User login
Hospitalized patients often have difficulty initiating and maintaining sleep, or complain of early awakening and nonrestorative sleep.1 The etiology of sleep disruption is multifactorial and includes the patient's underlying illness(es), medical treatments, and the hospital environment. Often unrecognized and untreated during hospitalization, sleep disruption may lead to sleep deprivation, or a chronic lack of restorative sleep.
Even in healthy individuals, sleep deprivation can result in numerous physical and psychological consequences. Sleep deprivation is associated with hypertension,2, 3 impaired postural control,4 decreased ventilatory drive,5 increased sympathetic cardiovascular activation,6 blunted hypothalamic‐pituitary‐adrenal axis,7 impaired host defenses, and possibly diabetes mellitus and obesity.810 The lack of restorative sleep increases the risk of developing anxiety and mood disorders and delirium, especially in acutely ill older patients.11 In the presence of acute physical infirmity, inadequate sleep may further compound illness and impair recovery. We provide an overview of normal sleep architecture and discuss factors that lead to sleep disruption in hospitalized medical patients.
NORMAL SLEEP ARCHITECTURE AND REGULATION
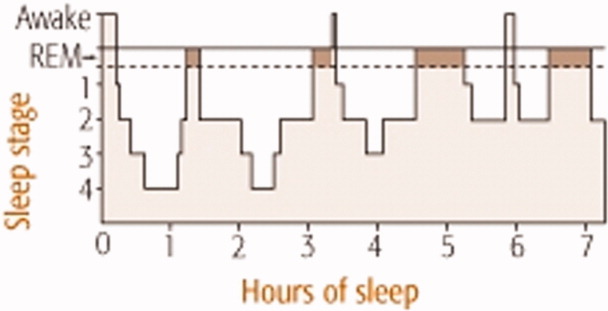
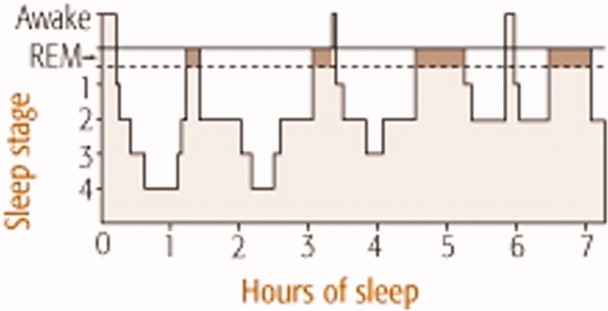
Normal sleep architecture refers to a characteristic pattern of sleep, and consists of two major stages: nonrapid eye movement (NREM, pronounced non‐rem) and rapid eye movement (REM). (For a table of pertinent abbreviations and terms, see Table 1) Sleep is quantified by polysomnography (PSG), which includes an electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). A PSG also includes an electrocardiogram (ECG), and measures of airflow, oxygen saturation, and body position. NREM sleep comprises 75% to 80% of total sleep time (TST), and is characterized by relatively quiescent brain activity and decreased metabolic rate.12 NREM sleep consists of four stages (S1‐S4), with each stage leading to a progressively deeper sleep (Figure 1). REM sleep follows slow wave sleep (SWS), or deep sleep, and increases over the night, comprising 20% to 25% of TST. REM sleep is characterized by an activated EEG pattern, muscle atonia, and episodic bursts of rapid eye movements.
| Acronym | Term |
|---|---|
| BiPAP | Bilevel positive airway pressure |
| CHF | Congestive heart failure |
| CPAP | Continuous positive airway pressure |
| COPD | Chronic obstructive pulmonary disease |
| EEG | Electroencephalogram |
| EOG | Electroculogram |
| EMG | Electromyogram |
| ESRD | End‐stage renal disease |
| NPPV | Noninvasive positive pressure ventilation |
| NREM | Nonrapid eye movement |
| OSA | Obstructive sleep apnea |
| PLMD | Periodic limb movement disorder |
| PSG | Polysomnography |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement |
| RLS | Restless leg syndrome |
| S1‐S4 | 4 Stages of sleep in NREM |
| SE | Sleep efficiency; TST divided by total time in bed |
| SWS | Slow wave sleep |
| TBI | Traumatic brain injury |
| TST | Total sleep time |
Normal sleep provides a period of physiologic and mental rest. During sleep, sympathetic tone decreases and parasympathetic tone increases, leading to a reduction in heart rate, arterial blood pressure, and cardiac output.13 Deep sleep is theorized to be necessary for physiologic restoration. REM sleep is associated with dreaming, and is essential for maintaining emotional and cognitive well‐being. Sleep architecture undergoes characteristic changes as people age.14 The duration of SWS peaks in childhood and decreases with age. Consequently, people >60 years old tend to have lower arousal thresholds and to have more frequent awakenings. The results of the Sleep Heart Health Study found that increased age was associated with decreased percentage of REM sleep, worse sleep efficiency (SE, which is TST divided by total time in bed), and lower arousal thresholds.14 With the reduction of SE, older people need to spend more hours in bed to achieve the same amount of restorative sleep as when they were younger. Although sleep tends to become more disrupted as people age, insomnia should not be considered a normal part of aging, and needs to be addressed clinically.15 The results of a National Sleep Foundation telephone survey of subjects between the ages of 55 and 84 years old (n = 1,506) suggested that sleep complaints in older adults are frequently secondary to comorbid medical conditions.16
Multiple anatomic structures, pathways, and neurotransmitter systems are involved in controlling wakefulness and sleep. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Sleep‐promoting neurotransmitters include gamma aminobutyric acid (GABA), adenosine, and melatonin. Specific stages of sleep are regulated by the turning on and off of various neurons. REM on cells use GABA, acetylcholine, and glutamine, whereas REM off cells use norepinephrine and serotonin. SWS is promoted by GABA and serotonin.17
Sleep regulation is a balance between a homeostatic sleep need and an intrinsic body clock, or circadian pacemaker. Located in the suprachiasmic nucleus, the circadian pacemaker determines the onset and termination of sleep, and is partially regulated by environmental cues such as light and ambient temperature. Melatonin, a physiologic sleep promoter, is inhibited by ambient light, and its circulation is decreased during daylight hours. The adrenal secretion of cortisol, which is associated with wakefulness, follows a circadian pattern. Regulated by the hypothalamic‐pituitary axis, cortisol levels peak in the early morning hours in preparation for the increased metabolic demands during wakefulness.
SLEEP PROBLEMS IN HOSPITALIZED PATIENTS
Insomnia, which is characterized by difficulty initiating or maintaining sleep, is the most common sleep disorder in the United States. About one‐third of the adult population in the United States experiences insomnia at some point in their lives,18 and it is a persistent problem in approximately 10% of U.S. adults.19 Insomnia can be exacerbated during hospitalization.
Studies investigating sleep in hospitalized patients using PSG have been limited primarily to the setting of the intensive care unit (ICU). Critically ill patients, particularly those requiring mechanical ventilation, are prone to sleep disturbances and an associated delirium.2022 Critically ill patients have fragmented sleep, with decreased SE and SWS, and increased S1 and S2.23 Physician awareness of the impact of sleep disturbance in hospitalized patients is vital. Surveys reveal that approximately one‐half of patients admitted on general medical wards complain of sleep disruption.24, 25 Meissner et al.25 examined the prevalence of sleep complaints and physician recognition of these complaints among general medical patients admitted to a Veterans Affairs tertiary care center. Results showed that 47% (n = 222) of patients had complaints of either insomnia and/or excessive daytime sleepiness.
FACTORS AFFECTING SLEEP DURING HOSPITALIZATION
Many medical and neurologic illnesses, psychiatric disorders, pain, medication therapy and the hospital environment may impair sleep, and hinder recovery from illness.
General Medical Disorders
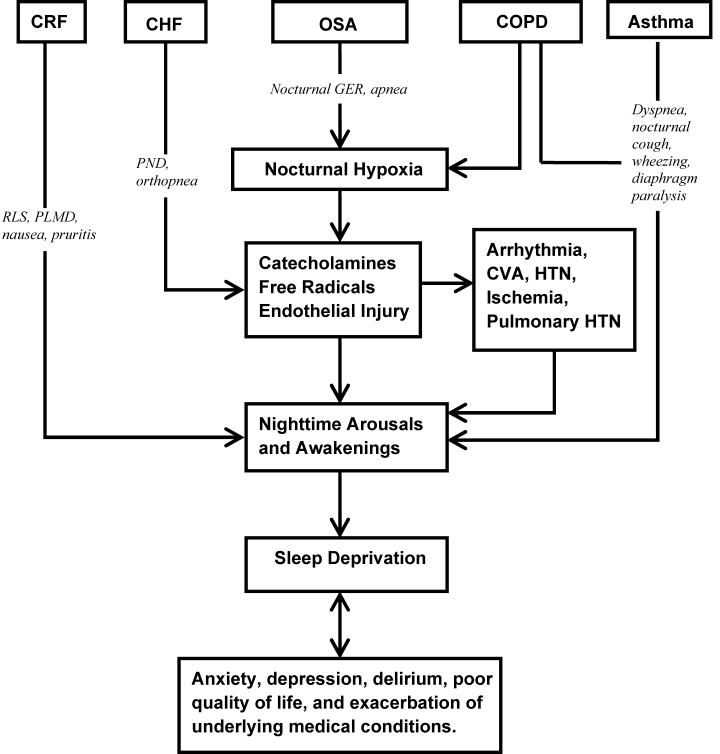
Primary sleep disorders, such as obstructive sleep apnea (OSA) and numerous other medical illnesses, can directly impair sleep physiology, leading to a cyclical interaction (Figure 2). Other conditions that disrupt sleep include congestive heart failure (CHF), diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux, cardiovascular disease, thyroid disorders, renal disease, and severe liver disease.26 Table 2 lists selected medical and neurological conditions, their associated sleep‐related problems, and suggestions on how to ameliorate these problems.
