User login
Rapidly Progressive Necrotizing Myositis Mimicking Pyoderma Gangrenosum
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
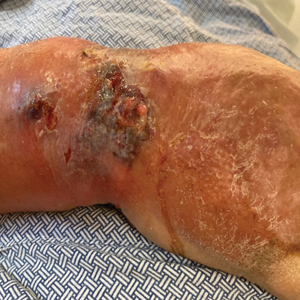
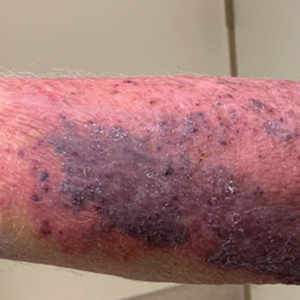
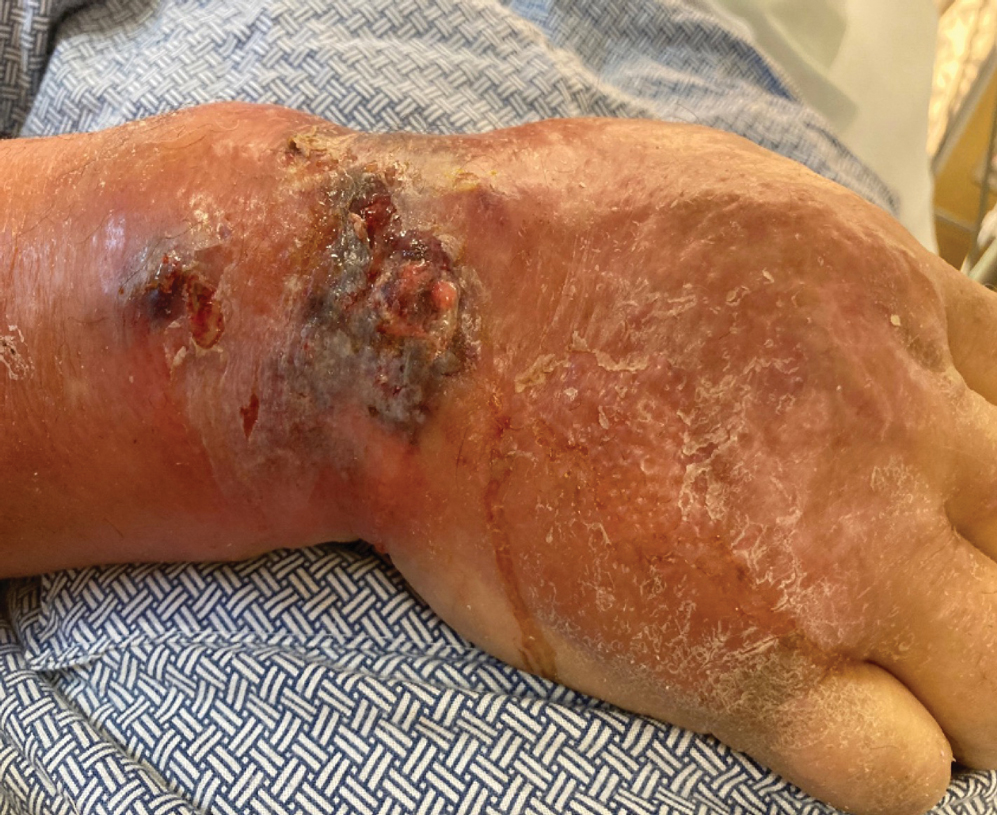
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.
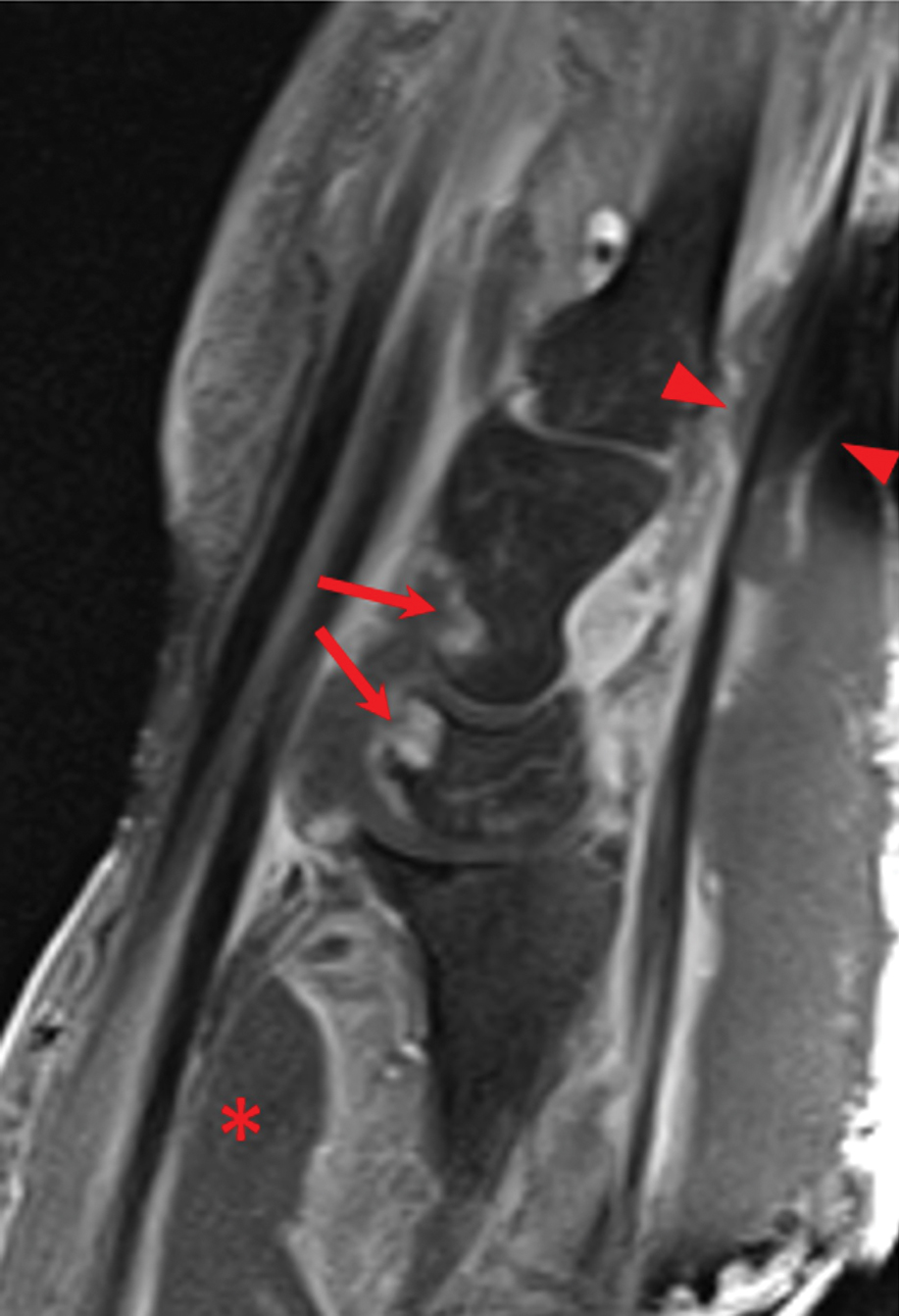
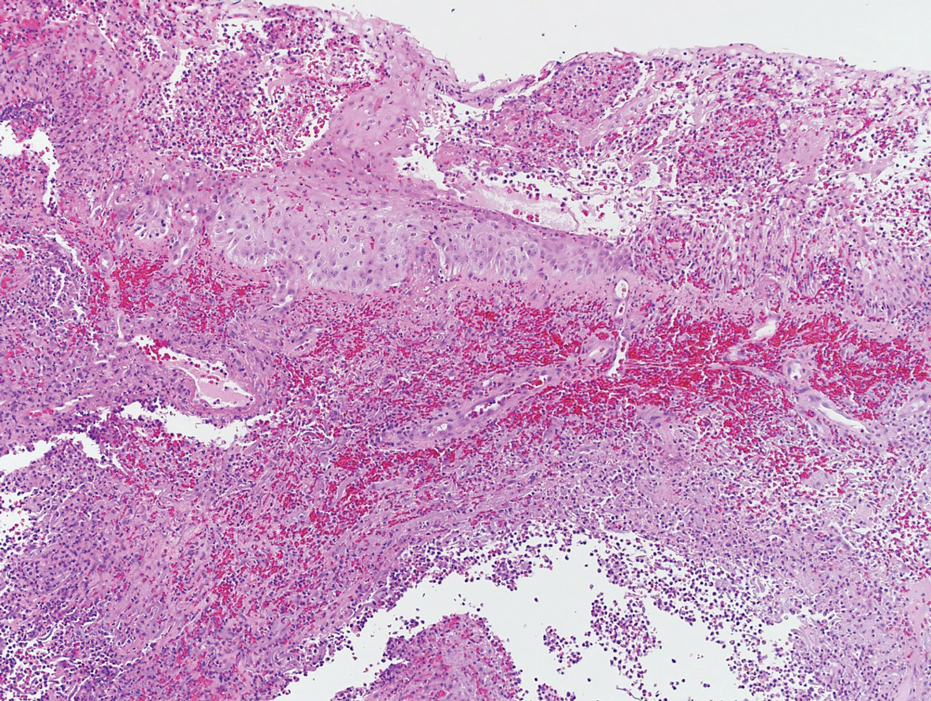
Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).
Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.
Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
To the Editor:
Necrotizing myositis (NM) is an exceedingly rare necrotizing soft-tissue infection (NSTI) that is characterized by skeletal muscle involvement. β -Hemolytic streptococci, such as Streptococcus pyogenes , are the most common causative organisms. The overall prevalence and incidence of NM is unknown. A review of the literature by Adams et al 2 identified only 21 cases between 1900 and 1985.
Timely treatment of this infection leads to improved outcomes, but diagnosis can be challenging due to the ambiguous presentation of NM and lack of specific cutaneous changes.3 Clinical manifestations including bullae, blisters, vesicles, and petechiae become more prominent as infection progresses.4 If NM is suspected due to cutaneous manifestations, it is imperative that the underlying cause be identified; for example, NM must be distinguished from the overlapping presentation of pyoderma gangrenosum (PG). Because NM has nearly 100% mortality without prompt surgical intervention, early identification is critical.5 Herein, we report a case of NM that illustrates the correlation of clinical, histological, and imaging findings required to diagnose this potentially fatal infection.
An 80-year-old man presented to the emergency department with worsening pain, edema, and spreading redness of the right wrist over the last 5 weeks. He had a history of atopic dermatitis that was refractory to topical steroids and methotrexate; he was dependent on an oral steroid (prednisone 30 mg/d) for symptom control. The patient reported minor trauma to the area after performing home renovations. He received numerous rounds of oral antibiotics as an outpatient for presumed cellulitis and reported he was “getting better” but that the signs and symptoms of the condition grew worse after outpatient arthrocentesis. Dermatology was consulted to evaluate for a necrotizing neutrophilic dermatosis such as PG.
