability of oral paliperidone extended-release tablets in elderly patients with schizophrenia: a double-blind, placebo-controlled study with six-month open-label extension. Am J Geriatr Psychiatry. 2008;16(1):31-43.
15. Howanitz E, Pardo M, Smelson DA, et al. The efficacy and safety of clozapine versus chlorpromazine in geriatric schizophrenia. J Clin Psychiatry. 1999;60(1):41-44.
16. Sproule BA, Lake J, Mamo DC, et al. Are antipsychotic prescribing patterns different in older and younger adults?: a survey of 1357 psychiatric inpatients in Toronto. Can J Psychiatry. 2010;55(4):248-254.
17. Uchida H, Suzuki T, Mamo DC, et al. Effects of age and age of onset on prescribed antipsychotic dose in schizophrenia spectrum disorders: a survey of 1,418 patients in Japan. Am J Geriatr Psychiatry. 2008;16(7):584-593.
18. Graff-Guerrero A, Rajji TK, Mulsant BH, et al. Evaluation of antipsychotic dose reduction in late-life schizophrenia: a prospective dopamine D2/3 occupancy study. JAMA Psychiatry. 2015;72(9):927-934.
19. Khan A, Schwartz K, Stern C, et al. Mortality risk in patients with schizophrenia participating in premarketing atypical antipsychotic clinical trials. J Clin Psychiatry. 2007;68(12):1828-1833.
20. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: a systematic review. Schizophr Res. 2009;113(1):1-11.
21. Novick D, Haro JM, Perrin E, et al. Tolerability of outpatient antipsychotic treatment: 36-month results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Eur Neuropsychopharmacol. 2009;19(8):542-550.
22. Sajatovic M, Blow FC, Ignacio RV, et al. Age-related modifiers of clinical presentation and health service use among veterans with bipolar disorder. Psychiatr Serv. 2004;55(9):1014-1021.
23. Jeste DV, Alexopoulos GS, Bartels SJ, et al. Consensus statement on the upcoming crisis in geriatric mental health: research agenda for the next 2 decades. Arch Gen Psychiatry. 1999;56(9):848-853.
24. Sajatovic M, Chen P. Geriatric bipolar disorder. Psychiatr Clin North Am. 2011;34(2):319-333,vii.
25. Sajatovic M, Strejilevich SA, Gildengers AG, et al. A report on older-age bipolar disorder from the International Society for Bipolar Disorders Task Force. Bipolar Disord. 2015;17(7):689-704.
26. Lala SV, Sajatovic M. Medical and psychiatric comorbidities among elderly individuals with bipolar disorder: a literature review. J Geriatr Psychiatry Neurol. 2012;25(1):20-25.
27. Dols A, Rhebergen D, Beekman A, et al. Psychiatric and medical comorbidities: results from a bipolar elderly cohort study. Am J Geriatr Psychiatry. 2014;22(11):1066-1074.
28. Pillarella J, Higashi A, Alexander GC, et al. Trends in use of second-generation antipsychotics for treatment of bipolar disorder in the United States, 1998-2009. Psychiatr Serv. 2012;63(1):83-86.
29. De Fruyt J, Deschepper E, Audenaert K, et al. Second generation antipsychotics in the treatment of bipolar depression: a systematic review and meta-analysis. J Psychopharmacol. 2012;26(5):603-617.
30. Nivoli AM, Murru A, Goikolea JM, et al. New treatment guidelines for acute bipolar mania: a critical review. J Affect Disord. 2012;140(2):125-141.
31. Sajatovic M, Paulsson B. Quetiapine for the treatment of depressive episodes in adults aged 55 to 65 years with bipolar disorder. Paper presented at: American Association of Geriatric Psychiatry Annual Meeting; 2007; New Orleans, LA.
32. Sajatovic M, Forester B, Tsai J, et al. Efficacy and safety of lurasidone in older adults with bipolar depression: analysis of two double-blind, placebo-controlled studies. Paper presented at: American College of Neuropsychopharmacology (ACNP) 53rd Annual Meeting; 2014; Phoenix, AZ.
33. Sajatovic M, Coconcea N, Ignacio RV, et al. Aripiprazole therapy in 20 older adults with bipolar disorder: a 12-week, open-label trial. J Clin Psychiatry. 2008;69(1):41-46.
34. Sajatovic M, Dines P, Fuentes-Casiano E, et al. Asenapine in the treatment of older adults with bipolar disorder. Int J Geriatr Psychiatry. 2015;30(7):710-719.
35. Sajatovic M, Calabrese JR, Mullen J. Quetiapine for the treatment of bipolar mania in older adults. Bipolar Disord. 2008;10(6):662-671.
36. Baruch Y, Tadger S, Plopski I, et al. Asenapine for elderly bipolar manic patients. J Affect Disord. 2013;145(1):130-132.
37. Beyer JL, Siegal A, Kennedy JS. Olanzapine, divalproex and placebo treatment, non-head to head comparisons of older adults acute mania. Paper presented at: 10th Congress of the International Psychogeriatric Association; 2001; Nice, France.
38. Al Jurdi RK, Marangell LB, Petersen NJ, et al. Prescription patterns of psychotropic medications in elderly compared with younger participants who achieved a “recovered” status in the systematic treatment enhancement program for bipolar disorder. Am J Geriatr Psychiatry. 2008;16(11):922-933.
39. Gildengers AG, Chung KH, Huang SH, et al. Neuroprogressive effects of lifetime illness duration in older adults with bipolar disorder. Bipolar Disord. 2014;16(6):617-623.
40. Ballard C, Lana MM, Theodoulou M, et al. A randomised, blinded, placebo-controlled trial in dementia patients continuing or stopping neuroleptics (the DART-AD trial). PLoS Med. 2008;5(4):e76.