| Disease | Problem | Clinical Implications and Strategies to Improve Sleep |
|---|---|---|
| ||
| Asthma | Nocturnal exacerbation, nocturnal GER | Inhaled corticosteroids and/or long‐acting inhaled beta‐adrenergic agents |
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep‐disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne‐Stokes respiration | Keep the head of bed elevated 30 degrees. Nocturnal O2 to keep O2 saturation >88%. Daytime diuresis. Optimize cardiac function to treat Cheyne‐Stokes respiration. Consider CPAP for CHF |
| COPD | Persistent nocturnal hypoxemia with complications (e.g., cor pulmonale, polycythemia) | O2 for COPD and persistent hypoxemia (PaO2 55‐60 mm Hg) |
| Sporadic nighttime desaturations | PaO2 55 mm Hg monitor O2 saturation by pulse oximetry. If patient desaturates to 88% at night consistently, start nocturnal O2. For hypercapnia, adjust O2 to maintain O2 saturation at 88% to 90% | |
| Early‐morning airflow obstruction | Consider bedtime tiotropium and inhaled long‐acting beta‐adrenergic agonist agents | |
| Inhibition of respiratory muscles in REM | Avoid sedative‐hypnotics that cause respiratory depression | |
| Decreased functional residual capacity from recumbent position during sleep | Minimize recumbancy by keeping the head of bed up at 30 degrees | |
| End‐stage renal disease | Pruritus, nausea; increased risk of RLS and PLMD | Ambulation may help with RLS. Consider ropinirole and pramipexole. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents |
| Nocturnal GER | Nocturnal GER decreased sleep, heartburn, coughing, asthma | Avoid eating or drinking 2 hours before bedtime, especially those that delay gastric emptying, increase acid secretion, or decrease lower esophageal sphincter pressure; e.g., high‐fat foods, ethanol, chocolate, peppers, peppermint. Keep head of bed 30 degrees. Minimize medications that could worsen nocturnal GER; e.g., theophylline, calcium channel blockers, prostaglandins, bisphosphonates |
| OSA | Snoring with upper airway obstruction | No ethanol 2 hours before bedtime. Minimize CNS depressants. Avoid supine position. Consider CPAP, oral mandibular advancement device, and/or surgical correction. Long‐term plan should include weight loss |
| Stroke | Focal neurologic deficits (e.g., dysphagia, weakness or paralysis) | Keep head of bed 30 degrees. Regularly suction secretions. Post‐stroke patients have an increased risk of hypersomnia, insomnia, and/or OSA |
Affecting approximately 24% of men and 9% of women in the United States, OSA is the most common primary sleep disorder,27, 28 and causes significant mental and physical morbidity. Risk factors for OSA include obesity, hypothyroidism‐induced muscle weakness, and structural abnormalities in the oropharynx region such as acromegaly, micrognathia, or retrognathia. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness.28 These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment (Figure 2). In addition, chronic sympathetic activation causes numerous derangements in the vascular endothelium and platelet activation.29, 30 Sleep‐disordered breathing has been independently associated with cardiovascular diseases such as hypertension, CHF, ischemic heart disease, atrial fibrillation, and cerebrovascular disease.31, 32
OSA is also associated with sleep‐related gastroesophageal reflux, which is characterized by pain and nocturnal cough, and can induce nocturnal asthma attacks and laryngospasm.33 Green et al.29 found that OSA patients treated with continuous positive airway pressure (CPAP) had a 48% improvement in nocturnal reflux symptoms. Although the pathophysiology connecting OSA to the renal system is unknown, OSA has been found in up to 60% of patients with end‐stage renal disease and chronic renal failure.34
Patients with pulmonary disorders can be profoundly affected by the normal physiologic changes during sleep, particularly in REM sleep. During REM sleep, all respiratory muscles except the diaphragm become paralyzed. Thus, episodes of marked oxygen desaturation can occur in patients who rely on their accessory muscles for respiration. COPD patients have decreased TST, SWS, and REM sleep. Shortness of breath, nocturnal cough, and wheezing worsen sleep.35 The resulting fatigue and sleep deprivation negatively impact the work of breathing and impair gas exchange. Airflow obstruction tends to worsen in the early morning hours in patients with COPD and asthma, and may be related to the effect of REM on the accessory muscles for respiration. Although used to target CO2 retention, investigations using bilevel positive airway pressure ventilators (BiPAP) for improving sleep in COPD patients have been limited. Noninvasive positive pressure ventilation (NPPV) appears to acutely improve SE and TST in patients with hypercapnic COPD without significantly improving gas exchange. Other sleep parameters such as sleep architecture and the number of arousals during the night, remain unchanged during NPPV.36
CPAP has several side effects that could worsen sleep, which may explain its poor adherence rate among ambulatory patients.37 Side effects include nasal bridge discomfort, nasal congestion, swallowing air, dry nose, dry or red eyes, noise, ear pain, and rhinitis.38 During hospitalization, efforts should be made to improve patient comfort by resizing ill‐fitting masks, adding heated humidification or nasal steroids to alleviate nasal congestion, or adding a chin strap to reduce air leak and ingestion of air.
Endocrine disorders have also been associated with sleep disruption. Studies suggest that patients with diabetes mellitus have decreased TST and impaired sleep quality due to nocturia and neuropathic pain.39 Inadequate sleep may also affect glucose control. Inadequate quality or quantity of sleep has been shown to be a risk factor for developing Type 2 diabetes mellitus in large prospective studies.40 Sleep duration and quality were significant predictors of increased levels of glycosylated hemoglobin (HbA1c) in patients with Type 2 diabetes mellitus. Thyroid diseases often coexist with diabetes mellitus. Both hypo‐ and hyperthyroidism have been associated with sleep disruption. Hypothyroidism is associated with daytime somnolence and fatigue. Patients with hypothyroidism tend to have reduced SWS. Hyperthyroid patients often complain of insomnia, which has been attributed to a hypermetabolic state.
Approximately 50% of patients with chronic end‐stage renal disease (ESRD) have insomnia and other sleep disorders.41 Patients often complain of restless leg syndrome (RLS), periodic limb movement disorder (PLMD), bone pain, nausea, and pruritus. The etiology of sleep disorders appears to be related to metabolic derangements associated with ESRD or from coexisting diabetes mellitus.
RLS and PLMD are distinct problems that affect sleep differently. RLS is characterized by an unpleasant crampy, creeping or crawling sensation in the lower extremities that is relieved by movement of the legs.42 RLS symptoms typically occur soon after going to bed, and therefore tend to disrupt sleep onset. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems.43 Since RLS may partially be caused by disrupted iron metabolism, serum ferritin levels should be evaluated.44 Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, ESRD, and Parkinson's disease. The differential diagnosis for RLS and PLMD includes neuroleptic‐induced akathisia, peripheral neuropathy, and positional or nocturnal leg cramps. PLMD occurs in about 80% of those with RLS, and is characterized by involuntary limb movements that occur every 20 to 40 seconds during NREM sleep. Unaware of these movements, patients often experience frequent arousals throughout the night, and complain of daytime somnolence and fatigue.42
A pilot study of 35 patients with minimal hepatic encephalopathy found that nearly 50% complained of sleep difficulties.45 Hypothesizing that a dysregulation of histaminergic neurotransmission in cirrhosis alters the sleep‐wake cycle, Spahr et al.46 found that 40% of their patients reported subjective improvement in sleep when administered 25 mg of hydroxyzine, compared to none who received placebo.
Neurologic Disorders
Since the brain and its various neurotransmitter systems are critical in regulating sleep and wakefulness, patients with neurologic disorders have an increased risk of developing sleep disorders. Patients with dementia, other neurodegenerative disorders, epilepsy, and traumatic brain injury (TBI) have a higher prevalence of sleep disturbance and sleep disorders.47 Poststroke patients can develop insomnia or hypersomnia, a reduction in sleep latency, increased sleep, or excessive daytime sleepiness, and are at higher risk for OSA during the first several months after a stroke.48 Specific neurologic lesions may lead to uncommon problems such as inversion of the sleep‐wake cycle, parasomnias, and hallucinatory dream‐like states.
Both Parkinson's disease and Alzheimer's disease are associated with multiple sleep disturbances, which tend to worsen with disease progression.14 Common problems include increased sleep fragmentation and wakefulness, with increases of stage 1 sleep and reductions of SWS and REM. Patients with neurodegenerative disorders also have an increased risk of REM sleep behavior disorder, or RBD.49 RBD is characterized by vivid and unusual dreams, and physically vigorous sleep behaviors that may result in ecchymoses, lacerations, and fractures.50 Fifty percent of patients with TBI reported insomnia symptoms.51 Disorders in initiating and maintaining sleep were the most common complaints among hospitalized patients with TBI. Some patients with TBI may develop circadian rhythm disturbances.52
Pain
A majority of patients with chronic pain, 50% to 70%, complain of impaired sleep.53 Sleep disruption is so common in fibromyalgia (75%) that it is considered to be a key diagnostic symptom.54 In a study investigating the affect of pain on sleep in burn patients, pain was associated with increased intermittent awakenings and prolonged periods of wake time during the night.55 The following day, these patients had poorer pain tolerance and greater pain intensity. Pain causes sleep fragmentation by increasing cortical arousals. Recent evidence suggests that sleep deprivation can increase pain sensitivity by inhibiting opioid protein synthesis or reducing opioid receptor affinity.56
Psychiatric Disorders
Sleep problems are so common in psychiatric conditions that the Diagnostic and Statistical Manual of Mental Disorders (DMS‐IV‐TR) includes sleep disturbance as a diagnostic criterion for a manic episode, and for various depressive, anxiety, and substance abuse disorders.57 The presence of sleep disturbance in hospitalized patients may suggest the presence of an underlying psychiatric disorder that would otherwise go unrecognized. In a survey of 200 general medical patients in a Brazilian hospital, Rocha et al.58 found that 112 (56.5%) complained of insomnia, and 100 (50%) met criteria for at least 1 psychiatric disorder. However, only 3 out of the total number of 200 surveyed (1.5%) were identified as having psychiatric diagnoses in the medical record, and sleep history was not noted in the clinical evaluation. An episode of major depressive disorder was the most common psychiatric diagnosis (35%). In this study, hospitalized patients with insomnia had a 3.6 times higher risk of having major depressive disorder than inpatients without insomnia.
Insomnia has a profound effect on mental health by worsening health‐related quality of life. In a study of outpatients at family medicine, internal medicine, endocrinology, cardiology, and psychiatry clinics in 3 U.S. cities (n = 3,445), insomnia worsened health‐related quality of life nearly as much as CHF or major depressive disorder did.59 Another survey of outpatients found that those with chronic insomnia were nearly 40 times more likely to have major depression and 6 times more likely to have an anxiety disorder compared to those without insomnia.60 Longitudinal studies have found that prior insomnia was associated with 2‐ to 5‐fold increase in the odds of mood and anxiety disorders and suicide.61, 62 Examining prodromes and precursors to mental disorders, Eaton et al.63 found that 47% of those with onset of depression at the 1‐year follow‐up had sleep problems at baseline.
An estimated 65% of patients with major depression have difficulty falling asleep, frequent awakenings, or early morning awakenings.64 Three patterns of sleep architecture abnormalities have been observed in patients with major depression: 1) sleep continuity disturbances characterized by prolonged sleep‐onset, increased wake time during sleep, increased early morning wake time, and decreased TST; 2) decreased proportion and length of SWS; and 3) REM sleep abnormalities such as reduced time to REM sleep, prolonged first REM sleep episode, and increased REM sleep percentage.65 Sleep during a manic episode has been less studied than in depression, but the data suggest that abnormal sleep in mania includes disrupted sleep continuity, shortened REM latency, and increased REM density (REM eye movement activity/total REM sleep time).65
Substance use disorders are also associated with sleep problems. In a survey by Brower et al.66 of patients who were undergoing alcohol rehabilitation, 61% (n = 172) had symptoms of insomnia such as increased sleep latency during the 6 months prior to entering treatment. Approximately 45% of these patients reported using alcohol for the purpose of initiating sleep. Alcohol and illicit substance intoxication and withdrawal are known to be associated with disrupted sleep. However, sleep disturbances may persist long after withdrawal symptoms have abated. Drummond et al. found that some patients continued to have alcohol‐associated sleep problems even after 27 months of abstinence.67 Evidence also suggests that untreated insomnia and other sleep problems may increase the risk of developing substance abuse problems due to self‐medicating with alcohol and other substances to help with sleep.68
Drugs that Affect Sleep
Numerous drugs can alter sleep quantity and quality. Sedatives and opioids may initially help with sleep onset, but impair sleep architecture. Medications used to treat medical and psychiatric illnesses also disrupt sleep (Table 3). The most common agents that impair sleep include antiepileptic drugs, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, antihypertensives, antihistamines, and corticosteroids.
