User login
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
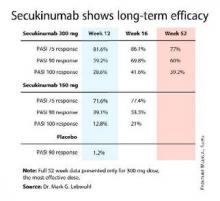
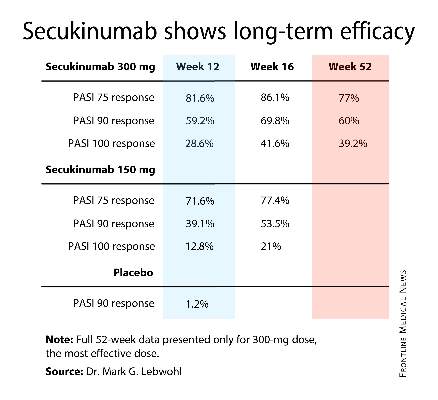
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AMSTERDAM – The investigational biologic agent secukinumab continued to show strong efficacy through 52 weeks of treatment in a new secondary analysis of the pivotal phase III ERASURE trial.
For example, 60% of patients treated for moderate-to-severe chronic plaque psoriasis at the 300-mg dose of secukinumab continued to maintain a PASI 90 response through 52 weeks of therapy, Dr. Mark G. Lebwohl reported at the annual congress of the European Academy of Dermatology and Venereology.
Secukinumab is a fully human monoclonal antibody directed against interleukin-17A. While the Food and Drug Administration requested that the primary outcome in ERASURE (Efficacy of Response and Safety of Two Fixed Secukinumab Regimens in Psoriasis) should be the degree of skin clearing at week 12, the efficacy actually peaked at 16 weeks and was then sustained with only modest tailoff through the remainder of the 52-week study (see graphic).
This is important new information, Dr. Lebwohl noted, because 12-week outcomes don’t provide a full picture regarding potent therapies. Psoriasis is a chronic disease requiring long-term therapy, and some biologic agents now marketed for psoriasis tend to show a loss of effect over time. Reassuringly, the long-term ERASURE data show that’s not the case for secukinumab, explained Dr. Lebwohl, professor and chairman of the department of dermatology at Mt. Sinai Medical Center in New York.
ERASURE involved 738 patients randomized double-blind to secukinumab at either 150 mg or 300 mg, or to placebo. Following an initial loading-dose phase when the biologic was given subcutaneously once weekly for 5 weeks, it was then administered every 4 weeks for the remainder of the year-long trial.
The safety profile of secukinumab was similar to placebo with one exception: Upper respiratory tract infections were three- to fourfold more common in patients on the IL-17A inhibitor.
Novartis funded the ERASURE trial, whose primary results were recently published (N. Engl. J. Med. 2014;371:326-38). Dr. Lebwohl reported serving as a consultant to Novartis and more than a dozen other pharmaceutical companies.
AT THE EADV CONGRESS
Key clinical point: Psoriasis patients’ initial strong clinical response to the interleukin-17A inhibitor secukinumab is sustained through 52 weeks of therapy.
Major finding: Sixty percent of patients with moderate-to-severe chronic plaque psoriasis had a PASI 90 response sustained through a full year of treatment.
Data source: The 52-week long, double-blind, multicenter ERASURE trial randomized 738 patients with moderate-to-severe chronic plaque psoriasis to secukinumab at 150 mg or 300 mg, or to placebo.
Disclosures: Novartis funded the study. Dr. Lebwohl reported serving as a consultant to the company.