User login
Sleep in hospitalized medical patients, Part 2: Behavioral and pharmacological management of sleep disturbances
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
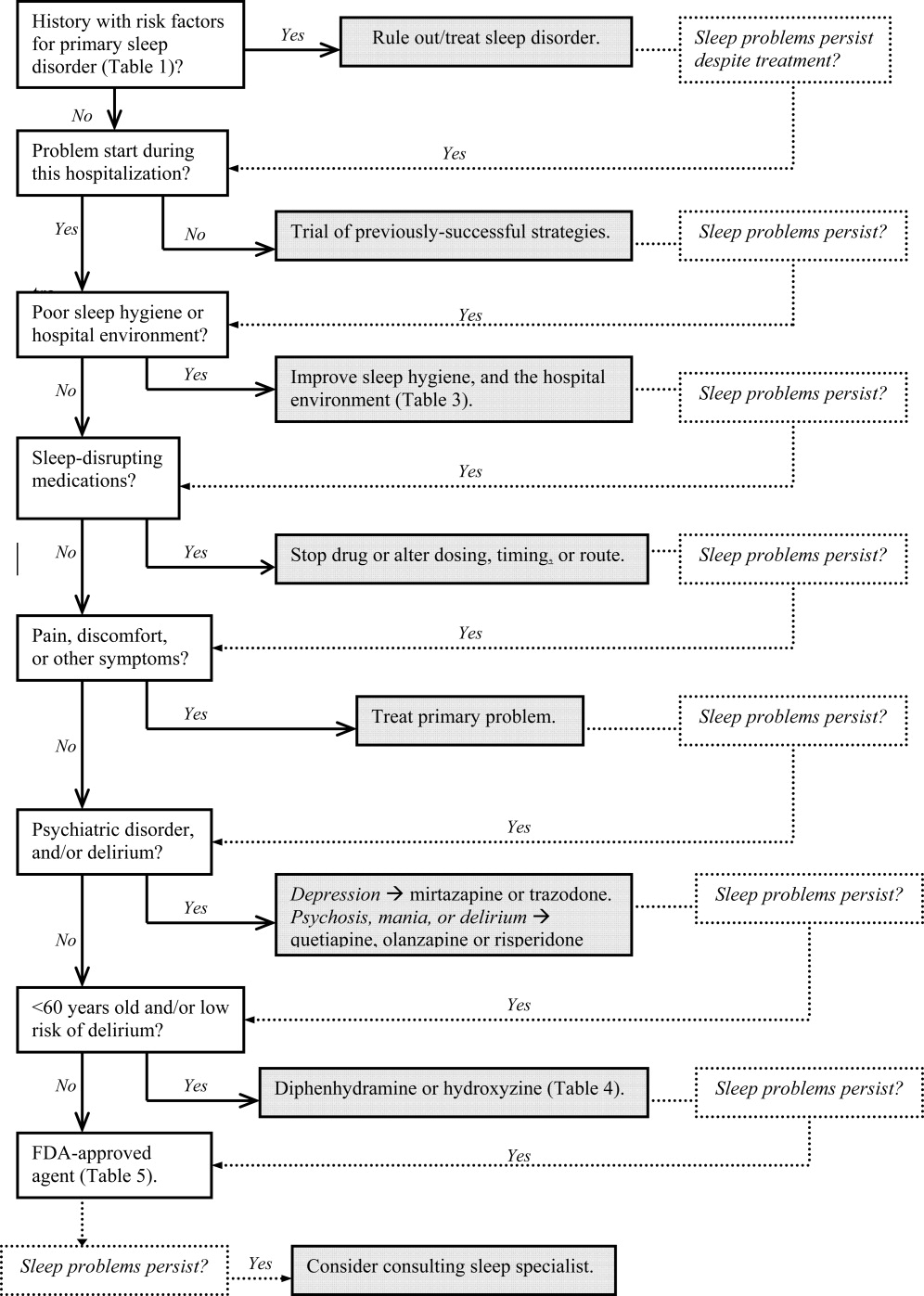
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.
| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (<2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, <45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | <2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
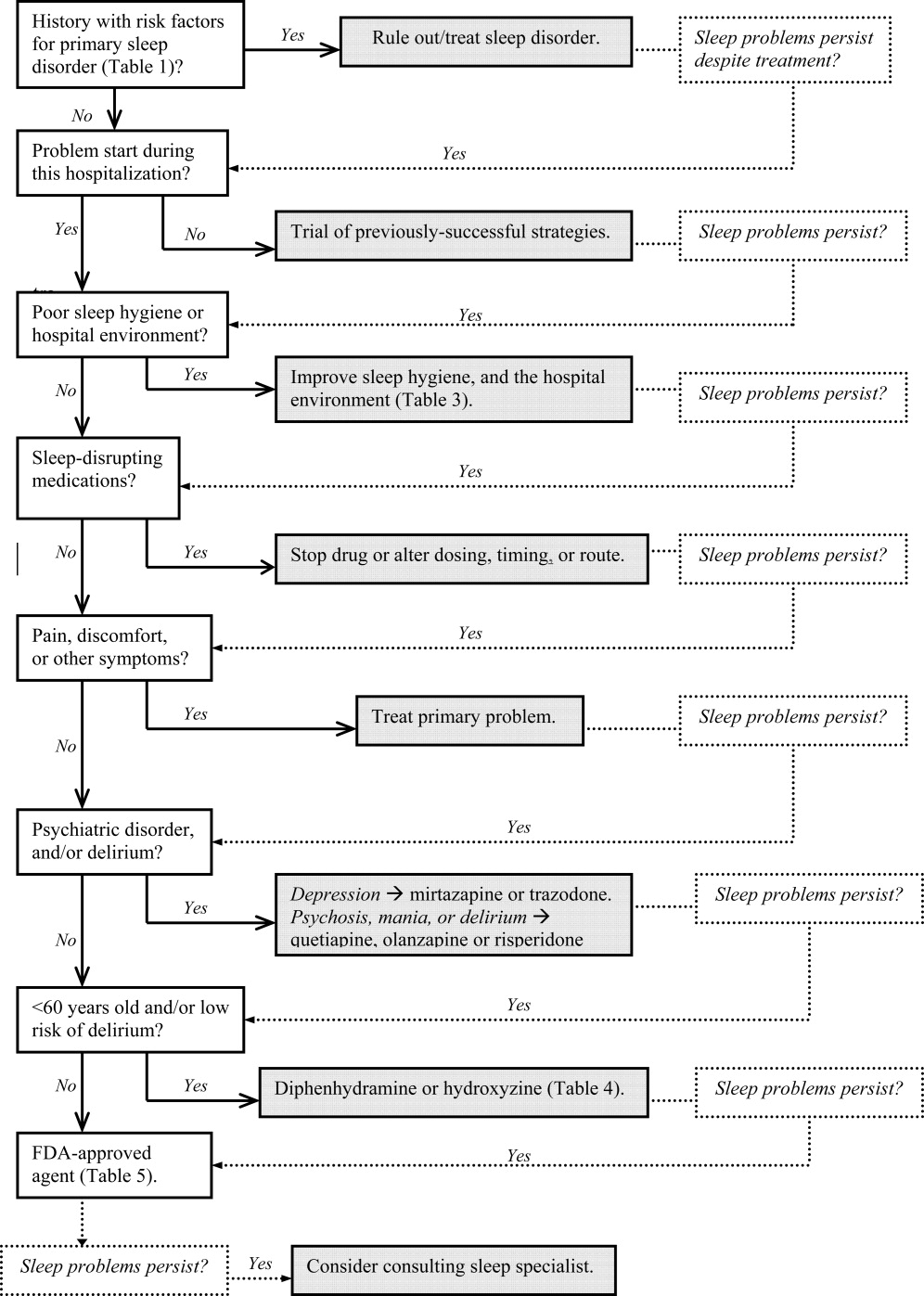
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.

| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (<2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, <45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | <2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.

| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (<2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, <45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | <2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
Sleep in Hospitalized Medical Patients: Part 1
Hospitalized patients often have difficulty initiating and maintaining sleep, or complain of early awakening and nonrestorative sleep.1 The etiology of sleep disruption is multifactorial and includes the patient's underlying illness(es), medical treatments, and the hospital environment. Often unrecognized and untreated during hospitalization, sleep disruption may lead to sleep deprivation, or a chronic lack of restorative sleep.
Even in healthy individuals, sleep deprivation can result in numerous physical and psychological consequences. Sleep deprivation is associated with hypertension,2, 3 impaired postural control,4 decreased ventilatory drive,5 increased sympathetic cardiovascular activation,6 blunted hypothalamic‐pituitary‐adrenal axis,7 impaired host defenses, and possibly diabetes mellitus and obesity.810 The lack of restorative sleep increases the risk of developing anxiety and mood disorders and delirium, especially in acutely ill older patients.11 In the presence of acute physical infirmity, inadequate sleep may further compound illness and impair recovery. We provide an overview of normal sleep architecture and discuss factors that lead to sleep disruption in hospitalized medical patients.
NORMAL SLEEP ARCHITECTURE AND REGULATION
Normal sleep architecture refers to a characteristic pattern of sleep, and consists of two major stages: nonrapid eye movement (NREM, pronounced non‐rem) and rapid eye movement (REM). (For a table of pertinent abbreviations and terms, see Table 1) Sleep is quantified by polysomnography (PSG), which includes an electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). A PSG also includes an electrocardiogram (ECG), and measures of airflow, oxygen saturation, and body position. NREM sleep comprises 75% to 80% of total sleep time (TST), and is characterized by relatively quiescent brain activity and decreased metabolic rate.12 NREM sleep consists of four stages (S1‐S4), with each stage leading to a progressively deeper sleep (Figure 1). REM sleep follows slow wave sleep (SWS), or deep sleep, and increases over the night, comprising 20% to 25% of TST. REM sleep is characterized by an activated EEG pattern, muscle atonia, and episodic bursts of rapid eye movements.
| Acronym | Term |
|---|---|
| BiPAP | Bilevel positive airway pressure |
| CHF | Congestive heart failure |
| CPAP | Continuous positive airway pressure |
| COPD | Chronic obstructive pulmonary disease |
| EEG | Electroencephalogram |
| EOG | Electroculogram |
| EMG | Electromyogram |
| ESRD | End‐stage renal disease |
| NPPV | Noninvasive positive pressure ventilation |
| NREM | Nonrapid eye movement |
| OSA | Obstructive sleep apnea |
| PLMD | Periodic limb movement disorder |
| PSG | Polysomnography |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement |
| RLS | Restless leg syndrome |
| S1‐S4 | 4 Stages of sleep in NREM |
| SE | Sleep efficiency; TST divided by total time in bed |
| SWS | Slow wave sleep |
| TBI | Traumatic brain injury |
| TST | Total sleep time |
