User login
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
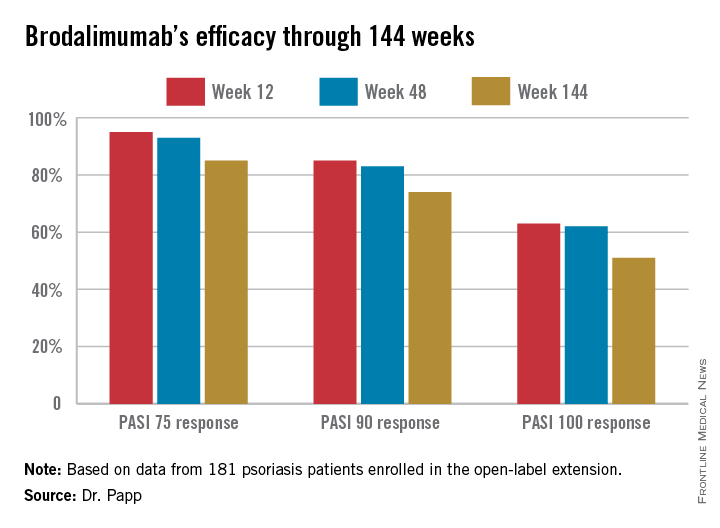
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AMSTERDAM – The majority of psoriasis patients placed on the investigational biologic agent brodalumab maintained a PASI 100 response throughout 144 weeks in a long-term, open-label extension of a phase II study.
“The benefit/risk ratio of brodalumab remains very favorable, and warrants continued development of this anti–interleukin-17 receptor A human monoclonal antibody as a potential treatment for psoriasis,” Dr. Kim Papp declared in presenting the study results at the annual congress of the European Academy of Dermatology and Venereology.
Large phase III clinical trials of brodalumab for the treatment of moderate to severe plaque psoriasis are ongoing.
The parent phase II study was 16 weeks long, double blinded, and placebo controlled. At the study’s end, 181 participants enrolled in the open-label extension, in which they received subcutaneous brodalumab at 210 mg every 2 weeks. The study protocol was amended after about a year, with the dose reduced to 140 mg every 2 weeks in the 119 patients weighing 100 kg or less. Six of those patients subsequently had an inadequate response to the lower dose, and were returned to the higher-dose regimen, explained Dr. Papp of Probity Medical Research in Waterloo, Ont.
Roughly 95% of patients became PASI 75 responders within the first few weeks. There was very little drop-off over time. The PASI 75 response rate was 95% at week 12, 93% at week 48, and 85% at week 144.
At week 144, roughly two-thirds of subjects were deemed clear or almost clear according to Physician Global Assessment.
No new safety signals emerged during this long-term study. The most common adverse events were minor upper respiratory infections. Two percent of patients developed a grade 2 absolute neutrophil count of less than 1,500 per 109/L; however, these were transitory events that resolved without a change in treatment.
In response to an audience question, Dr. Papp said antidrug antibodies did develop in some patients during the course of 144 weeks of treatment, but he had no information as to the clinical effect, if any.
“This is a fairly small study population. I think it makes sense to wait for the phase III results to see if the antibodies affect safety and/or efficacy,” he added.
Dr. Papp reported receiving research support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.
AT THE EADV CONGRESS
Key clinical point: The investigational interleukin-17 receptor A inhibitor brodalumab maintained strong clinical efficacy throughout 144 weeks of psoriasis treatment.
Major finding: The PASI 90 response rate to brodalumab was 85% at week 12, 83% at week 48, and 74% at week 144.
Data source: A 181-patient, prospective, open-label extension of a phase II study.
Disclosures: Dr. Papp reported receiving financial support from Amgen, which is codeveloping brodalumab with AstraZeneca/MedImmune.