| Drug Class | Examples of Drugs | Affect on Sleep Architecture | Potential Mechanism | Clinical Implications |
|---|---|---|---|---|
| ||||
| CNS | ||||
| AEDs | Phenobarbital, carbamazepine, phenytoin | Very sedating. AEDs tend to TST, sleep latency | Inhibit neuronal calcium influx, adenosine, or 5HT activity | Sedation is dose‐dependent, and tends to occur with acute use |
| TCAs | Amoxapine, amitriptyline, imipramine, nortriptyline, desipramine, doxepin, clomipramine | Very sedating. Suppresses REM sleep, TST, stage‐2 sleep | Stimulate antimuscarinic‐receptor and alpha1‐receptor | Suppressed REM sleep motor inhibition restlessness, psychomotor agitation during sleep subjectively sleep quality, daytime sleepiness |
| BzRAs | Alprazolam, lorazepam, chlordiazepoxide, diazepam, oxazepam | Very sedating. TST, sleep latency, SWS duration, REM, stage‐2 sleep | Stimulate GABA type A receptor | Minimize daytime use. Chronic BzRAs SWS long‐term sequelae unknown |
| MAOIs | Phenylzine, tranylcypromine | Very sedating. TST, REM, REM rebound if stop MAOIs | Mechanism unknown | Daytime sleepiness; dosing time does not affect daytime somnolence |
| SSRIs | Sedating: paroxetine, fluvoxamine. Activating: fluoxetine, sertraline, citalopram | TST, are less sedating than TCAs and MAOIs. May REM, TWT, TST, SE | 5HT activity | Some patients get the opposite reaction |
| SNRI | Venlafaxine, duloxetine | Activating in some patients; sedating in 12% to 31%. TST | 5HT and NE activity | If activating, switch to AM dosing. If sedating, switch to PM dosing |
| Mood stabilizer | Lithium | Sedating. TST, SWS, REM, REM latency | daytime sedation. Dose at night | |
| Stimulants | Ephedrine, pseudoephedrine, modafinil | Activating. TST, SWS, sleep latency | DOPA, NE, and 5HT activity | Avoid after 6 PM |
| Anti‐Parkinson | Bromocriptine, levodopa | Sedating. Nightmares, SWS | DOPA | Dose at night, if possible |
| Cardiac | ||||
| Lipophilic beta‐blockers | Propranolol, pindolol, metoprolol, timolol. Hydrophilic agents (atenolol and sotalol) lack these effects | Activating. awakenings, TWT, REM, nightmares | CNS beta‐blockade | Lipophilic beta‐blockers daytime sleep when dosed in AM |
| CNS agents | Norepinephrine, epinephrine | Activating. REM, SWS | Stimulate alpha1‐receptor | Minimize use at night |
| Dopamine | Activating. REM, SWS | Stimulate dopamine2‐receptor and alpha1‐receptor | Minimize use at night | |
| Ca++ channel blockers | Amlodipine, verapamil, nifedipine | Exacerbate underlying medical condition | Lower esophageal sphincter tone nocturnal GER sleep disturbance | |
| Alpha2‐receptor agonist | Clonidine | Stage 1, REM, nightmares | Stimulate alpha2‐receptor | Alpha2‐agonists daytime sleep and sleepiness directly. Dose at night |
| Alpha1‐receptor blockers | Doxazosin, prazosin, terazosin | Inhibit alpha1‐receptor | Alpha1‐receptor blockers daytime sleepiness | |
| Diuretics | HCTZ, furosemide | Sedating. | PM diuresis frequent awakenings | |
| Other | ||||
| Opioids | Codeine, morphine | Sedating. SWS, REM | Stimulate mu‐receptor | Minimize use at night |
| NSAIDs | Ibuprofen, indomethcin, celecoxib | TST, SE | Inhibit prostaglandin synthesis | Minimize use at night |
| Methylxanthine | Theophylline | Activating. stage 1, REM | Causes less restful sleep | |
| Antihistamines | Diphenhydramine, promethazine | Sedating | H1 receptor blockade | Minimize use at night |
| Corticosteroids | Dexamethasone, prednisone | Activating. REM, SWS, nightmares | Melatonin secretion | Can disrupt sleep, anxiety, induce mania or psychosis |
| H2 blockers | Cimetidine, ranitidine, famotidine | Sedating. TST | H2 receptor blockade | Sedating if >60 years old, renal impairment |
| Quinolone | Ciprofloxacin, sparfloxacin, ofloxacin, grepafloxacin, levofloxacin | Activating | Stimulate GABA type A receptor | Consider sleep agent after maximizing sleep hygiene. Linezolid rarely causes sleep disturbances |
Lipophilic beta antagonists such as propranolol and timolol can increase total wake time, decrease REM sleep, and increase the incidence of nightmares and insomnia.69 Anabolic steroids and beta‐agonist bronchodilator therapy can cause severe anxiety, sleeplessness, and even psychosis. Vasopressor agents such as dopamine can cause cortical activation, leading to increased arousal and reduced SWS.
Hospital Environment
Environmental noise and patient care activities often interfere with sleep in the hospital. They account for about 30% of patient awakenings in ICU patients.70 Noise levels in the ICU have average sound peaks of 150 to 200 dB, and evening peaks >80 dB between midnight and 6 AM.71 By comparison, the front row seats at a rock concert have sound levels of 110 dB. The high noise level in hospitals has long been implicated as a sleep disruptor,72 but studies in the past decade have found that patient care activities probably contribute more to awakenings than does environmental noise.73 An analysis of critical care nursing routines found that activities such as taking vital signs and giving baths occurred a mean 42.6 times a night per patient.74 Tamburri et al.74 found that patients experienced 2 to 3 hours without interruption on only 6% of the 147 nights studied. Routine daily baths were provided on 55 of the 147 study nights between 2 AM and 5 AM, which is unlikely to be an opportune time for most patients.
CONCLUSION
Hospitalization often prevents patients from achieving adequate sleep and can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing sedative/hypnotic agents, the treatment for sleep disruption includes addressing multiple medical, behavioral, and environmental factors, which will be discussed in Part 2 of this article.
- NIH State‐of‐the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults.NIH Consens Sci Statements.2005;22(2):1–30.
- ,,, et al.Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey.Hypertension.2006;47(5):833–839.
- ,,, et al.Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique.Sleep.2003;26(8):986–989.
- ,,,,.Postural control after a night without sleep.Neuropsychologia.2006;44(12):2520–2525.
- .Sleep deprivation decreases ventilatory responses to CO2 but not load compensation.Chest.1983;84(6):695–698.
- ,,, et al.Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation.J Appl Physiol.2005;98(6):2024–2032.
- .Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats.Am J Physiol Endocrinol Metab.2004;286(6):E1060–E1070.
- ,,,.The metabolic consequences of sleep deprivation.Sleep Med Rev.2007;11(3):163–178.
- ,,,,.Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.Arch Int Med.2006;166:1756–1752.
- ,,,.Effects of sleep and sleep deprivation on immunoglobulins and complement in humans.Brain Behav Immun.2007;21:308–310.
- ,,,,.The effects of sleep deprivation on symptoms of psychopathology in healthy adults.Sleep Med.2007;8:215–221.
- ,.Normal human sleep. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:15–16.
- .Update on the Science, Diagnosis and Management of Insomnia.International Congress and Symposium Series 262.London:Royal Society of Medicine Press Ltd;2006.
- ,,,,,.The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture.Arch Intern Med.2004;164:406–18.
- ,.Sleep disruption in older adults.Am J Nurs.2007;107(5):40–49.
- ,,,.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey.J Psychosom Res.2004;56(5):497–502.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Insomnia and its treatment: prevalence and correlates.Arch Gen Psychiatry.1985;42(3):225–232.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117:809–818.
- ,.Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill.Crit Care Med.2006;22:313–327.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,,,.Failure of physician documentation of sleep complaints in hospitalized patients.West J Med.1998;169:146–149.
- .Sleep and medical disorders.Prim Care.2005;35:511–533.
- ,,,,,.The occurrence of sleep‐disordered breathing among middle‐aged adults.N Engl J Med.1993;328:1230–1235.
- ,.Clinical features and evaluation of obstructive sleep apnea‐hypopnea syndrome. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:869–872.
- ,,.Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea.Arch Intern Med.2003;163:41–45.
- ,.Sleep and medical disorders.Med Clin North Am.2004;88:679–703.
- ,,,.Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness.Atherosclerosis.2008;197(1):125– 131.
- ,,, et al.Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med.2001;163:19–25.
- .Gastroesophageal reflux during sleep.Sleep Med Clin.2007;2:41–50.
- ,.Sleep apnea in renal failure.Adv Perit Dial.1997;13:88–92.
- .Sleep in chronic obstructive pulmonary disease.Sleep Med Clin.2007;2:1–8.
- ,,,.Effects of non‐invasive positive pressure ventilation on gas exchange and sleep in COPD patients.Chest.1997;112:623–628.
- ,,, et al.Night‐to‐night variability in CPAP use over the first three months of treatment.Sleep.1997;20(4):278–283.
- ,.Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS).Sleep Med Rev.2003;7(1):81–99.
- ,,.Factors predicting sleep disruption in type II diabetes.Sleep.2000;23:415–416.
- ,,.Sleep duration as a risk factor for the development of type 2 diabetes.Diabetes Care.2006;29:657–661.
- .Sleep disorders and end‐stage renal disease.Sleep Med Clin.2007;2:59–66.
- ,,,.Sleep and aging: 1. Sleep disorders commonly found in older people.CMAJ.2007;176(9):1299–1304.
- ,,,,,.Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS Epidemiology, Symptoms, and Treatment) primary care study.Sleep Med.2004;5(3):237–246.
- ,.Epidemiology and clinical findings of restless leg syndrome.Sleep Med.2004;5(3):293–299.
- ,,,,,.High prevalence of sleep disturbance in cirrhosis.Hepatology.1998;27:339–345.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Obstructive sleep apnea: implications for cardiac and vascular disease.JAMA.2003;290(14):1906–1914.
- ,,, et al.Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.Brain.2007;130(Pt 11):2770–2788.
- ,.REM sleep parasomnias. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:724–725.
- ,,.Insomnia in patients with traumatic brain injury.J Head Trauma Rehabil.2006;21(3):199–212.
- ,,,,.Circadian rhythm sleep disorders following mild traumatic brain injury.Neurology.2007;68(14):1136–1140.
- ,.Comorbidities: psychiatric, medical, medications, and substances.Sleep Med Clin.2006;231–245.
- ,,.Sleep disturbance and fibromyalgia.Sleep Med Clin.2007;2:31–39.
- ,,.Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries.Sleep Med.2004;5:551–559.
- ,,.Sleep deprivation and pain perception.Sleep Med Rev.2006;10:357–369.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision.Washington, DC:American Psychiatric Association;2000.
- ,,, et al.Is insomnia a marker for psychiatric disorders in general hospitals?Sleep Med.2005;6:549–553.
- ,.The relationship between insomnia and health‐related quality of life in patients with chronic illness.J Fam Pract.2002;51(3):229–235.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,.Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults.Biol Psychiatry.1996;39:411–418.
- ,,,.The morbidity of insomnia uncomplicated by psychiatric disorders.Gen Hosp Psychiatry.1997;19:245–250.
- ,,.Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset.Am J Psychiatry.1995;152:967–972.
- ,,,,,.Which depressive symptoms are related to which sleep electroencephalographic variables?Biol Psychol.1997;42:904–913.
- ,.Sleep in mood disorders.Psychiatr Clin North Am.2006;29:1009–1032.
- ,,,,.Insomnia, self‐medication, and relapse to alcoholism.Am J Psychiatry.2001;158:399–404.
- ,,,.The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse.Alcohol Clin Exp Res.1998;22:1796–1802.
- ,,,,.Screening for substance use patterns among patients referred for a variety of sleep complaints.Am J Drug Alcohol Abuse.2006;32:111–120.
- .Drugs that disturb sleep and wakefulness. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:441–462.
- ,,, et al.Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects.Am J Respir Crit Care Med.2003;167(5):708–715.
- ,,.Adverse environmental conditions in the respiratory and medical ICU settings.Chest.1994;105:1211–1216.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118(6):3629–3645.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,,.Nocturnal care interactions with patients in critical care units.Am J Crit Care.2004;13(2):102–115.
Hospitalized patients often have difficulty initiating and maintaining sleep, or complain of early awakening and nonrestorative sleep.1 The etiology of sleep disruption is multifactorial and includes the patient's underlying illness(es), medical treatments, and the hospital environment. Often unrecognized and untreated during hospitalization, sleep disruption may lead to sleep deprivation, or a chronic lack of restorative sleep.
Even in healthy individuals, sleep deprivation can result in numerous physical and psychological consequences. Sleep deprivation is associated with hypertension,2, 3 impaired postural control,4 decreased ventilatory drive,5 increased sympathetic cardiovascular activation,6 blunted hypothalamic‐pituitary‐adrenal axis,7 impaired host defenses, and possibly diabetes mellitus and obesity.810 The lack of restorative sleep increases the risk of developing anxiety and mood disorders and delirium, especially in acutely ill older patients.11 In the presence of acute physical infirmity, inadequate sleep may further compound illness and impair recovery. We provide an overview of normal sleep architecture and discuss factors that lead to sleep disruption in hospitalized medical patients.
NORMAL SLEEP ARCHITECTURE AND REGULATION
Normal sleep architecture refers to a characteristic pattern of sleep, and consists of two major stages: nonrapid eye movement (NREM, pronounced non‐rem) and rapid eye movement (REM). (For a table of pertinent abbreviations and terms, see Table 1) Sleep is quantified by polysomnography (PSG), which includes an electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). A PSG also includes an electrocardiogram (ECG), and measures of airflow, oxygen saturation, and body position. NREM sleep comprises 75% to 80% of total sleep time (TST), and is characterized by relatively quiescent brain activity and decreased metabolic rate.12 NREM sleep consists of four stages (S1‐S4), with each stage leading to a progressively deeper sleep (Figure 1). REM sleep follows slow wave sleep (SWS), or deep sleep, and increases over the night, comprising 20% to 25% of TST. REM sleep is characterized by an activated EEG pattern, muscle atonia, and episodic bursts of rapid eye movements.