Normal sleep provides a period of physiologic and mental rest. During sleep, sympathetic tone decreases and parasympathetic tone increases, leading to a reduction in heart rate, arterial blood pressure, and cardiac output.13 Deep sleep is theorized to be necessary for physiologic restoration. REM sleep is associated with dreaming, and is essential for maintaining emotional and cognitive well‐being. Sleep architecture undergoes characteristic changes as people age.14 The duration of SWS peaks in childhood and decreases with age. Consequently, people >60 years old tend to have lower arousal thresholds and to have more frequent awakenings. The results of the Sleep Heart Health Study found that increased age was associated with decreased percentage of REM sleep, worse sleep efficiency (SE, which is TST divided by total time in bed), and lower arousal thresholds.14 With the reduction of SE, older people need to spend more hours in bed to achieve the same amount of restorative sleep as when they were younger. Although sleep tends to become more disrupted as people age, insomnia should not be considered a normal part of aging, and needs to be addressed clinically.15 The results of a National Sleep Foundation telephone survey of subjects between the ages of 55 and 84 years old (n = 1,506) suggested that sleep complaints in older adults are frequently secondary to comorbid medical conditions.16
Multiple anatomic structures, pathways, and neurotransmitter systems are involved in controlling wakefulness and sleep. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Sleep‐promoting neurotransmitters include gamma aminobutyric acid (GABA), adenosine, and melatonin. Specific stages of sleep are regulated by the turning on and off of various neurons. REM on cells use GABA, acetylcholine, and glutamine, whereas REM off cells use norepinephrine and serotonin. SWS is promoted by GABA and serotonin.17
Sleep regulation is a balance between a homeostatic sleep need and an intrinsic body clock, or circadian pacemaker. Located in the suprachiasmic nucleus, the circadian pacemaker determines the onset and termination of sleep, and is partially regulated by environmental cues such as light and ambient temperature. Melatonin, a physiologic sleep promoter, is inhibited by ambient light, and its circulation is decreased during daylight hours. The adrenal secretion of cortisol, which is associated with wakefulness, follows a circadian pattern. Regulated by the hypothalamic‐pituitary axis, cortisol levels peak in the early morning hours in preparation for the increased metabolic demands during wakefulness.
SLEEP PROBLEMS IN HOSPITALIZED PATIENTS
Insomnia, which is characterized by difficulty initiating or maintaining sleep, is the most common sleep disorder in the United States. About one‐third of the adult population in the United States experiences insomnia at some point in their lives,18 and it is a persistent problem in approximately 10% of U.S. adults.19 Insomnia can be exacerbated during hospitalization.
Studies investigating sleep in hospitalized patients using PSG have been limited primarily to the setting of the intensive care unit (ICU). Critically ill patients, particularly those requiring mechanical ventilation, are prone to sleep disturbances and an associated delirium.2022 Critically ill patients have fragmented sleep, with decreased SE and SWS, and increased S1 and S2.23 Physician awareness of the impact of sleep disturbance in hospitalized patients is vital. Surveys reveal that approximately one‐half of patients admitted on general medical wards complain of sleep disruption.24, 25 Meissner et al.25 examined the prevalence of sleep complaints and physician recognition of these complaints among general medical patients admitted to a Veterans Affairs tertiary care center. Results showed that 47% (n = 222) of patients had complaints of either insomnia and/or excessive daytime sleepiness.
FACTORS AFFECTING SLEEP DURING HOSPITALIZATION
Many medical and neurologic illnesses, psychiatric disorders, pain, medication therapy and the hospital environment may impair sleep, and hinder recovery from illness.
General Medical Disorders
Primary sleep disorders, such as obstructive sleep apnea (OSA) and numerous other medical illnesses, can directly impair sleep physiology, leading to a cyclical interaction (Figure 2). Other conditions that disrupt sleep include congestive heart failure (CHF), diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux, cardiovascular disease, thyroid disorders, renal disease, and severe liver disease.26 Table 2 lists selected medical and neurological conditions, their associated sleep‐related problems, and suggestions on how to ameliorate these problems.