At the current presentation, the patient was tachycardic and afebrile (temperature, 98.2 °F [36.8 °C]). Physical examination revealed large, exquisitely tender, ill-defined necrotic ulceration of the right wrist with purulent debris and diffuse edema (Figure 1). Sequential evaluation at 6-hour intervals revealed notably increasing purulence, edema, and tenderness. Interconnected sinus tracts that extended to the fascial plane were observed.

Laboratory workup was notable for a markedly elevated C-reactive protein level of 18.9 mg/dL (reference range, 0–0.8 mg/dL) and an elevated white blood cell count of 19.92×109/L (reference range, 4.5–11.0×109/L). Blood and tissue cultures were positive for methicillin-sensitive Staphylococcus aureus. Computed tomography and magnetic resonance imaging (MRI) prior to biopsy demonstrated findings consistent with extensive subcutaneous and intramuscular areas of loculation and foci of gas (Figure 2). These findings were consistent with intramuscular involvement. A punch biopsy revealed a necrotic epidermis filled with neutrophilic pustules and a dense dermal infiltrate of neutrophilic inflammation consistent with infection (Figure 3).

Emergency surgery was performed with debridement of necrotic tissue and muscle. Postoperatively, he became more clinically stable after being placed on cefazolin through a peripherally inserted central catheter. He underwent 4 additional washouts over the ensuing month, as well as tendon reconstructions, a radial forearm flap, and reverse radial forearm flap reconstruction of the forearm. At the time of publication, there has been no recurrence. The patient’s atopic dermatitis is well controlled on dupilumab and topical fluocinonide alone, with a recent IgA level of 1 g/L and a body surface area measurement of 2%. Dupilumab was started 3 months after surgery.