| Acronym | Term |
|---|---|
| BiPAP | Bilevel positive airway pressure |
| CHF | Congestive heart failure |
| CPAP | Continuous positive airway pressure |
| COPD | Chronic obstructive pulmonary disease |
| EEG | Electroencephalogram |
| EOG | Electroculogram |
| EMG | Electromyogram |
| ESRD | End‐stage renal disease |
| NPPV | Noninvasive positive pressure ventilation |
| NREM | Nonrapid eye movement |
| OSA | Obstructive sleep apnea |
| PLMD | Periodic limb movement disorder |
| PSG | Polysomnography |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement |
| RLS | Restless leg syndrome |
| S1‐S4 | 4 Stages of sleep in NREM |
| SE | Sleep efficiency; TST divided by total time in bed |
| SWS | Slow wave sleep |
| TBI | Traumatic brain injury |
| TST | Total sleep time |
Normal sleep provides a period of physiologic and mental rest. During sleep, sympathetic tone decreases and parasympathetic tone increases, leading to a reduction in heart rate, arterial blood pressure, and cardiac output.13 Deep sleep is theorized to be necessary for physiologic restoration. REM sleep is associated with dreaming, and is essential for maintaining emotional and cognitive well‐being. Sleep architecture undergoes characteristic changes as people age.14 The duration of SWS peaks in childhood and decreases with age. Consequently, people >60 years old tend to have lower arousal thresholds and to have more frequent awakenings. The results of the Sleep Heart Health Study found that increased age was associated with decreased percentage of REM sleep, worse sleep efficiency (SE, which is TST divided by total time in bed), and lower arousal thresholds.14 With the reduction of SE, older people need to spend more hours in bed to achieve the same amount of restorative sleep as when they were younger. Although sleep tends to become more disrupted as people age, insomnia should not be considered a normal part of aging, and needs to be addressed clinically.15 The results of a National Sleep Foundation telephone survey of subjects between the ages of 55 and 84 years old (n = 1,506) suggested that sleep complaints in older adults are frequently secondary to comorbid medical conditions.16
Multiple anatomic structures, pathways, and neurotransmitter systems are involved in controlling wakefulness and sleep. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Sleep‐promoting neurotransmitters include gamma aminobutyric acid (GABA), adenosine, and melatonin. Specific stages of sleep are regulated by the turning on and off of various neurons. REM on cells use GABA, acetylcholine, and glutamine, whereas REM off cells use norepinephrine and serotonin. SWS is promoted by GABA and serotonin.17
Sleep regulation is a balance between a homeostatic sleep need and an intrinsic body clock, or circadian pacemaker. Located in the suprachiasmic nucleus, the circadian pacemaker determines the onset and termination of sleep, and is partially regulated by environmental cues such as light and ambient temperature. Melatonin, a physiologic sleep promoter, is inhibited by ambient light, and its circulation is decreased during daylight hours. The adrenal secretion of cortisol, which is associated with wakefulness, follows a circadian pattern. Regulated by the hypothalamic‐pituitary axis, cortisol levels peak in the early morning hours in preparation for the increased metabolic demands during wakefulness.
SLEEP PROBLEMS IN HOSPITALIZED PATIENTS
Insomnia, which is characterized by difficulty initiating or maintaining sleep, is the most common sleep disorder in the United States. About one‐third of the adult population in the United States experiences insomnia at some point in their lives,18 and it is a persistent problem in approximately 10% of U.S. adults.19 Insomnia can be exacerbated during hospitalization.
Studies investigating sleep in hospitalized patients using PSG have been limited primarily to the setting of the intensive care unit (ICU). Critically ill patients, particularly those requiring mechanical ventilation, are prone to sleep disturbances and an associated delirium.2022 Critically ill patients have fragmented sleep, with decreased SE and SWS, and increased S1 and S2.23 Physician awareness of the impact of sleep disturbance in hospitalized patients is vital. Surveys reveal that approximately one‐half of patients admitted on general medical wards complain of sleep disruption.24, 25 Meissner et al.25 examined the prevalence of sleep complaints and physician recognition of these complaints among general medical patients admitted to a Veterans Affairs tertiary care center. Results showed that 47% (n = 222) of patients had complaints of either insomnia and/or excessive daytime sleepiness.
FACTORS AFFECTING SLEEP DURING HOSPITALIZATION
Many medical and neurologic illnesses, psychiatric disorders, pain, medication therapy and the hospital environment may impair sleep, and hinder recovery from illness.
General Medical Disorders
Primary sleep disorders, such as obstructive sleep apnea (OSA) and numerous other medical illnesses, can directly impair sleep physiology, leading to a cyclical interaction (Figure 2). Other conditions that disrupt sleep include congestive heart failure (CHF), diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux, cardiovascular disease, thyroid disorders, renal disease, and severe liver disease.26 Table 2 lists selected medical and neurological conditions, their associated sleep‐related problems, and suggestions on how to ameliorate these problems.

| Disease | Problem | Clinical Implications and Strategies to Improve Sleep |
|---|---|---|
| ||
| Asthma | Nocturnal exacerbation, nocturnal GER | Inhaled corticosteroids and/or long‐acting inhaled beta‐adrenergic agents |
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep‐disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne‐Stokes respiration | Keep the head of bed elevated 30 degrees. Nocturnal O2 to keep O2 saturation >88%. Daytime diuresis. Optimize cardiac function to treat Cheyne‐Stokes respiration. Consider CPAP for CHF |
| COPD | Persistent nocturnal hypoxemia with complications (e.g., cor pulmonale, polycythemia) | O2 for COPD and persistent hypoxemia (PaO2 55‐60 mm Hg) |
| Sporadic nighttime desaturations | PaO2 55 mm Hg monitor O2 saturation by pulse oximetry. If patient desaturates to 88% at night consistently, start nocturnal O2. For hypercapnia, adjust O2 to maintain O2 saturation at 88% to 90% | |
| Early‐morning airflow obstruction | Consider bedtime tiotropium and inhaled long‐acting beta‐adrenergic agonist agents | |
| Inhibition of respiratory muscles in REM | Avoid sedative‐hypnotics that cause respiratory depression | |
| Decreased functional residual capacity from recumbent position during sleep | Minimize recumbancy by keeping the head of bed up at 30 degrees | |
| End‐stage renal disease | Pruritus, nausea; increased risk of RLS and PLMD | Ambulation may help with RLS. Consider ropinirole and pramipexole. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents |
| Nocturnal GER | Nocturnal GER decreased sleep, heartburn, coughing, asthma | Avoid eating or drinking 2 hours before bedtime, especially those that delay gastric emptying, increase acid secretion, or decrease lower esophageal sphincter pressure; e.g., high‐fat foods, ethanol, chocolate, peppers, peppermint. Keep head of bed 30 degrees. Minimize medications that could worsen nocturnal GER; e.g., theophylline, calcium channel blockers, prostaglandins, bisphosphonates |
| OSA | Snoring with upper airway obstruction | No ethanol 2 hours before bedtime. Minimize CNS depressants. Avoid supine position. Consider CPAP, oral mandibular advancement device, and/or surgical correction. Long‐term plan should include weight loss |
| Stroke | Focal neurologic deficits (e.g., dysphagia, weakness or paralysis) | Keep head of bed 30 degrees. Regularly suction secretions. Post‐stroke patients have an increased risk of hypersomnia, insomnia, and/or OSA |
Affecting approximately 24% of men and 9% of women in the United States, OSA is the most common primary sleep disorder,27, 28 and causes significant mental and physical morbidity. Risk factors for OSA include obesity, hypothyroidism‐induced muscle weakness, and structural abnormalities in the oropharynx region such as acromegaly, micrognathia, or retrognathia. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness.28 These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment (Figure 2). In addition, chronic sympathetic activation causes numerous derangements in the vascular endothelium and platelet activation.29, 30 Sleep‐disordered breathing has been independently associated with cardiovascular diseases such as hypertension, CHF, ischemic heart disease, atrial fibrillation, and cerebrovascular disease.31, 32
OSA is also associated with sleep‐related gastroesophageal reflux, which is characterized by pain and nocturnal cough, and can induce nocturnal asthma attacks and laryngospasm.33 Green et al.29 found that OSA patients treated with continuous positive airway pressure (CPAP) had a 48% improvement in nocturnal reflux symptoms. Although the pathophysiology connecting OSA to the renal system is unknown, OSA has been found in up to 60% of patients with end‐stage renal disease and chronic renal failure.34
Patients with pulmonary disorders can be profoundly affected by the normal physiologic changes during sleep, particularly in REM sleep. During REM sleep, all respiratory muscles except the diaphragm become paralyzed. Thus, episodes of marked oxygen desaturation can occur in patients who rely on their accessory muscles for respiration. COPD patients have decreased TST, SWS, and REM sleep. Shortness of breath, nocturnal cough, and wheezing worsen sleep.35 The resulting fatigue and sleep deprivation negatively impact the work of breathing and impair gas exchange. Airflow obstruction tends to worsen in the early morning hours in patients with COPD and asthma, and may be related to the effect of REM on the accessory muscles for respiration. Although used to target CO2 retention, investigations using bilevel positive airway pressure ventilators (BiPAP) for improving sleep in COPD patients have been limited. Noninvasive positive pressure ventilation (NPPV) appears to acutely improve SE and TST in patients with hypercapnic COPD without significantly improving gas exchange. Other sleep parameters such as sleep architecture and the number of arousals during the night, remain unchanged during NPPV.36
CPAP has several side effects that could worsen sleep, which may explain its poor adherence rate among ambulatory patients.37 Side effects include nasal bridge discomfort, nasal congestion, swallowing air, dry nose, dry or red eyes, noise, ear pain, and rhinitis.38 During hospitalization, efforts should be made to improve patient comfort by resizing ill‐fitting masks, adding heated humidification or nasal steroids to alleviate nasal congestion, or adding a chin strap to reduce air leak and ingestion of air.
Endocrine disorders have also been associated with sleep disruption. Studies suggest that patients with diabetes mellitus have decreased TST and impaired sleep quality due to nocturia and neuropathic pain.39 Inadequate sleep may also affect glucose control. Inadequate quality or quantity of sleep has been shown to be a risk factor for developing Type 2 diabetes mellitus in large prospective studies.40 Sleep duration and quality were significant predictors of increased levels of glycosylated hemoglobin (HbA1c) in patients with Type 2 diabetes mellitus. Thyroid diseases often coexist with diabetes mellitus. Both hypo‐ and hyperthyroidism have been associated with sleep disruption. Hypothyroidism is associated with daytime somnolence and fatigue. Patients with hypothyroidism tend to have reduced SWS. Hyperthyroid patients often complain of insomnia, which has been attributed to a hypermetabolic state.
Approximately 50% of patients with chronic end‐stage renal disease (ESRD) have insomnia and other sleep disorders.41 Patients often complain of restless leg syndrome (RLS), periodic limb movement disorder (PLMD), bone pain, nausea, and pruritus. The etiology of sleep disorders appears to be related to metabolic derangements associated with ESRD or from coexisting diabetes mellitus.
RLS and PLMD are distinct problems that affect sleep differently. RLS is characterized by an unpleasant crampy, creeping or crawling sensation in the lower extremities that is relieved by movement of the legs.42 RLS symptoms typically occur soon after going to bed, and therefore tend to disrupt sleep onset. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems.43 Since RLS may partially be caused by disrupted iron metabolism, serum ferritin levels should be evaluated.44 Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, ESRD, and Parkinson's disease. The differential diagnosis for RLS and PLMD includes neuroleptic‐induced akathisia, peripheral neuropathy, and positional or nocturnal leg cramps. PLMD occurs in about 80% of those with RLS, and is characterized by involuntary limb movements that occur every 20 to 40 seconds during NREM sleep. Unaware of these movements, patients often experience frequent arousals throughout the night, and complain of daytime somnolence and fatigue.42
A pilot study of 35 patients with minimal hepatic encephalopathy found that nearly 50% complained of sleep difficulties.45 Hypothesizing that a dysregulation of histaminergic neurotransmission in cirrhosis alters the sleep‐wake cycle, Spahr et al.46 found that 40% of their patients reported subjective improvement in sleep when administered 25 mg of hydroxyzine, compared to none who received placebo.
