User login
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
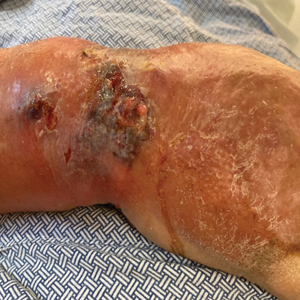
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.
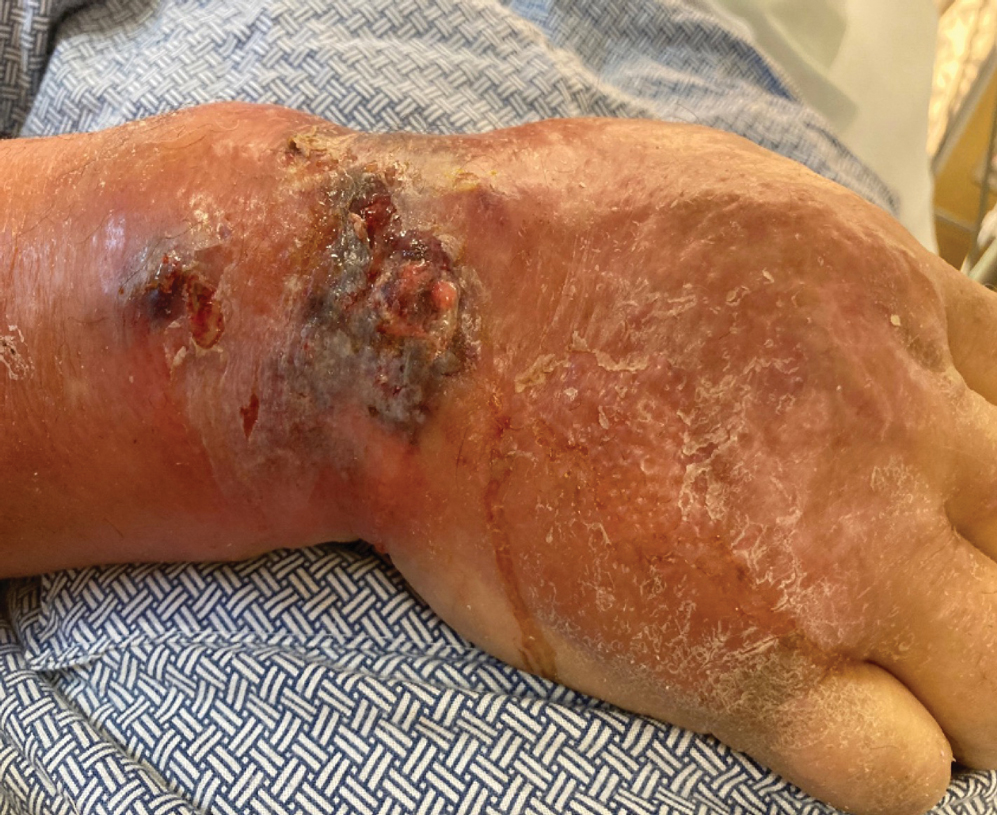
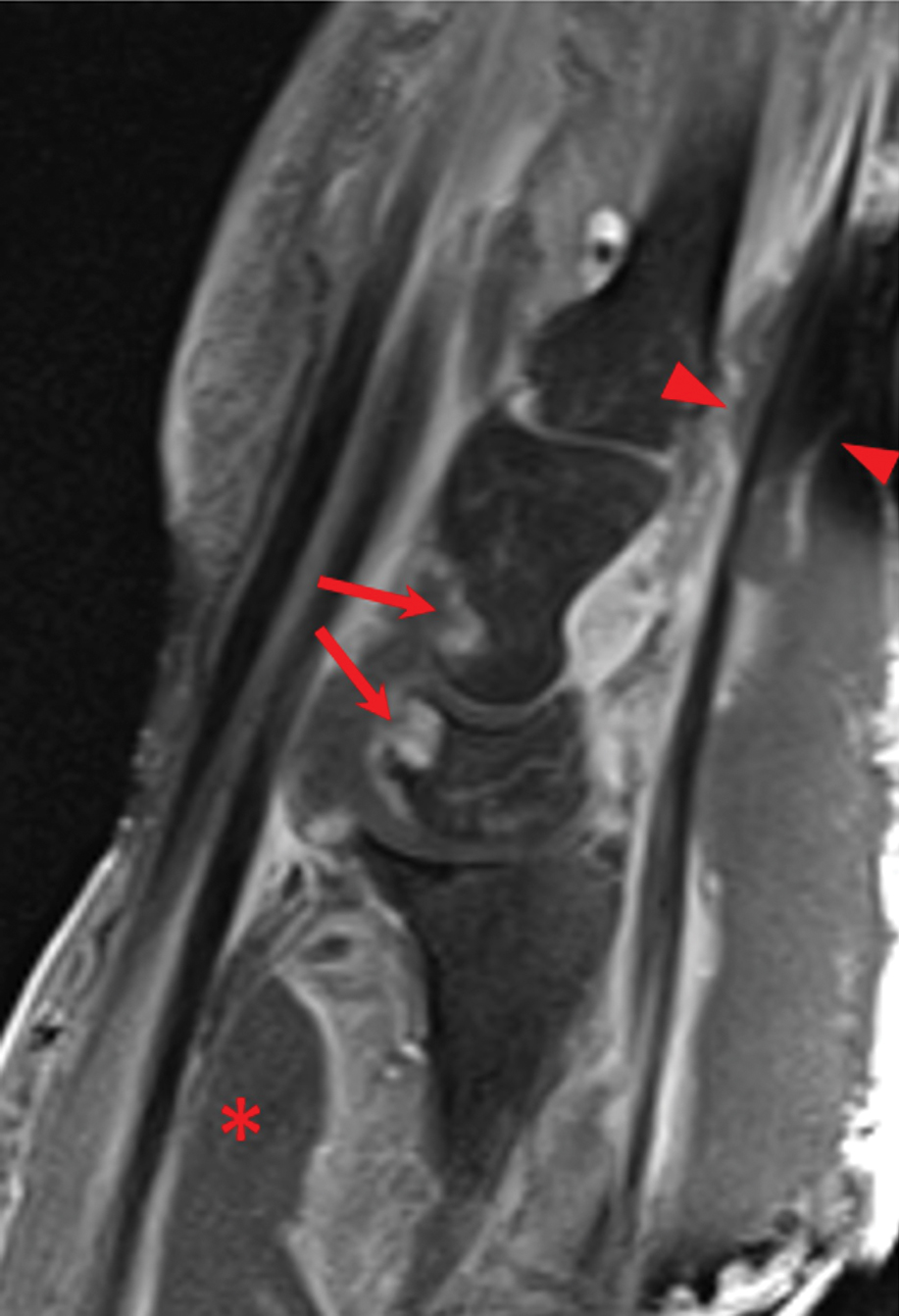
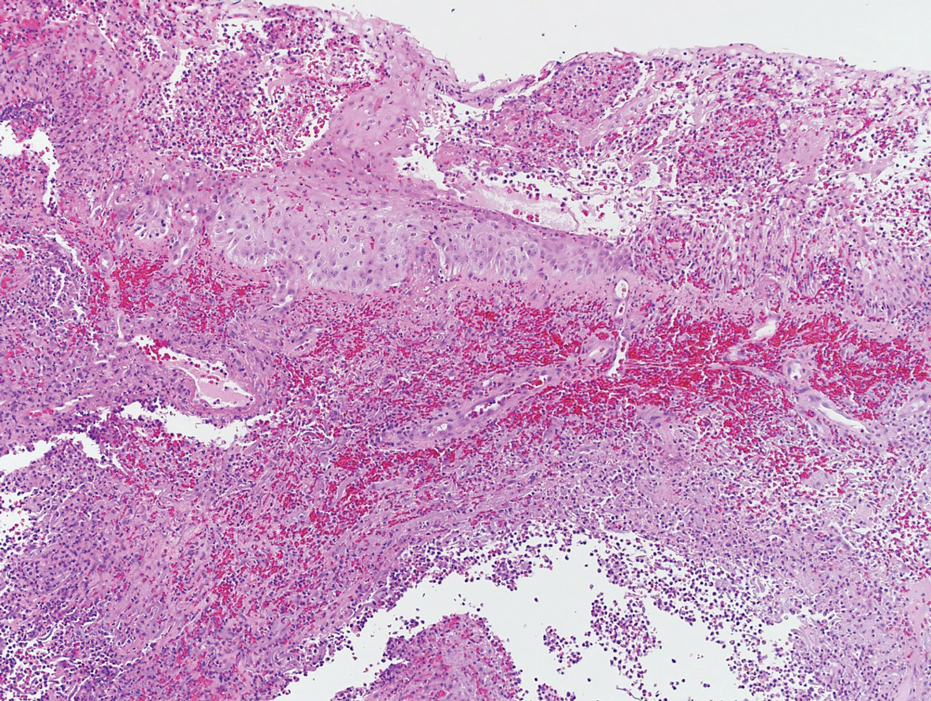
Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).
Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.
Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
Practice Points
- The accurate diagnosis of necrotizing myositis (NM) requires a multimodal approach with complete clinical, histological, and radiographic correlation.
- Necrotizing myositis can manifest as violaceous erythematous plaques, bullae, blisters, or vesicles with petechiae, marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane.
- The differential diagnosis of NM includes pyoderma gangrenosum.