Necrotizing myositis is a rare, rapidly progressive infection involving muscle that can manifest as superficial cutaneous involvement. The clinical manifestation of NM is harder to recognize than other NSTIs such as necrotizing fasciitis, likely due to the initial prodromal phase of NM, which consists of nonspecific constitutional symptoms.3 Systemic findings such as tachycardia, fever, hypotension, and shock occur in only 10% to 40% of NM patients.4,5
In our patient, clues of NM included fulfillment of criteria for systemic inflammatory response syndrome at admission and a presumed source of infection; taken together, these findings should lead to a diagnosis of sepsis until otherwise proven. The patient also reported pain that was not proportional to the skin findings, which suggested an NSTI. His lack of constitutional symptoms may have been due to the effects of prednisone, which was changed to dupilumab during hospitalization.
The clinical and histological findings of NM are nonspecific. Clinical findings include skin discoloration with bullae, blisters, vesicles, or petechiae.4 Our case adds to the descriptive morphology by including marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane. Histologic findings can include confluent necrosis extending from the epidermis to the underlying muscle with dense neutrophilic inflammation. Notably, these findings can mirror necrotizing neutrophilic dermatoses in the absence of an infectious cause. Failure to recognize simple systemic inflammatory response syndrome criteria in NM patients due to slow treatment response or incorrect treatment can can lead to loss of a limb or death.
Workup reveals overlap with necrotizing neutrophilic dermatoses including PG, which is the prototypical neutrophilic dermatosis. Morphologically, PG presents as an ulcer with a purple and undermined border, often having developed from an initial papule, vesicle, or pustule. A neutrophilic infiltrate of the ulcer edge is the major criterion required to diagnose PG6; minor criteria include a positive pathergy test, history of inflammatory arthritis or inflammatory bowel disease, and exclusion of infection.6 When compared directly to an NSTI such as NM, the most important variable that sets PG apart is the absence of bacterial growth on blood and tissue cultures.7
Imaging studies can aid in the clinical diagnosis of NM and help distinguish the disease from PG. Computed tomography and MRI may demonstrate hallmarks of extensive necrotizing infection, such as gas formation and consequent fascial swelling, thickening and edema of involved muscle, and subfascial fluid collection.3,4 Distinct from NM, imaging findings in PG are more subtle, suggesting cellulitic inflammation with edema.8 A defining radiographic feature of NM can be foci of gas within muscle or fascia, though absence of this finding does not exclude NM.1,4
In conclusion, NM is a rare intramuscular infection that can be difficult to diagnose due to its nonspecific presentation and lack of constitutional symptoms. Dermatologists should maintain a high level of suspicion for NM in the setting of rapidly progressive clinical findings; accurate diagnosis requires a multimodal approach with complete correlation of clinical, histological, and imaging findings. Computed tomography and MRI can heighten the approach, even when necrotizing neutrophilic dermatoses and NM have similar clinical and histological appearances. Once a diagnosis of NM is established, prompt surgical and medical intervention improves the prognosis.
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
- Stevens DL, Baddour LM. Necrotizing soft tissue infections. UpToDate. Updated October 7, 2022. Accessed February 13, 2024. https://www.uptodate.com/contents/necrotizing-soft-tissue-infections?search=Necrotizing%20soft%20tissue%20infections&source=search_result&selectedTitle=1~150&usage_type=default&display_rank=1
- Adams EM, Gudmundsson S, Yocum DE, et al. Streptococcal myositis. Arch Intern Med . 1985;145:1020-1023.
- Khanna A, Gurusinghe D, Taylor D. Necrotizing myositis: highlighting the hidden depths—case series and review of the literature. ANZ J Surg . 2020;90:130-134. doi:10.1111/ans.15429
- Boinpally H, Howell RS, Ram B, et al. Necrotizing myositis: a rare necrotizing soft tissue infection involving muscle. Wounds . 2018;30:E116-E120.
- Anaya DA, Dellinger EP. Necrotizing soft-tissue infection: diagnosis and management. Clin Infect Dis . 2007;44:705-710. doi:10.1086/511638
- Maverakis E, Ma C, Shinkai K, et al. Diagnostic criteria of ulcerative pyoderma gangrenosum: a Delphi consensus of international experts. JAMA Dermatol . 2018;154:461-466. doi:10.1001/jamadermatol.2017.5980
- Sanchez IM, Lowenstein S, Johnson KA, et al. Clinical features of neutrophilic dermatosis variants resembling necrotizing fasciitis. JAMA Dermatol . 2019;155:79-84. doi:10.1001/jamadermatol.2018.3890
- Demirdover C, Geyik A, Vayvada H. Necrotising fasciitis or pyoderma gangrenosum: a fatal dilemma. Int Wound J . 2019;16:1347-1353. doi:10.1111/iwj.13196
Practice Points
- The accurate diagnosis of necrotizing myositis (NM) requires a multimodal approach with complete clinical, histological, and radiographic correlation.
- Necrotizing myositis can manifest as violaceous erythematous plaques, bullae, blisters, or vesicles with petechiae, marked edema with ulceration, progressive purulence, and interconnected sinuses tracking to the fascial plane.
- The differential diagnosis of NM includes pyoderma gangrenosum.
Mycetomalike Skin Infection Due to Gordonia bronchialis in an Immunocompetent Patient
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.