Neurologic Disorders
Since the brain and its various neurotransmitter systems are critical in regulating sleep and wakefulness, patients with neurologic disorders have an increased risk of developing sleep disorders. Patients with dementia, other neurodegenerative disorders, epilepsy, and traumatic brain injury (TBI) have a higher prevalence of sleep disturbance and sleep disorders.47 Poststroke patients can develop insomnia or hypersomnia, a reduction in sleep latency, increased sleep, or excessive daytime sleepiness, and are at higher risk for OSA during the first several months after a stroke.48 Specific neurologic lesions may lead to uncommon problems such as inversion of the sleep‐wake cycle, parasomnias, and hallucinatory dream‐like states.
Both Parkinson's disease and Alzheimer's disease are associated with multiple sleep disturbances, which tend to worsen with disease progression.14 Common problems include increased sleep fragmentation and wakefulness, with increases of stage 1 sleep and reductions of SWS and REM. Patients with neurodegenerative disorders also have an increased risk of REM sleep behavior disorder, or RBD.49 RBD is characterized by vivid and unusual dreams, and physically vigorous sleep behaviors that may result in ecchymoses, lacerations, and fractures.50 Fifty percent of patients with TBI reported insomnia symptoms.51 Disorders in initiating and maintaining sleep were the most common complaints among hospitalized patients with TBI. Some patients with TBI may develop circadian rhythm disturbances.52
Pain
A majority of patients with chronic pain, 50% to 70%, complain of impaired sleep.53 Sleep disruption is so common in fibromyalgia (75%) that it is considered to be a key diagnostic symptom.54 In a study investigating the affect of pain on sleep in burn patients, pain was associated with increased intermittent awakenings and prolonged periods of wake time during the night.55 The following day, these patients had poorer pain tolerance and greater pain intensity. Pain causes sleep fragmentation by increasing cortical arousals. Recent evidence suggests that sleep deprivation can increase pain sensitivity by inhibiting opioid protein synthesis or reducing opioid receptor affinity.56
Psychiatric Disorders
Sleep problems are so common in psychiatric conditions that the Diagnostic and Statistical Manual of Mental Disorders (DMS‐IV‐TR) includes sleep disturbance as a diagnostic criterion for a manic episode, and for various depressive, anxiety, and substance abuse disorders.57 The presence of sleep disturbance in hospitalized patients may suggest the presence of an underlying psychiatric disorder that would otherwise go unrecognized. In a survey of 200 general medical patients in a Brazilian hospital, Rocha et al.58 found that 112 (56.5%) complained of insomnia, and 100 (50%) met criteria for at least 1 psychiatric disorder. However, only 3 out of the total number of 200 surveyed (1.5%) were identified as having psychiatric diagnoses in the medical record, and sleep history was not noted in the clinical evaluation. An episode of major depressive disorder was the most common psychiatric diagnosis (35%). In this study, hospitalized patients with insomnia had a 3.6 times higher risk of having major depressive disorder than inpatients without insomnia.
Insomnia has a profound effect on mental health by worsening health‐related quality of life. In a study of outpatients at family medicine, internal medicine, endocrinology, cardiology, and psychiatry clinics in 3 U.S. cities (n = 3,445), insomnia worsened health‐related quality of life nearly as much as CHF or major depressive disorder did.59 Another survey of outpatients found that those with chronic insomnia were nearly 40 times more likely to have major depression and 6 times more likely to have an anxiety disorder compared to those without insomnia.60 Longitudinal studies have found that prior insomnia was associated with 2‐ to 5‐fold increase in the odds of mood and anxiety disorders and suicide.61, 62 Examining prodromes and precursors to mental disorders, Eaton et al.63 found that 47% of those with onset of depression at the 1‐year follow‐up had sleep problems at baseline.
An estimated 65% of patients with major depression have difficulty falling asleep, frequent awakenings, or early morning awakenings.64 Three patterns of sleep architecture abnormalities have been observed in patients with major depression: 1) sleep continuity disturbances characterized by prolonged sleep‐onset, increased wake time during sleep, increased early morning wake time, and decreased TST; 2) decreased proportion and length of SWS; and 3) REM sleep abnormalities such as reduced time to REM sleep, prolonged first REM sleep episode, and increased REM sleep percentage.65 Sleep during a manic episode has been less studied than in depression, but the data suggest that abnormal sleep in mania includes disrupted sleep continuity, shortened REM latency, and increased REM density (REM eye movement activity/total REM sleep time).65
Substance use disorders are also associated with sleep problems. In a survey by Brower et al.66 of patients who were undergoing alcohol rehabilitation, 61% (n = 172) had symptoms of insomnia such as increased sleep latency during the 6 months prior to entering treatment. Approximately 45% of these patients reported using alcohol for the purpose of initiating sleep. Alcohol and illicit substance intoxication and withdrawal are known to be associated with disrupted sleep. However, sleep disturbances may persist long after withdrawal symptoms have abated. Drummond et al. found that some patients continued to have alcohol‐associated sleep problems even after 27 months of abstinence.67 Evidence also suggests that untreated insomnia and other sleep problems may increase the risk of developing substance abuse problems due to self‐medicating with alcohol and other substances to help with sleep.68
Drugs that Affect Sleep
Numerous drugs can alter sleep quantity and quality. Sedatives and opioids may initially help with sleep onset, but impair sleep architecture. Medications used to treat medical and psychiatric illnesses also disrupt sleep (Table 3). The most common agents that impair sleep include antiepileptic drugs, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, antihypertensives, antihistamines, and corticosteroids.
| Drug Class | Examples of Drugs | Affect on Sleep Architecture | Potential Mechanism | Clinical Implications |
|---|---|---|---|---|
| ||||
| CNS | ||||
| AEDs | Phenobarbital, carbamazepine, phenytoin | Very sedating. AEDs tend to TST, sleep latency | Inhibit neuronal calcium influx, adenosine, or 5HT activity | Sedation is dose‐dependent, and tends to occur with acute use |
| TCAs | Amoxapine, amitriptyline, imipramine, nortriptyline, desipramine, doxepin, clomipramine | Very sedating. Suppresses REM sleep, TST, stage‐2 sleep | Stimulate antimuscarinic‐receptor and alpha1‐receptor | Suppressed REM sleep motor inhibition restlessness, psychomotor agitation during sleep subjectively sleep quality, daytime sleepiness |
| BzRAs | Alprazolam, lorazepam, chlordiazepoxide, diazepam, oxazepam | Very sedating. TST, sleep latency, SWS duration, REM, stage‐2 sleep | Stimulate GABA type A receptor | Minimize daytime use. Chronic BzRAs SWS long‐term sequelae unknown |
| MAOIs | Phenylzine, tranylcypromine | Very sedating. TST, REM, REM rebound if stop MAOIs | Mechanism unknown | Daytime sleepiness; dosing time does not affect daytime somnolence |
| SSRIs | Sedating: paroxetine, fluvoxamine. Activating: fluoxetine, sertraline, citalopram | TST, are less sedating than TCAs and MAOIs. May REM, TWT, TST, SE | 5HT activity | Some patients get the opposite reaction |
| SNRI | Venlafaxine, duloxetine | Activating in some patients; sedating in 12% to 31%. TST | 5HT and NE activity | If activating, switch to AM dosing. If sedating, switch to PM dosing |
| Mood stabilizer | Lithium | Sedating. TST, SWS, REM, REM latency | daytime sedation. Dose at night | |
| Stimulants | Ephedrine, pseudoephedrine, modafinil | Activating. TST, SWS, sleep latency | DOPA, NE, and 5HT activity | Avoid after 6 PM |
| Anti‐Parkinson | Bromocriptine, levodopa | Sedating. Nightmares, SWS | DOPA | Dose at night, if possible |
| Cardiac | ||||
| Lipophilic beta‐blockers | Propranolol, pindolol, metoprolol, timolol. Hydrophilic agents (atenolol and sotalol) lack these effects | Activating. awakenings, TWT, REM, nightmares | CNS beta‐blockade | Lipophilic beta‐blockers daytime sleep when dosed in AM |
| CNS agents | Norepinephrine, epinephrine | Activating. REM, SWS | Stimulate alpha1‐receptor | Minimize use at night |
| Dopamine | Activating. REM, SWS | Stimulate dopamine2‐receptor and alpha1‐receptor | Minimize use at night | |
| Ca++ channel blockers | Amlodipine, verapamil, nifedipine | Exacerbate underlying medical condition | Lower esophageal sphincter tone nocturnal GER sleep disturbance | |
| Alpha2‐receptor agonist | Clonidine | Stage 1, REM, nightmares | Stimulate alpha2‐receptor | Alpha2‐agonists daytime sleep and sleepiness directly. Dose at night |
| Alpha1‐receptor blockers | Doxazosin, prazosin, terazosin | Inhibit alpha1‐receptor | Alpha1‐receptor blockers daytime sleepiness | |
| Diuretics | HCTZ, furosemide | Sedating. | PM diuresis frequent awakenings | |
| Other | ||||
| Opioids | Codeine, morphine | Sedating. SWS, REM | Stimulate mu‐receptor | Minimize use at night |
| NSAIDs | Ibuprofen, indomethcin, celecoxib | TST, SE | Inhibit prostaglandin synthesis | Minimize use at night |
| Methylxanthine | Theophylline | Activating. stage 1, REM | Causes less restful sleep | |
| Antihistamines | Diphenhydramine, promethazine | Sedating | H1 receptor blockade | Minimize use at night |
| Corticosteroids | Dexamethasone, prednisone | Activating. REM, SWS, nightmares | Melatonin secretion | Can disrupt sleep, anxiety, induce mania or psychosis |
| H2 blockers | Cimetidine, ranitidine, famotidine | Sedating. TST | H2 receptor blockade | Sedating if >60 years old, renal impairment |
| Quinolone | Ciprofloxacin, sparfloxacin, ofloxacin, grepafloxacin, levofloxacin | Activating | Stimulate GABA type A receptor | Consider sleep agent after maximizing sleep hygiene. Linezolid rarely causes sleep disturbances |
Lipophilic beta antagonists such as propranolol and timolol can increase total wake time, decrease REM sleep, and increase the incidence of nightmares and insomnia.69 Anabolic steroids and beta‐agonist bronchodilator therapy can cause severe anxiety, sleeplessness, and even psychosis. Vasopressor agents such as dopamine can cause cortical activation, leading to increased arousal and reduced SWS.
Hospital Environment
Environmental noise and patient care activities often interfere with sleep in the hospital. They account for about 30% of patient awakenings in ICU patients.70 Noise levels in the ICU have average sound peaks of 150 to 200 dB, and evening peaks >80 dB between midnight and 6 AM.71 By comparison, the front row seats at a rock concert have sound levels of 110 dB. The high noise level in hospitals has long been implicated as a sleep disruptor,72 but studies in the past decade have found that patient care activities probably contribute more to awakenings than does environmental noise.73 An analysis of critical care nursing routines found that activities such as taking vital signs and giving baths occurred a mean 42.6 times a night per patient.74 Tamburri et al.74 found that patients experienced 2 to 3 hours without interruption on only 6% of the 147 nights studied. Routine daily baths were provided on 55 of the 147 study nights between 2 AM and 5 AM, which is unlikely to be an opportune time for most patients.
CONCLUSION
Hospitalization often prevents patients from achieving adequate sleep and can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing sedative/hypnotic agents, the treatment for sleep disruption includes addressing multiple medical, behavioral, and environmental factors, which will be discussed in Part 2 of this article.
Hospitalized patients often have difficulty initiating and maintaining sleep, or complain of early awakening and nonrestorative sleep.1 The etiology of sleep disruption is multifactorial and includes the patient's underlying illness(es), medical treatments, and the hospital environment. Often unrecognized and untreated during hospitalization, sleep disruption may lead to sleep deprivation, or a chronic lack of restorative sleep.
Even in healthy individuals, sleep deprivation can result in numerous physical and psychological consequences. Sleep deprivation is associated with hypertension,2, 3 impaired postural control,4 decreased ventilatory drive,5 increased sympathetic cardiovascular activation,6 blunted hypothalamic‐pituitary‐adrenal axis,7 impaired host defenses, and possibly diabetes mellitus and obesity.810 The lack of restorative sleep increases the risk of developing anxiety and mood disorders and delirium, especially in acutely ill older patients.11 In the presence of acute physical infirmity, inadequate sleep may further compound illness and impair recovery. We provide an overview of normal sleep architecture and discuss factors that lead to sleep disruption in hospitalized medical patients.