Case Report
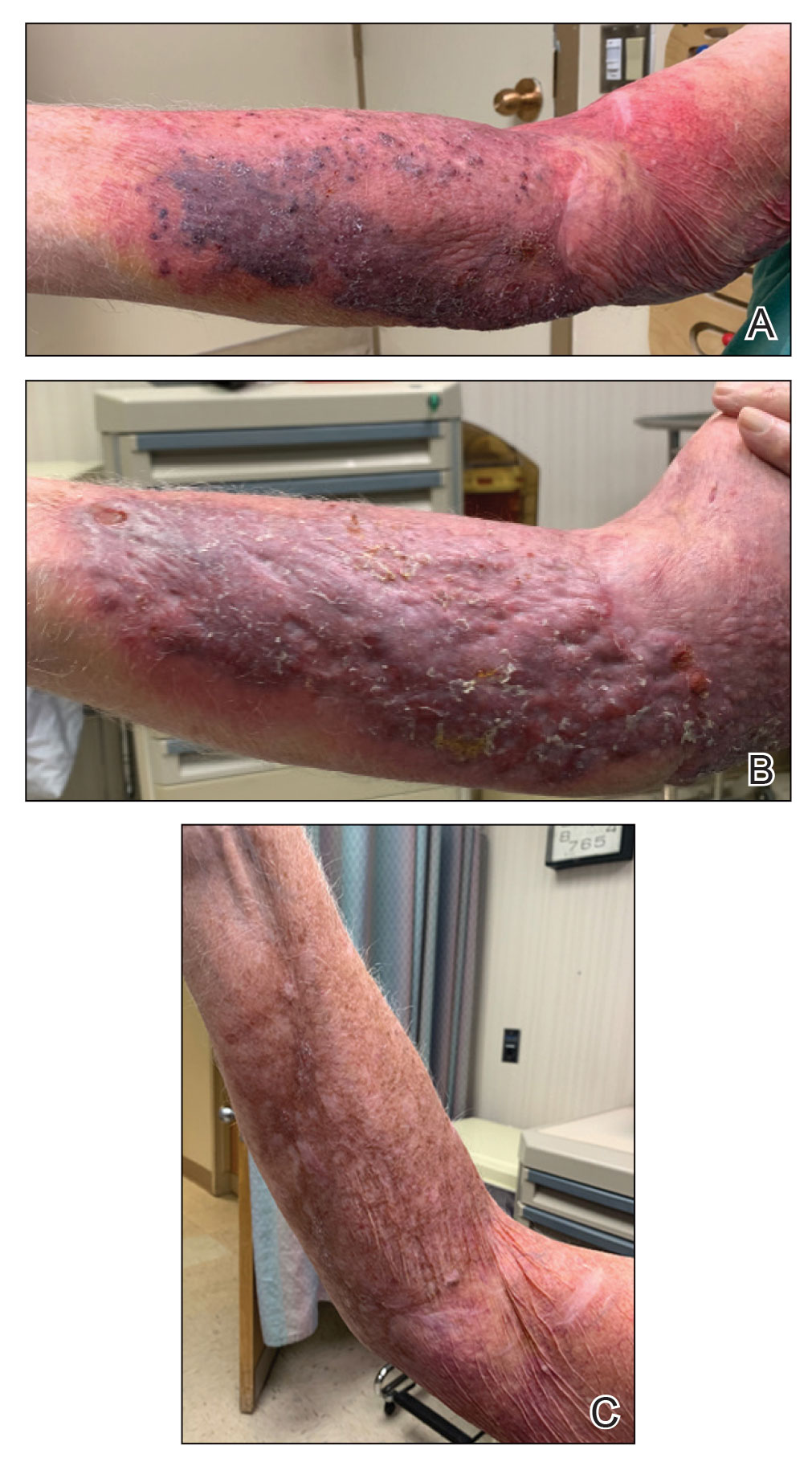
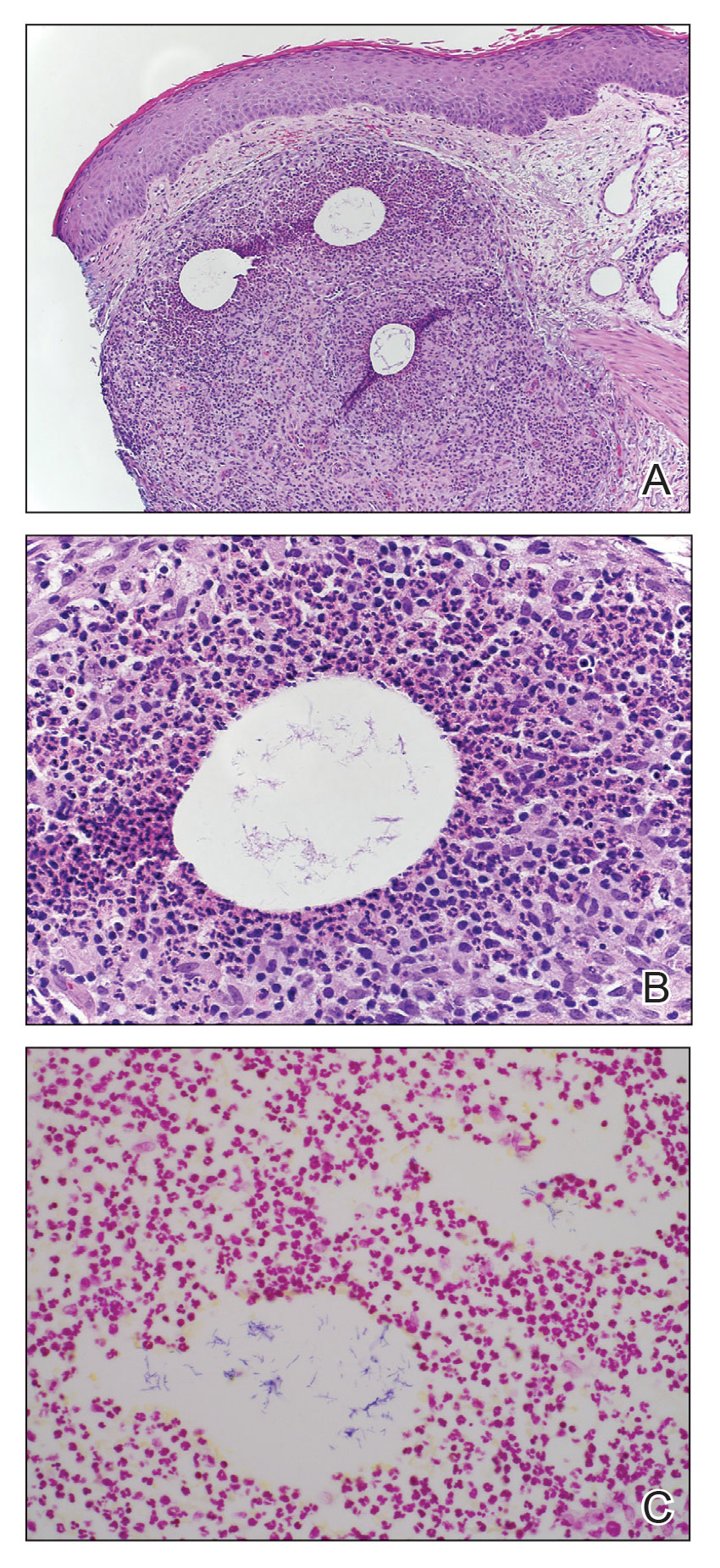
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.
Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
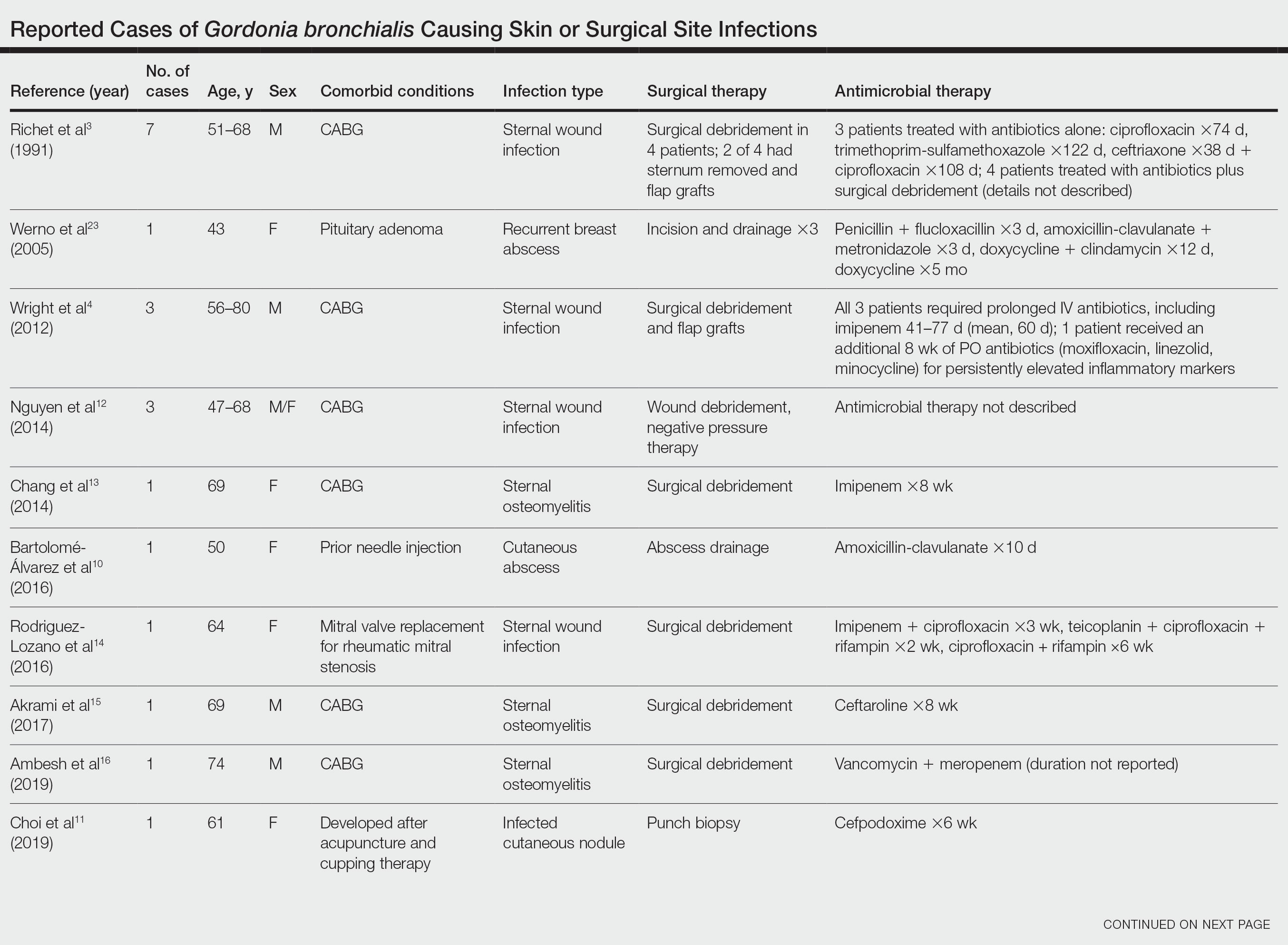
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.
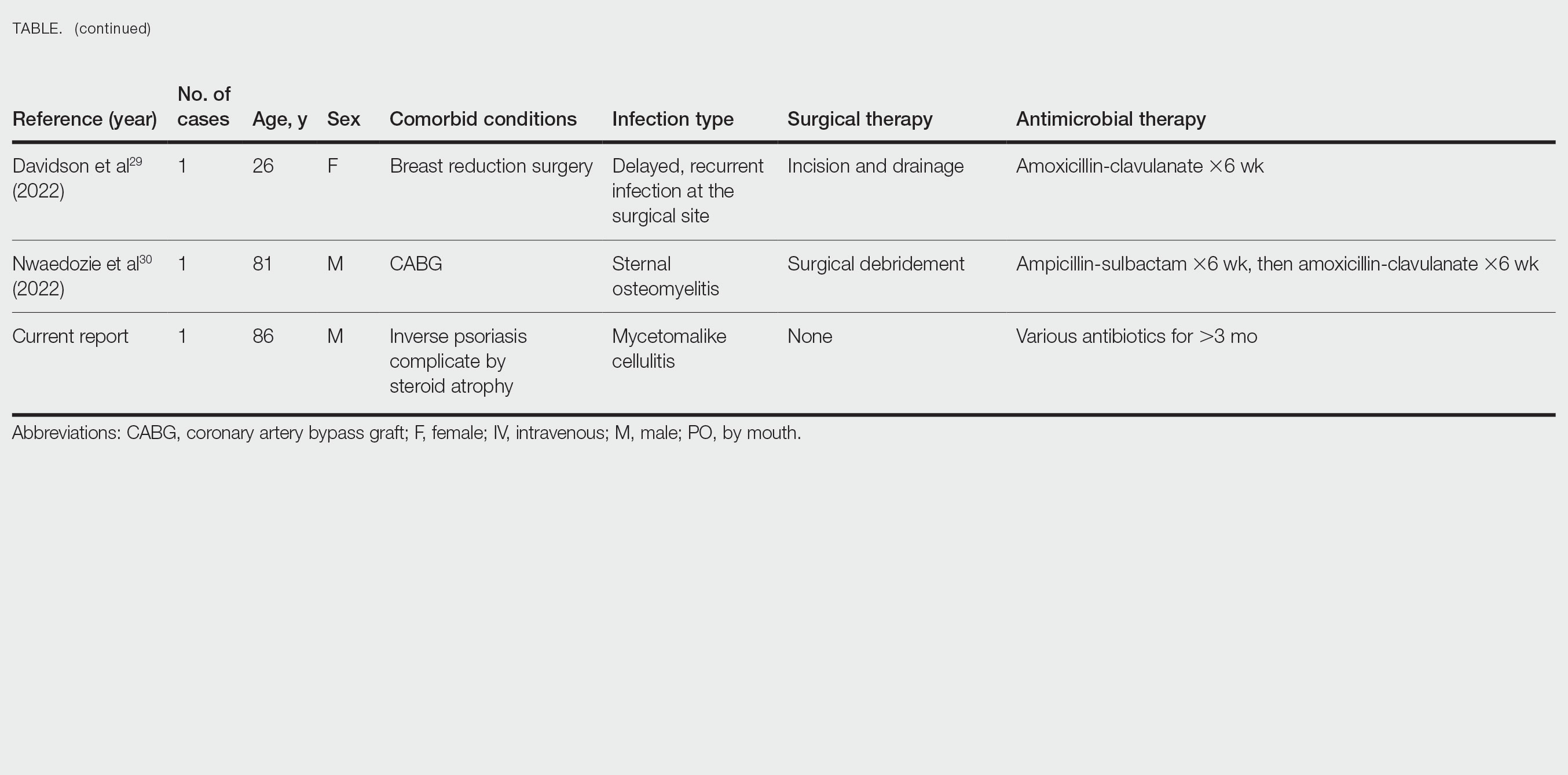
Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.

Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.

Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.


Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
Mycetoma is a chronic subcutaneous infection due to fungal (eumycetoma) or aerobic actinomycetes (actinomycetoma) organisms. Clinical lesions develop from a granulomatous infiltrate organizing around the infectious organism. Patients can present with extensive subcutaneous nodularity and draining sinuses that can lead to deformation of the affected extremity. These infections are rare in developed countries, and the prevalence and incidence remain unknown. It has been reported that actinomycetes represent 60% of mycetoma cases worldwide, with the majority of cases in Central America from Nocardia (86%) and Actinomadura madurae (10%). 1Gordonia species are aerobic, partially acid-fast, gram-positive actinobacteria that may comprise a notable minority of actinomycete isolates. 2 The species Gordonia bronchialis is of particular interest as a human pathogen because of increasing reports of nosocomial infections. 3,4 We describe a case of a mycetomalike infection due to G bronchialis in an immunocompetent patient with complete resolution after 3 months of antibiotics.

Case Report
An 86-year-old man presented to the emergency department with a pruritic rash on the right forearm. He had a history of chronic kidney disease, hypertension, and inverse psoriasis complicated by steroid atrophy. He reported trauma to the right antecubital fossa approximately 1 to 2 months prior from a car door; he received wound care over several weeks at an outside hospital. The initial wound healed completely, but he subsequently noticed erythema spreading down the forearm. At the current presentation, he was empirically treated with mid-potency topical steroids and cefuroxime for 7 days. Initial laboratory results were notable for a white blood cell count of 5.7×103 cells/μL (reference range,3.7–8.4×103 cells/μL) and a creatinine level of 1.5 mg/dL (reference range, 0.57–1.25 mg/dL). The patient returned to the emergency department 2 weeks later with spreading of the initial rash and worsening pruritus. Dermatologic evaluation revealed the patient was afebrile and had violaceous papules and nodules that coalesced into plaques on the right arm, with the largest measuring approximately 15 cm. Areas of superficial erosion and crusting were noted (Figure 1A). The patient denied constitutional symptoms and had no axillary or cervical lymphadenopathy. The differential initially included an atypical infection vs a neoplasm. Two 5-mm punch biopsies were performed, which demonstrated a suppurative granulomatous infiltrate in the dermis with extension into the subcutis (Figure 2A). Focal vacuolations within the dermis demonstrated aggregates of gram-positive pseudofilamentous organisms (Figures 2B and 2C). Aerobic tissue cultures grew G bronchialis that was susceptible to all antibiotics tested and Staphylococcus epidermidis. Fungal and mycobacterial cultures were negative. The patient was placed on amoxicillin 875 mg–clavulanate 125 mg twice daily for 3 weeks. However, he demonstrated progression of the rash, with increased induration and confluence of plaques on the forearm (Figure 1B). A repeat excisional biopsy was performed, and a tissue sample was sent for 16S ribosomal RNA sequencing identification. However, neither conventional cultures nor sequencing demonstrated evidence of G bronchialis or any other pathogen. Additionally, bacterial, fungal, and mycobacterial blood cultures were negative. Amoxicillin-clavulanate was stopped, and he was placed on trimethoprim-sulfamethoxazole for 2 weeks, then changed to linezolid (600 mg twice daily) due to continued lack of improvement of the rash. After 2 weeks of linezolid, the rash was slightly improved, but the patient had notable side effects (eg, nausea, mucositis). Therefore, he was switched back to trimethoprim-sulfamethoxazole for another 6 weeks. Antibiotic therapy was discontinued after there was notable regression of indurated plaques (Figure 1C); he received more than 3 months of antibiotics in all. At 1 month after completion of antibiotic therapy, the patient had no evidence of recurrence.

Comment
Microbiology of Gordonia Species—Gordonia bronchialis originally was isolated in 1971 by Tsukamura et al5 from the sputum of patients with cavitary tuberculosis and bronchiectasis in Japan. Other Gordonia species (formerly Rhodococcus or Gordona) later were identified in soil, seawater, sediment, and wastewater. Gordonia bronchialis is a gram-positive aerobic actinomycete short rod that organizes in cordlike compact groups. It is weakly acid fast, nonmotile, and nonsporulating. Colonies exhibit pinkish-brown pigmentation. Our understanding of the clinical significance of this organism continues to evolve, and it is not always clearly pathogenic. Because Gordonia isolates may be dismissed as commensals or misidentified as Nocardia or Rhodococcus by routine biochemical tests, it is possible that infections may go undetected. Speciation requires gene sequencing; as our utilization of molecular methods has increased, the identification of clinically relevant aerobic actinomycetes, including Gordonia, has improved,6 and the following species have been recognized as pathogens: Gordonia araii, G bronchialis, Gordonia effusa, Gordonia otitidis, Gordonia polyisoprenivorans, Gordonia rubirpertincta, Gordonia sputi, and Gordonia terrae.7
Cases Reported in the Literature—A PubMed search of articles indexed for MEDLINE using the term Gordonia bronchialis yielded 35 previously reported human cases of G bronchialis infection, most often associated with medical devices or procedures.8-31 Eighteen of these cases were sternal surgical site infections in patients with a history of cardiac surgery,3,4,12-16,30 including 2 outbreaks following coronary artery bypass grafting that were thought to be related to intraoperative transmission from a nurse.3,4 Of the remaining cases, 12 were linked to a procedure or an indwelling catheter: 4 cases of peritonitis in the setting of continuous ambulatory peritoneal dialysis17,18,26,27; 3 cases of skin and soft tissue infection (1 at the site of a prior needle injection,10 1 after acupuncture,11 and 1 after breast reduction surgery29); 1 case of ventriculitis in a premature neonate with an underlying intraventricular shunt19; 2 cases of pacemaker-induced endocarditis20,28; 1 case of tibial osteomyelitis related to a bioresorbable polymer screw21; and 1 case of chronic endophthalmitis with underlying intraocular lens implants.22 The Table lists all cases of G bronchialis skin or surgical site infections encountered in our literature search as well as the treatment provided in each case.


Only 4 of these 35 cases of G bronchialis infections were skin and soft tissue infections. All 4 occurred in immunocompetent hosts, and 3 were associated with needle punctures or surgery. The fourth case involved a recurrent breast abscess that occurred in a patient without known risk factors or recent procedures.23 Other Gordonia species have been associated with cutaneous infections, including Gordonia amicalis, G terrae, and recently Gordonia westfalica, with the latter 2 demonstrating actinomycetoma formation.32-34 Our case is remarkable in that it represents actinomycetoma due to G bronchialis. Of note, our patient was immunocompetent and did not have any radiation or chronic lymphedema involving the affected extremity. However, his history of steroid-induced skin atrophy may have predisposed him to this rare infection.
Clinical Presentation—Classic mycetoma demonstrate organismal granules within the dermis, surrounded by a neutrophilic infiltrate, which is in turn surrounded by histiocytes and multinucleated giant cells. Periodic acid–Schiff and silver stains can identify fungal organisms, while Gram stain helps to elucidate bacterial etiologies.1 In our patient, a biopsy revealed several dermal aggregates of pseudofilamentous gram-positive organisms surrounded by a neutrophilic and histiocytic infiltrate.8 Because this case presented over weeks to months rather than months to years, it progressed more rapidly than a classic mycetoma. However, the dermatologic and histologic features were consistent with mycetoma.
Management—General treatment of actinomycetoma requires identification of the causative organism and prolonged administration of antibiotics, typically in combination.35-37 Most G bronchialis infections associated with surgical intervention or implants in the literature required surgical debridement and removal of contaminated material for clinical cure, with the exception of 3 cases of sternal wound infection and 1 case of peritonitis that recovered with antimicrobial therapy alone.3,17 Combination therapy often was used, but monotherapy, particularly with a fluoroquinolone, has been reported. Susceptibility data are limited, but in general, Gordonia species appear susceptible to imipenem, ciprofloxacin, amikacin, gentamicin, and linezolid, with variable susceptibility to vancomycin (89% of isolates), third-generation cephalosporins (80%–90% of isolates), tetracyclines (≤85% of isolates), penicillin (≤70% of isolates), and trimethoprim-sulfamethoxazole (≤65% of isolates).7,10,19,38-40 Although there are no standardized recommendations for the treatment of these infections, the most commonly used drugs to treat Gordonia are carbapenems and fluoroquinolones, with or without an aminoglycoside, followed by third-generation cephalosporins and vancomycin, depending on susceptibilities. Additional antibiotics (alone or in combination) that have previously been used with favorable outcomes include amoxicillin or amoxicillin-clavulanate, piperacillin-tazobactam, rifampicin, trimethoprim-sulfamethoxazole, minocycline, doxycycline, and daptomycin.
Our patient received amoxicillin-clavulanate, trimethoprim-sulfamethoxazole, and linezolid. We considered combination therapy but decided against it due to concern for toxicity, given his age and poor renal function. The antibiotic that was most important to his recovery was unclear; the patient insisted that his body, not antibiotics, deserved most of the credit for healing his arm. Although cultures and polymerase chain reaction assays were negative after 3 weeks of amoxicillin-clavulanate, the patient did not show clinical improvement—reasons could be because the antibiotic reduced but did not eliminate the bacterial burden, sampling error of the biopsy, or it takes much longer for the body to heal than it takes to kill the bacteria. Most likely a combination of factors was at play.
Conclusion
Gordonia bronchialis is an emerging cause of human infections typically occurring after trauma, inoculation, or surgery. Most infections are localized; however, the present case highlights the ability of this species to form a massive cutaneous infection. Treatment should be tailored to susceptibility, with close follow-up to ensure improvement and resolution. For clinicians encountering a similar case, we encourage biopsy prior to empiric antibiotics, as antibiotic therapy can decrease the yield of subsequent testing. Treatment should be guided by the clinical course and may need to last weeks to months. Combination therapy for Gordonia infections should be considered in severe cases, in cases presenting as actinomycetoma, in those not responding to therapy, or when the susceptibility profile is unknown or unreliable.
Acknowledgments—The authors thank this veteran for allowing us to participate in his care and to learn from his experience. He gave his consent for us to share his story and the photographs of the arm.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
- Arenas R, Fernandez Martinez RF, Torres-Guerrero E, et al. Actinomycetoma: an update on diagnosis and treatment. Cutis. 2017;99:E11-E15.
- Poonwan N, Mekha N, Yazawa K, et al. Characterization of clinical isolates of pathogenic Nocardia strains and related actinomycetes in Thailand from 1996 to 2003. Mycopathologia. 2005;159:361-368.
- Richet HM, Craven PC, Brown JM, et al. A cluster of Rhodococcus (Gordona) bronchialis sternal-wound infections after coronary-artery bypass surgery. N Engl J Med. 1991;324:104-109.
- Wright SN, Gerry JS, Busowski MT, et al. Gordonia bronchialis sternal wound infection in 3 patients following open heart surgery: intraoperative transmission from a healthcare worker. Infect Control Hosp Epidemiol. 2012;33:1238-1241.
- Tsukamura M. Proposal of a new genus, Gordona, for slightly acid-fast organisms occurring in sputa of patients with pulmonary disease and in soil. J Gen Microbiol. 1971;68:15-26.
- Wang T, Kong F, Chen S, et al. Improved identification of Gordonia, Rhodococcus and Tsukamurella species by 5′-end 16s rRNA gene sequencing. Pathology. 2011;43:58-63.
- Aoyama K, Kang Y, Yazawa K, et al. Characterization of clinical isolates of Gordonia species in Japanese clinical samples during 1998-2008. Mycopathologia. 2009;168:175-183.
- Ivanova N, Sikorski J, Jando M, et al. Complete genome sequence of Gordonia bronchialis type strain (3410 T). Stand Genomic Sci. 2010;2:19-28.
- Johnson JA, Onderdonk AB, Cosimi LA, et al. Gordonia bronchialis bacteremia and pleural infection: case report and review of the literature. J Clin Microbiol. 2011;49:1662-1666.