NORMAL SLEEP ARCHITECTURE AND REGULATION
Normal sleep architecture refers to a characteristic pattern of sleep, and consists of two major stages: nonrapid eye movement (NREM, pronounced non‐rem) and rapid eye movement (REM). (For a table of pertinent abbreviations and terms, see Table 1) Sleep is quantified by polysomnography (PSG), which includes an electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). A PSG also includes an electrocardiogram (ECG), and measures of airflow, oxygen saturation, and body position. NREM sleep comprises 75% to 80% of total sleep time (TST), and is characterized by relatively quiescent brain activity and decreased metabolic rate.12 NREM sleep consists of four stages (S1‐S4), with each stage leading to a progressively deeper sleep (Figure 1). REM sleep follows slow wave sleep (SWS), or deep sleep, and increases over the night, comprising 20% to 25% of TST. REM sleep is characterized by an activated EEG pattern, muscle atonia, and episodic bursts of rapid eye movements.

| Acronym | Term |
|---|---|
| BiPAP | Bilevel positive airway pressure |
| CHF | Congestive heart failure |
| CPAP | Continuous positive airway pressure |
| COPD | Chronic obstructive pulmonary disease |
| EEG | Electroencephalogram |
| EOG | Electroculogram |
| EMG | Electromyogram |
| ESRD | End‐stage renal disease |
| NPPV | Noninvasive positive pressure ventilation |
| NREM | Nonrapid eye movement |
| OSA | Obstructive sleep apnea |
| PLMD | Periodic limb movement disorder |
| PSG | Polysomnography |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement |
| RLS | Restless leg syndrome |
| S1‐S4 | 4 Stages of sleep in NREM |
| SE | Sleep efficiency; TST divided by total time in bed |
| SWS | Slow wave sleep |
| TBI | Traumatic brain injury |
| TST | Total sleep time |
Normal sleep provides a period of physiologic and mental rest. During sleep, sympathetic tone decreases and parasympathetic tone increases, leading to a reduction in heart rate, arterial blood pressure, and cardiac output.13 Deep sleep is theorized to be necessary for physiologic restoration. REM sleep is associated with dreaming, and is essential for maintaining emotional and cognitive well‐being. Sleep architecture undergoes characteristic changes as people age.14 The duration of SWS peaks in childhood and decreases with age. Consequently, people >60 years old tend to have lower arousal thresholds and to have more frequent awakenings. The results of the Sleep Heart Health Study found that increased age was associated with decreased percentage of REM sleep, worse sleep efficiency (SE, which is TST divided by total time in bed), and lower arousal thresholds.14 With the reduction of SE, older people need to spend more hours in bed to achieve the same amount of restorative sleep as when they were younger. Although sleep tends to become more disrupted as people age, insomnia should not be considered a normal part of aging, and needs to be addressed clinically.15 The results of a National Sleep Foundation telephone survey of subjects between the ages of 55 and 84 years old (n = 1,506) suggested that sleep complaints in older adults are frequently secondary to comorbid medical conditions.16
Multiple anatomic structures, pathways, and neurotransmitter systems are involved in controlling wakefulness and sleep. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Sleep‐promoting neurotransmitters include gamma aminobutyric acid (GABA), adenosine, and melatonin. Specific stages of sleep are regulated by the turning on and off of various neurons. REM on cells use GABA, acetylcholine, and glutamine, whereas REM off cells use norepinephrine and serotonin. SWS is promoted by GABA and serotonin.17
Sleep regulation is a balance between a homeostatic sleep need and an intrinsic body clock, or circadian pacemaker. Located in the suprachiasmic nucleus, the circadian pacemaker determines the onset and termination of sleep, and is partially regulated by environmental cues such as light and ambient temperature. Melatonin, a physiologic sleep promoter, is inhibited by ambient light, and its circulation is decreased during daylight hours. The adrenal secretion of cortisol, which is associated with wakefulness, follows a circadian pattern. Regulated by the hypothalamic‐pituitary axis, cortisol levels peak in the early morning hours in preparation for the increased metabolic demands during wakefulness.
SLEEP PROBLEMS IN HOSPITALIZED PATIENTS
Insomnia, which is characterized by difficulty initiating or maintaining sleep, is the most common sleep disorder in the United States. About one‐third of the adult population in the United States experiences insomnia at some point in their lives,18 and it is a persistent problem in approximately 10% of U.S. adults.19 Insomnia can be exacerbated during hospitalization.
Studies investigating sleep in hospitalized patients using PSG have been limited primarily to the setting of the intensive care unit (ICU). Critically ill patients, particularly those requiring mechanical ventilation, are prone to sleep disturbances and an associated delirium.2022 Critically ill patients have fragmented sleep, with decreased SE and SWS, and increased S1 and S2.23 Physician awareness of the impact of sleep disturbance in hospitalized patients is vital. Surveys reveal that approximately one‐half of patients admitted on general medical wards complain of sleep disruption.24, 25 Meissner et al.25 examined the prevalence of sleep complaints and physician recognition of these complaints among general medical patients admitted to a Veterans Affairs tertiary care center. Results showed that 47% (n = 222) of patients had complaints of either insomnia and/or excessive daytime sleepiness.
FACTORS AFFECTING SLEEP DURING HOSPITALIZATION
Many medical and neurologic illnesses, psychiatric disorders, pain, medication therapy and the hospital environment may impair sleep, and hinder recovery from illness.
General Medical Disorders
Primary sleep disorders, such as obstructive sleep apnea (OSA) and numerous other medical illnesses, can directly impair sleep physiology, leading to a cyclical interaction (Figure 2). Other conditions that disrupt sleep include congestive heart failure (CHF), diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux, cardiovascular disease, thyroid disorders, renal disease, and severe liver disease.26 Table 2 lists selected medical and neurological conditions, their associated sleep‐related problems, and suggestions on how to ameliorate these problems.

| Disease | Problem | Clinical Implications and Strategies to Improve Sleep |
|---|---|---|
| ||
| Asthma | Nocturnal exacerbation, nocturnal GER | Inhaled corticosteroids and/or long‐acting inhaled beta‐adrenergic agents |
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep‐disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne‐Stokes respiration | Keep the head of bed elevated 30 degrees. Nocturnal O2 to keep O2 saturation >88%. Daytime diuresis. Optimize cardiac function to treat Cheyne‐Stokes respiration. Consider CPAP for CHF |
| COPD | Persistent nocturnal hypoxemia with complications (e.g., cor pulmonale, polycythemia) | O2 for COPD and persistent hypoxemia (PaO2 55‐60 mm Hg) |
| Sporadic nighttime desaturations | PaO2 55 mm Hg monitor O2 saturation by pulse oximetry. If patient desaturates to 88% at night consistently, start nocturnal O2. For hypercapnia, adjust O2 to maintain O2 saturation at 88% to 90% | |
| Early‐morning airflow obstruction | Consider bedtime tiotropium and inhaled long‐acting beta‐adrenergic agonist agents | |
| Inhibition of respiratory muscles in REM | Avoid sedative‐hypnotics that cause respiratory depression | |
| Decreased functional residual capacity from recumbent position during sleep | Minimize recumbancy by keeping the head of bed up at 30 degrees | |
| End‐stage renal disease | Pruritus, nausea; increased risk of RLS and PLMD | Ambulation may help with RLS. Consider ropinirole and pramipexole. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents |
| Nocturnal GER | Nocturnal GER decreased sleep, heartburn, coughing, asthma | Avoid eating or drinking 2 hours before bedtime, especially those that delay gastric emptying, increase acid secretion, or decrease lower esophageal sphincter pressure; e.g., high‐fat foods, ethanol, chocolate, peppers, peppermint. Keep head of bed 30 degrees. Minimize medications that could worsen nocturnal GER; e.g., theophylline, calcium channel blockers, prostaglandins, bisphosphonates |
| OSA | Snoring with upper airway obstruction | No ethanol 2 hours before bedtime. Minimize CNS depressants. Avoid supine position. Consider CPAP, oral mandibular advancement device, and/or surgical correction. Long‐term plan should include weight loss |
| Stroke | Focal neurologic deficits (e.g., dysphagia, weakness or paralysis) | Keep head of bed 30 degrees. Regularly suction secretions. Post‐stroke patients have an increased risk of hypersomnia, insomnia, and/or OSA |
Affecting approximately 24% of men and 9% of women in the United States, OSA is the most common primary sleep disorder,27, 28 and causes significant mental and physical morbidity. Risk factors for OSA include obesity, hypothyroidism‐induced muscle weakness, and structural abnormalities in the oropharynx region such as acromegaly, micrognathia, or retrognathia. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness.28 These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment (Figure 2). In addition, chronic sympathetic activation causes numerous derangements in the vascular endothelium and platelet activation.29, 30 Sleep‐disordered breathing has been independently associated with cardiovascular diseases such as hypertension, CHF, ischemic heart disease, atrial fibrillation, and cerebrovascular disease.31, 32
OSA is also associated with sleep‐related gastroesophageal reflux, which is characterized by pain and nocturnal cough, and can induce nocturnal asthma attacks and laryngospasm.33 Green et al.29 found that OSA patients treated with continuous positive airway pressure (CPAP) had a 48% improvement in nocturnal reflux symptoms. Although the pathophysiology connecting OSA to the renal system is unknown, OSA has been found in up to 60% of patients with end‐stage renal disease and chronic renal failure.34
Patients with pulmonary disorders can be profoundly affected by the normal physiologic changes during sleep, particularly in REM sleep. During REM sleep, all respiratory muscles except the diaphragm become paralyzed. Thus, episodes of marked oxygen desaturation can occur in patients who rely on their accessory muscles for respiration. COPD patients have decreased TST, SWS, and REM sleep. Shortness of breath, nocturnal cough, and wheezing worsen sleep.35 The resulting fatigue and sleep deprivation negatively impact the work of breathing and impair gas exchange. Airflow obstruction tends to worsen in the early morning hours in patients with COPD and asthma, and may be related to the effect of REM on the accessory muscles for respiration. Although used to target CO2 retention, investigations using bilevel positive airway pressure ventilators (BiPAP) for improving sleep in COPD patients have been limited. Noninvasive positive pressure ventilation (NPPV) appears to acutely improve SE and TST in patients with hypercapnic COPD without significantly improving gas exchange. Other sleep parameters such as sleep architecture and the number of arousals during the night, remain unchanged during NPPV.36
CPAP has several side effects that could worsen sleep, which may explain its poor adherence rate among ambulatory patients.37 Side effects include nasal bridge discomfort, nasal congestion, swallowing air, dry nose, dry or red eyes, noise, ear pain, and rhinitis.38 During hospitalization, efforts should be made to improve patient comfort by resizing ill‐fitting masks, adding heated humidification or nasal steroids to alleviate nasal congestion, or adding a chin strap to reduce air leak and ingestion of air.
Endocrine disorders have also been associated with sleep disruption. Studies suggest that patients with diabetes mellitus have decreased TST and impaired sleep quality due to nocturia and neuropathic pain.39 Inadequate sleep may also affect glucose control. Inadequate quality or quantity of sleep has been shown to be a risk factor for developing Type 2 diabetes mellitus in large prospective studies.40 Sleep duration and quality were significant predictors of increased levels of glycosylated hemoglobin (HbA1c) in patients with Type 2 diabetes mellitus. Thyroid diseases often coexist with diabetes mellitus. Both hypo‐ and hyperthyroidism have been associated with sleep disruption. Hypothyroidism is associated with daytime somnolence and fatigue. Patients with hypothyroidism tend to have reduced SWS. Hyperthyroid patients often complain of insomnia, which has been attributed to a hypermetabolic state.
Approximately 50% of patients with chronic end‐stage renal disease (ESRD) have insomnia and other sleep disorders.41 Patients often complain of restless leg syndrome (RLS), periodic limb movement disorder (PLMD), bone pain, nausea, and pruritus. The etiology of sleep disorders appears to be related to metabolic derangements associated with ESRD or from coexisting diabetes mellitus.