- Bartolomé-Álvarez J, Sáez-Nieto JA, Escudero-Jiménez A, et al. Cutaneous abscess due to Gordonia bronchialis: case report and literature review. Rev Esp Quimioter. 2016;29:170-173.
- Choi ME, Jung CJ, Won CH, et al. Case report of cutaneous nodule caused by Gordonia bronchialis in an immunocompetent patient after receiving acupuncture. J Dermatol. 2019;46:343-346.
- Nguyen DB, Gupta N, Abou-Daoud A, et al. A polymicrobial outbreak of surgical site infections following cardiac surgery at a community hospital in Florida, 2011-2012. Am J Infect Control. 2014;42:432-435.
- Chang JH, Ji M, Hong HL, et al. Sternal osteomyelitis caused byGordonia bronchialis after open-heart surgery. Infect Chemother. 2014;46:110-114.
- Rodriguez-Lozano J, Pérez-Llantada E, Agüero J, et al. Sternal wound infection caused by Gordonia bronchialis: identification by MALDI-TOF MS. JMM Case Rep. 2016;3:e005067.
- Akrami K, Coletta J, Mehta S, et al. Gordonia sternal wound infection treated with ceftaroline: case report and literature review. JMM Case Rep. 2017;4:e005113.
- Ambesh P, Kapoor A, Kazmi D, et al. Sternal osteomyelitis by Gordonia bronchialis in an immunocompetent patient after open heart surgery. Ann Card Anaesth. 2019;22:221-224.
- Ma TKW, Chow KM, Kwan BCH, et al. Peritoneal-dialysis related peritonitis caused by Gordonia species: report of four cases and literature review. Nephrology. 2014;19:379-383.
- Lam JYW, Wu AKL, Leung WS, et al. Gordonia species as emerging causes of continuous-ambulatory-peritoneal-dialysis-related peritonitis identified by 16S rRNA and secA1 gene sequencing and matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). J Clin Microbiol. 2015;53:671-676.
- Blaschke AJ, Bender J, Byington CL, et al. Gordonia species: emerging pathogens in pediatric patients that are identified by 16S ribosomal RNA gene sequencing. Clin Infect Dis. 2007;45:483-486.
- Titécat M, Loïez C, Courcol RJ, et al. Difficulty with Gordonia bronchialis identification by Microflex mass spectrometer in a pacemaker‐induced endocarditis. JMM Case Rep. 2014;1:E003681.
- Siddiqui N, Toumeh A, Georgescu C. Tibial osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2012;50:3119-3121.
- Choi R, Strnad L, Flaxel CJ, et al. Gordonia bronchialis–associated endophthalmitis. Emerg Infect Dis. 2019;25:1017-1019.
- Werno AM, Anderson TP, Chambers ST, et al. Recurrent breast abscess caused by Gordonia bronchialis in an immunocompetent patient. J Clin Microbiol. 2005;43:3009-3010.
- Sng LH, Koh TH, Toney SR, et al. Bacteremia caused by Gordonia bronchialis in a patient with sequestrated lung. J Clin Microbiol. 2004;42:2870-2871.
- Ramanan P, Deziel PJ, Wengenack NL. Gordonia bacteremia. J Clin Microbiol. 2013;51:3443-3447.
- Sukackiene D, Rimsevicius L, Kiveryte S, et al. A case of successfully treated relapsing peritoneal dialysis-associated peritonitis caused by Gordonia bronchialis in a farmer. Nephrol Ther. 2018;14:109-111.
- Bruno V, Tjon J, Lin S, et al. Peritoneal dialysis-related peritonitis caused by Gordonia bronchialis: first pediatric report. Pediatr Nephrol. 2022;37:217-220. doi: 10.1007/s00467-021-05313-3
- Mormeneo Bayo S, Palacián Ruíz MP, Asin Samper U, et al. Pacemaker-induced endocarditis by Gordonia bronchialis. Enferm Infecc Microbiol Clin (Engl Ed). 2022;40:255-257.
- Davidson AL, Driscoll CR, Luther VP, et al. Recurrent skin and soft tissue infection following breast reduction surgery caused by Gordonia bronchialis: a case report. Plast Reconstr Surg Glob Open. 2022;10:E4395.
- Nwaedozie S, Mojarrab JN, Gopinath P, et al. Sternal osteomyelitis caused by Gordonia bronchialis in an immunocompetent patient following coronary artery bypass surgery. IDCases. 2022;29:E01548.
- Nakahama H, Hanada S, Takada K, et al. Obstructive pneumonia caused by Gordonia bronchialis with a bronchial foreign body. Int J Infect Dis. 2022;124:157-158. doi:10.1016/j.ijid.2022.09.028
- Lai CC, Hsieh JH, Tsai HY, et al. Cutaneous infection caused by Gordonia amicalis after a traumatic injury. J Clin Microbiol. 2012;50:1821-1822.
- Bakker XR, Spauwen PHM, Dolmans WMV. Mycetoma of the hand caused by Gordona terrae: a case report. J Hand Surg Am. 2004;29:188-190.
- Gueneau R, Blanchet D, Rodriguez-Nava V, et al. Actinomycetoma caused by Gordonia westfalica: first reported case of human infection. New Microbes New Infect. 2020;34:100658.
- Auwaerter PG, ed. The Johns Hopkins POC-IT ABX Guide. Johns Hopkins Medicine; 2021.
- Welsh O, Sauceda E, Gonzalez J, et al. Amikacin alone andin combination with trimethoprim-sulfamethoxazole in the treatment of actinomycotic mycetoma. J Am Acad Dermatol. 1987;17:443-448.
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112.
- Pham AS, Dé I, Rolston KV, et al. Catheter-related bacteremia caused by the nocardioform actinomycete Gordonia terrae. Clin Infect Dis. 2003;36:524-527.
- Renvoise A, Harle JR, Raoult D, et al. Gordonia sputi bacteremia. Emerg Infect Dis. 2009;15:1535-1537.
- Moser BD, Pellegrini GJ, Lasker BA, et al. Pattern of antimicrobial susceptibility obtained from blood isolates of a rare but emerging human pathogen, Gordonia polyisoprenivorans. Antimicrob Agents Chemother. 2012;56:4991-4993.
Practice Points
- Gordonia bronchialis is an emerging cause of human skin and soft tissue infection, typically occurring after trauma, inoculation, or surgery.
- Gordonia species can cause a mycetomalike skin infection.
- Increasing use of molecular methods to identify bacteria has improved identification of clinically relevant actinomycetes, such as Helvetica Neue LT StdGordonia, and increases the likelihood that clinicians will see these organisms on culture results.