RLS and PLMD are distinct problems that affect sleep differently. RLS is characterized by an unpleasant crampy, creeping or crawling sensation in the lower extremities that is relieved by movement of the legs.42 RLS symptoms typically occur soon after going to bed, and therefore tend to disrupt sleep onset. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems.43 Since RLS may partially be caused by disrupted iron metabolism, serum ferritin levels should be evaluated.44 Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, ESRD, and Parkinson's disease. The differential diagnosis for RLS and PLMD includes neuroleptic‐induced akathisia, peripheral neuropathy, and positional or nocturnal leg cramps. PLMD occurs in about 80% of those with RLS, and is characterized by involuntary limb movements that occur every 20 to 40 seconds during NREM sleep. Unaware of these movements, patients often experience frequent arousals throughout the night, and complain of daytime somnolence and fatigue.42
A pilot study of 35 patients with minimal hepatic encephalopathy found that nearly 50% complained of sleep difficulties.45 Hypothesizing that a dysregulation of histaminergic neurotransmission in cirrhosis alters the sleep‐wake cycle, Spahr et al.46 found that 40% of their patients reported subjective improvement in sleep when administered 25 mg of hydroxyzine, compared to none who received placebo.
Neurologic Disorders
Since the brain and its various neurotransmitter systems are critical in regulating sleep and wakefulness, patients with neurologic disorders have an increased risk of developing sleep disorders. Patients with dementia, other neurodegenerative disorders, epilepsy, and traumatic brain injury (TBI) have a higher prevalence of sleep disturbance and sleep disorders.47 Poststroke patients can develop insomnia or hypersomnia, a reduction in sleep latency, increased sleep, or excessive daytime sleepiness, and are at higher risk for OSA during the first several months after a stroke.48 Specific neurologic lesions may lead to uncommon problems such as inversion of the sleep‐wake cycle, parasomnias, and hallucinatory dream‐like states.
Both Parkinson's disease and Alzheimer's disease are associated with multiple sleep disturbances, which tend to worsen with disease progression.14 Common problems include increased sleep fragmentation and wakefulness, with increases of stage 1 sleep and reductions of SWS and REM. Patients with neurodegenerative disorders also have an increased risk of REM sleep behavior disorder, or RBD.49 RBD is characterized by vivid and unusual dreams, and physically vigorous sleep behaviors that may result in ecchymoses, lacerations, and fractures.50 Fifty percent of patients with TBI reported insomnia symptoms.51 Disorders in initiating and maintaining sleep were the most common complaints among hospitalized patients with TBI. Some patients with TBI may develop circadian rhythm disturbances.52
Pain
A majority of patients with chronic pain, 50% to 70%, complain of impaired sleep.53 Sleep disruption is so common in fibromyalgia (75%) that it is considered to be a key diagnostic symptom.54 In a study investigating the affect of pain on sleep in burn patients, pain was associated with increased intermittent awakenings and prolonged periods of wake time during the night.55 The following day, these patients had poorer pain tolerance and greater pain intensity. Pain causes sleep fragmentation by increasing cortical arousals. Recent evidence suggests that sleep deprivation can increase pain sensitivity by inhibiting opioid protein synthesis or reducing opioid receptor affinity.56
Psychiatric Disorders
Sleep problems are so common in psychiatric conditions that the Diagnostic and Statistical Manual of Mental Disorders (DMS‐IV‐TR) includes sleep disturbance as a diagnostic criterion for a manic episode, and for various depressive, anxiety, and substance abuse disorders.57 The presence of sleep disturbance in hospitalized patients may suggest the presence of an underlying psychiatric disorder that would otherwise go unrecognized. In a survey of 200 general medical patients in a Brazilian hospital, Rocha et al.58 found that 112 (56.5%) complained of insomnia, and 100 (50%) met criteria for at least 1 psychiatric disorder. However, only 3 out of the total number of 200 surveyed (1.5%) were identified as having psychiatric diagnoses in the medical record, and sleep history was not noted in the clinical evaluation. An episode of major depressive disorder was the most common psychiatric diagnosis (35%). In this study, hospitalized patients with insomnia had a 3.6 times higher risk of having major depressive disorder than inpatients without insomnia.
Insomnia has a profound effect on mental health by worsening health‐related quality of life. In a study of outpatients at family medicine, internal medicine, endocrinology, cardiology, and psychiatry clinics in 3 U.S. cities (n = 3,445), insomnia worsened health‐related quality of life nearly as much as CHF or major depressive disorder did.59 Another survey of outpatients found that those with chronic insomnia were nearly 40 times more likely to have major depression and 6 times more likely to have an anxiety disorder compared to those without insomnia.60 Longitudinal studies have found that prior insomnia was associated with 2‐ to 5‐fold increase in the odds of mood and anxiety disorders and suicide.61, 62 Examining prodromes and precursors to mental disorders, Eaton et al.63 found that 47% of those with onset of depression at the 1‐year follow‐up had sleep problems at baseline.
An estimated 65% of patients with major depression have difficulty falling asleep, frequent awakenings, or early morning awakenings.64 Three patterns of sleep architecture abnormalities have been observed in patients with major depression: 1) sleep continuity disturbances characterized by prolonged sleep‐onset, increased wake time during sleep, increased early morning wake time, and decreased TST; 2) decreased proportion and length of SWS; and 3) REM sleep abnormalities such as reduced time to REM sleep, prolonged first REM sleep episode, and increased REM sleep percentage.65 Sleep during a manic episode has been less studied than in depression, but the data suggest that abnormal sleep in mania includes disrupted sleep continuity, shortened REM latency, and increased REM density (REM eye movement activity/total REM sleep time).65
Substance use disorders are also associated with sleep problems. In a survey by Brower et al.66 of patients who were undergoing alcohol rehabilitation, 61% (n = 172) had symptoms of insomnia such as increased sleep latency during the 6 months prior to entering treatment. Approximately 45% of these patients reported using alcohol for the purpose of initiating sleep. Alcohol and illicit substance intoxication and withdrawal are known to be associated with disrupted sleep. However, sleep disturbances may persist long after withdrawal symptoms have abated. Drummond et al. found that some patients continued to have alcohol‐associated sleep problems even after 27 months of abstinence.67 Evidence also suggests that untreated insomnia and other sleep problems may increase the risk of developing substance abuse problems due to self‐medicating with alcohol and other substances to help with sleep.68
Drugs that Affect Sleep
Numerous drugs can alter sleep quantity and quality. Sedatives and opioids may initially help with sleep onset, but impair sleep architecture. Medications used to treat medical and psychiatric illnesses also disrupt sleep (Table 3). The most common agents that impair sleep include antiepileptic drugs, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, antihypertensives, antihistamines, and corticosteroids.
| Drug Class | Examples of Drugs | Affect on Sleep Architecture | Potential Mechanism | Clinical Implications |
|---|---|---|---|---|
| ||||
| CNS | ||||
| AEDs | Phenobarbital, carbamazepine, phenytoin | Very sedating. AEDs tend to TST, sleep latency | Inhibit neuronal calcium influx, adenosine, or 5HT activity | Sedation is dose‐dependent, and tends to occur with acute use |
| TCAs | Amoxapine, amitriptyline, imipramine, nortriptyline, desipramine, doxepin, clomipramine | Very sedating. Suppresses REM sleep, TST, stage‐2 sleep | Stimulate antimuscarinic‐receptor and alpha1‐receptor | Suppressed REM sleep motor inhibition restlessness, psychomotor agitation during sleep subjectively sleep quality, daytime sleepiness |
| BzRAs | Alprazolam, lorazepam, chlordiazepoxide, diazepam, oxazepam | Very sedating. TST, sleep latency, SWS duration, REM, stage‐2 sleep | Stimulate GABA type A receptor | Minimize daytime use. Chronic BzRAs SWS long‐term sequelae unknown |
| MAOIs | Phenylzine, tranylcypromine | Very sedating. TST, REM, REM rebound if stop MAOIs | Mechanism unknown | Daytime sleepiness; dosing time does not affect daytime somnolence |
| SSRIs | Sedating: paroxetine, fluvoxamine. Activating: fluoxetine, sertraline, citalopram | TST, are less sedating than TCAs and MAOIs. May REM, TWT, TST, SE | 5HT activity | Some patients get the opposite reaction |
| SNRI | Venlafaxine, duloxetine | Activating in some patients; sedating in 12% to 31%. TST | 5HT and NE activity | If activating, switch to AM dosing. If sedating, switch to PM dosing |
| Mood stabilizer | Lithium | Sedating. TST, SWS, REM, REM latency | daytime sedation. Dose at night | |
| Stimulants | Ephedrine, pseudoephedrine, modafinil | Activating. TST, SWS, sleep latency | DOPA, NE, and 5HT activity | Avoid after 6 PM |
| Anti‐Parkinson | Bromocriptine, levodopa | Sedating. Nightmares, SWS | DOPA | Dose at night, if possible |
| Cardiac | ||||
| Lipophilic beta‐blockers | Propranolol, pindolol, metoprolol, timolol. Hydrophilic agents (atenolol and sotalol) lack these effects | Activating. awakenings, TWT, REM, nightmares | CNS beta‐blockade | Lipophilic beta‐blockers daytime sleep when dosed in AM |
| CNS agents | Norepinephrine, epinephrine | Activating. REM, SWS | Stimulate alpha1‐receptor | Minimize use at night |
| Dopamine | Activating. REM, SWS | Stimulate dopamine2‐receptor and alpha1‐receptor | Minimize use at night | |
| Ca++ channel blockers | Amlodipine, verapamil, nifedipine | Exacerbate underlying medical condition | Lower esophageal sphincter tone nocturnal GER sleep disturbance | |
| Alpha2‐receptor agonist | Clonidine | Stage 1, REM, nightmares | Stimulate alpha2‐receptor | Alpha2‐agonists daytime sleep and sleepiness directly. Dose at night |
| Alpha1‐receptor blockers | Doxazosin, prazosin, terazosin | Inhibit alpha1‐receptor | Alpha1‐receptor blockers daytime sleepiness | |
| Diuretics | HCTZ, furosemide | Sedating. | PM diuresis frequent awakenings | |
| Other | ||||
| Opioids | Codeine, morphine | Sedating. SWS, REM | Stimulate mu‐receptor | Minimize use at night |
| NSAIDs | Ibuprofen, indomethcin, celecoxib | TST, SE | Inhibit prostaglandin synthesis | Minimize use at night |
| Methylxanthine | Theophylline | Activating. stage 1, REM | Causes less restful sleep | |
| Antihistamines | Diphenhydramine, promethazine | Sedating | H1 receptor blockade | Minimize use at night |
| Corticosteroids | Dexamethasone, prednisone | Activating. REM, SWS, nightmares | Melatonin secretion | Can disrupt sleep, anxiety, induce mania or psychosis |
| H2 blockers | Cimetidine, ranitidine, famotidine | Sedating. TST | H2 receptor blockade | Sedating if >60 years old, renal impairment |
| Quinolone | Ciprofloxacin, sparfloxacin, ofloxacin, grepafloxacin, levofloxacin | Activating | Stimulate GABA type A receptor | Consider sleep agent after maximizing sleep hygiene. Linezolid rarely causes sleep disturbances |
Lipophilic beta antagonists such as propranolol and timolol can increase total wake time, decrease REM sleep, and increase the incidence of nightmares and insomnia.69 Anabolic steroids and beta‐agonist bronchodilator therapy can cause severe anxiety, sleeplessness, and even psychosis. Vasopressor agents such as dopamine can cause cortical activation, leading to increased arousal and reduced SWS.
Hospital Environment
Environmental noise and patient care activities often interfere with sleep in the hospital. They account for about 30% of patient awakenings in ICU patients.70 Noise levels in the ICU have average sound peaks of 150 to 200 dB, and evening peaks >80 dB between midnight and 6 AM.71 By comparison, the front row seats at a rock concert have sound levels of 110 dB. The high noise level in hospitals has long been implicated as a sleep disruptor,72 but studies in the past decade have found that patient care activities probably contribute more to awakenings than does environmental noise.73 An analysis of critical care nursing routines found that activities such as taking vital signs and giving baths occurred a mean 42.6 times a night per patient.74 Tamburri et al.74 found that patients experienced 2 to 3 hours without interruption on only 6% of the 147 nights studied. Routine daily baths were provided on 55 of the 147 study nights between 2 AM and 5 AM, which is unlikely to be an opportune time for most patients.
CONCLUSION
Hospitalization often prevents patients from achieving adequate sleep and can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing sedative/hypnotic agents, the treatment for sleep disruption includes addressing multiple medical, behavioral, and environmental factors, which will be discussed in Part 2 of this article.
- NIH State‐of‐the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults.NIH Consens Sci Statements.2005;22(2):1–30.
- ,,, et al.Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey.Hypertension.2006;47(5):833–839.
- ,,, et al.Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique.Sleep.2003;26(8):986–989.
- ,,,,.Postural control after a night without sleep.Neuropsychologia.2006;44(12):2520–2525.
- .Sleep deprivation decreases ventilatory responses to CO2 but not load compensation.Chest.1983;84(6):695–698.
- ,,, et al.Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation.J Appl Physiol.2005;98(6):2024–2032.
- .Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats.Am J Physiol Endocrinol Metab.2004;286(6):E1060–E1070.
- ,,,.The metabolic consequences of sleep deprivation.Sleep Med Rev.2007;11(3):163–178.
- ,,,,.Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.Arch Int Med.2006;166:1756–1752.
- ,,,.Effects of sleep and sleep deprivation on immunoglobulins and complement in humans.Brain Behav Immun.2007;21:308–310.
- ,,,,.The effects of sleep deprivation on symptoms of psychopathology in healthy adults.Sleep Med.2007;8:215–221.
- ,.Normal human sleep. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:15–16.
- .Update on the Science, Diagnosis and Management of Insomnia.International Congress and Symposium Series 262.London:Royal Society of Medicine Press Ltd;2006.
- ,,,,,.The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture.Arch Intern Med.2004;164:406–18.
- ,.Sleep disruption in older adults.Am J Nurs.2007;107(5):40–49.
- ,,,.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey.J Psychosom Res.2004;56(5):497–502.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Insomnia and its treatment: prevalence and correlates.Arch Gen Psychiatry.1985;42(3):225–232.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117:809–818.
- ,.Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill.Crit Care Med.2006;22:313–327.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,,,.Failure of physician documentation of sleep complaints in hospitalized patients.West J Med.1998;169:146–149.
- .Sleep and medical disorders.Prim Care.2005;35:511–533.
- ,,,,,.The occurrence of sleep‐disordered breathing among middle‐aged adults.N Engl J Med.1993;328:1230–1235.
- ,.Clinical features and evaluation of obstructive sleep apnea‐hypopnea syndrome. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:869–872.
- ,,.Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea.Arch Intern Med.2003;163:41–45.
- ,.Sleep and medical disorders.Med Clin North Am.2004;88:679–703.
- ,,,.Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness.Atherosclerosis.2008;197(1):125– 131.
- ,,, et al.Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med.2001;163:19–25.
- .Gastroesophageal reflux during sleep.Sleep Med Clin.2007;2:41–50.
- ,.Sleep apnea in renal failure.Adv Perit Dial.1997;13:88–92.
- .Sleep in chronic obstructive pulmonary disease.Sleep Med Clin.2007;2:1–8.
- ,,,.Effects of non‐invasive positive pressure ventilation on gas exchange and sleep in COPD patients.Chest.1997;112:623–628.
- ,,, et al.Night‐to‐night variability in CPAP use over the first three months of treatment.Sleep.1997;20(4):278–283.
- ,.Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS).Sleep Med Rev.2003;7(1):81–99.
- ,,.Factors predicting sleep disruption in type II diabetes.Sleep.2000;23:415–416.
- ,,.Sleep duration as a risk factor for the development of type 2 diabetes.Diabetes Care.2006;29:657–661.
- .Sleep disorders and end‐stage renal disease.Sleep Med Clin.2007;2:59–66.
- ,,,.Sleep and aging: 1. Sleep disorders commonly found in older people.CMAJ.2007;176(9):1299–1304.
- ,,,,,.Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS Epidemiology, Symptoms, and Treatment) primary care study.Sleep Med.2004;5(3):237–246.
- ,.Epidemiology and clinical findings of restless leg syndrome.Sleep Med.2004;5(3):293–299.
- ,,,,,.High prevalence of sleep disturbance in cirrhosis.Hepatology.1998;27:339–345.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Obstructive sleep apnea: implications for cardiac and vascular disease.JAMA.2003;290(14):1906–1914.
- ,,, et al.Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.Brain.2007;130(Pt 11):2770–2788.
- ,.REM sleep parasomnias. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:724–725.
- ,,.Insomnia in patients with traumatic brain injury.J Head Trauma Rehabil.2006;21(3):199–212.
- ,,,,.Circadian rhythm sleep disorders following mild traumatic brain injury.Neurology.2007;68(14):1136–1140.
- ,.Comorbidities: psychiatric, medical, medications, and substances.Sleep Med Clin.2006;231–245.
- ,,.Sleep disturbance and fibromyalgia.Sleep Med Clin.2007;2:31–39.
- ,,.Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries.Sleep Med.2004;5:551–559.
- ,,.Sleep deprivation and pain perception.Sleep Med Rev.2006;10:357–369.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision.Washington, DC:American Psychiatric Association;2000.
- ,,, et al.Is insomnia a marker for psychiatric disorders in general hospitals?Sleep Med.2005;6:549–553.
- ,.The relationship between insomnia and health‐related quality of life in patients with chronic illness.J Fam Pract.2002;51(3):229–235.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,.Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults.Biol Psychiatry.1996;39:411–418.
- ,,,.The morbidity of insomnia uncomplicated by psychiatric disorders.Gen Hosp Psychiatry.1997;19:245–250.
- ,,.Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset.Am J Psychiatry.1995;152:967–972.
- ,,,,,.Which depressive symptoms are related to which sleep electroencephalographic variables?Biol Psychol.1997;42:904–913.
- ,.Sleep in mood disorders.Psychiatr Clin North Am.2006;29:1009–1032.
- ,,,,.Insomnia, self‐medication, and relapse to alcoholism.Am J Psychiatry.2001;158:399–404.
- ,,,.The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse.Alcohol Clin Exp Res.1998;22:1796–1802.
- ,,,,.Screening for substance use patterns among patients referred for a variety of sleep complaints.Am J Drug Alcohol Abuse.2006;32:111–120.
- .Drugs that disturb sleep and wakefulness. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:441–462.
- ,,, et al.Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects.Am J Respir Crit Care Med.2003;167(5):708–715.
- ,,.Adverse environmental conditions in the respiratory and medical ICU settings.Chest.1994;105:1211–1216.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118(6):3629–3645.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,,.Nocturnal care interactions with patients in critical care units.Am J Crit Care.2004;13(2):102–115.
- NIH State‐of‐the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults.NIH Consens Sci Statements.2005;22(2):1–30.
- ,,, et al.Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey.Hypertension.2006;47(5):833–839.
- ,,, et al.Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique.Sleep.2003;26(8):986–989.
- ,,,,.Postural control after a night without sleep.Neuropsychologia.2006;44(12):2520–2525.
- .Sleep deprivation decreases ventilatory responses to CO2 but not load compensation.Chest.1983;84(6):695–698.
- ,,, et al.Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation.J Appl Physiol.2005;98(6):2024–2032.
- .Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats.Am J Physiol Endocrinol Metab.2004;286(6):E1060–E1070.
- ,,,.The metabolic consequences of sleep deprivation.Sleep Med Rev.2007;11(3):163–178.
- ,,,,.Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.Arch Int Med.2006;166:1756–1752.
- ,,,.Effects of sleep and sleep deprivation on immunoglobulins and complement in humans.Brain Behav Immun.2007;21:308–310.
- ,,,,.The effects of sleep deprivation on symptoms of psychopathology in healthy adults.Sleep Med.2007;8:215–221.
- ,.Normal human sleep. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:15–16.
- .Update on the Science, Diagnosis and Management of Insomnia.International Congress and Symposium Series 262.London:Royal Society of Medicine Press Ltd;2006.
- ,,,,,.The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture.Arch Intern Med.2004;164:406–18.
- ,.Sleep disruption in older adults.Am J Nurs.2007;107(5):40–49.
- ,,,.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey.J Psychosom Res.2004;56(5):497–502.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Insomnia and its treatment: prevalence and correlates.Arch Gen Psychiatry.1985;42(3):225–232.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117:809–818.
- ,.Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill.Crit Care Med.2006;22:313–327.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,,,.Failure of physician documentation of sleep complaints in hospitalized patients.West J Med.1998;169:146–149.
- .Sleep and medical disorders.Prim Care.2005;35:511–533.
- ,,,,,.The occurrence of sleep‐disordered breathing among middle‐aged adults.N Engl J Med.1993;328:1230–1235.
- ,.Clinical features and evaluation of obstructive sleep apnea‐hypopnea syndrome. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:869–872.
- ,,.Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea.Arch Intern Med.2003;163:41–45.
- ,.Sleep and medical disorders.Med Clin North Am.2004;88:679–703.
- ,,,.Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness.Atherosclerosis.2008;197(1):125– 131.
- ,,, et al.Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med.2001;163:19–25.
- .Gastroesophageal reflux during sleep.Sleep Med Clin.2007;2:41–50.
- ,.Sleep apnea in renal failure.Adv Perit Dial.1997;13:88–92.
- .Sleep in chronic obstructive pulmonary disease.Sleep Med Clin.2007;2:1–8.
- ,,,.Effects of non‐invasive positive pressure ventilation on gas exchange and sleep in COPD patients.Chest.1997;112:623–628.
- ,,, et al.Night‐to‐night variability in CPAP use over the first three months of treatment.Sleep.1997;20(4):278–283.
- ,.Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS).Sleep Med Rev.2003;7(1):81–99.
- ,,.Factors predicting sleep disruption in type II diabetes.Sleep.2000;23:415–416.
- ,,.Sleep duration as a risk factor for the development of type 2 diabetes.Diabetes Care.2006;29:657–661.
- .Sleep disorders and end‐stage renal disease.Sleep Med Clin.2007;2:59–66.
- ,,,.Sleep and aging: 1. Sleep disorders commonly found in older people.CMAJ.2007;176(9):1299–1304.
- ,,,,,.Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS Epidemiology, Symptoms, and Treatment) primary care study.Sleep Med.2004;5(3):237–246.
- ,.Epidemiology and clinical findings of restless leg syndrome.Sleep Med.2004;5(3):293–299.
- ,,,,,.High prevalence of sleep disturbance in cirrhosis.Hepatology.1998;27:339–345.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Obstructive sleep apnea: implications for cardiac and vascular disease.JAMA.2003;290(14):1906–1914.
- ,,, et al.Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.Brain.2007;130(Pt 11):2770–2788.
- ,.REM sleep parasomnias. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:724–725.
- ,,.Insomnia in patients with traumatic brain injury.J Head Trauma Rehabil.2006;21(3):199–212.
- ,,,,.Circadian rhythm sleep disorders following mild traumatic brain injury.Neurology.2007;68(14):1136–1140.
- ,.Comorbidities: psychiatric, medical, medications, and substances.Sleep Med Clin.2006;231–245.
- ,,.Sleep disturbance and fibromyalgia.Sleep Med Clin.2007;2:31–39.
- ,,.Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries.Sleep Med.2004;5:551–559.
- ,,.Sleep deprivation and pain perception.Sleep Med Rev.2006;10:357–369.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision.Washington, DC:American Psychiatric Association;2000.
- ,,, et al.Is insomnia a marker for psychiatric disorders in general hospitals?Sleep Med.2005;6:549–553.
- ,.The relationship between insomnia and health‐related quality of life in patients with chronic illness.J Fam Pract.2002;51(3):229–235.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,.Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults.Biol Psychiatry.1996;39:411–418.
- ,,,.The morbidity of insomnia uncomplicated by psychiatric disorders.Gen Hosp Psychiatry.1997;19:245–250.
- ,,.Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset.Am J Psychiatry.1995;152:967–972.
- ,,,,,.Which depressive symptoms are related to which sleep electroencephalographic variables?Biol Psychol.1997;42:904–913.
- ,.Sleep in mood disorders.Psychiatr Clin North Am.2006;29:1009–1032.
- ,,,,.Insomnia, self‐medication, and relapse to alcoholism.Am J Psychiatry.2001;158:399–404.
- ,,,.The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse.Alcohol Clin Exp Res.1998;22:1796–1802.
- ,,,,.Screening for substance use patterns among patients referred for a variety of sleep complaints.Am J Drug Alcohol Abuse.2006;32:111–120.
- .Drugs that disturb sleep and wakefulness. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:441–462.
- ,,, et al.Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects.Am J Respir Crit Care Med.2003;167(5):708–715.
- ,,.Adverse environmental conditions in the respiratory and medical ICU settings.Chest.1994;105:1211–1216.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118(6):3629–3645.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,,.Nocturnal care interactions with patients in critical care units.Am J Crit Care.2004;13(2):102–115.