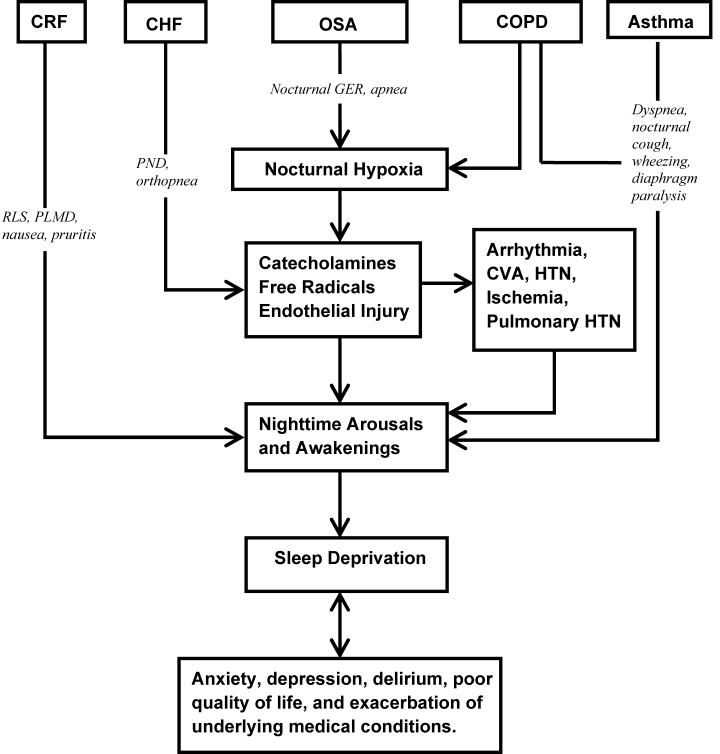
| Disease | Problem | Clinical Implications and Strategies to Improve Sleep |
|---|---|---|
| ||
| Asthma | Nocturnal exacerbation, nocturnal GER | Inhaled corticosteroids and/or long‐acting inhaled beta‐adrenergic agents |
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep‐disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne‐Stokes respiration | Keep the head of bed elevated 30 degrees. Nocturnal O2 to keep O2 saturation >88%. Daytime diuresis. Optimize cardiac function to treat Cheyne‐Stokes respiration. Consider CPAP for CHF |
| COPD | Persistent nocturnal hypoxemia with complications (e.g., cor pulmonale, polycythemia) | O2 for COPD and persistent hypoxemia (PaO2 55‐60 mm Hg) |
| Sporadic nighttime desaturations | PaO2 55 mm Hg monitor O2 saturation by pulse oximetry. If patient desaturates to 88% at night consistently, start nocturnal O2. For hypercapnia, adjust O2 to maintain O2 saturation at 88% to 90% | |
| Early‐morning airflow obstruction | Consider bedtime tiotropium and inhaled long‐acting beta‐adrenergic agonist agents | |
| Inhibition of respiratory muscles in REM | Avoid sedative‐hypnotics that cause respiratory depression | |
| Decreased functional residual capacity from recumbent position during sleep | Minimize recumbancy by keeping the head of bed up at 30 degrees | |
| End‐stage renal disease | Pruritus, nausea; increased risk of RLS and PLMD | Ambulation may help with RLS. Consider ropinirole and pramipexole. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents |
| Nocturnal GER | Nocturnal GER decreased sleep, heartburn, coughing, asthma | Avoid eating or drinking 2 hours before bedtime, especially those that delay gastric emptying, increase acid secretion, or decrease lower esophageal sphincter pressure; e.g., high‐fat foods, ethanol, chocolate, peppers, peppermint. Keep head of bed 30 degrees. Minimize medications that could worsen nocturnal GER; e.g., theophylline, calcium channel blockers, prostaglandins, bisphosphonates |
| OSA | Snoring with upper airway obstruction | No ethanol 2 hours before bedtime. Minimize CNS depressants. Avoid supine position. Consider CPAP, oral mandibular advancement device, and/or surgical correction. Long‐term plan should include weight loss |
| Stroke | Focal neurologic deficits (e.g., dysphagia, weakness or paralysis) | Keep head of bed 30 degrees. Regularly suction secretions. Post‐stroke patients have an increased risk of hypersomnia, insomnia, and/or OSA |
Affecting approximately 24% of men and 9% of women in the United States, OSA is the most common primary sleep disorder,27, 28 and causes significant mental and physical morbidity. Risk factors for OSA include obesity, hypothyroidism‐induced muscle weakness, and structural abnormalities in the oropharynx region such as acromegaly, micrognathia, or retrognathia. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness.28 These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment (Figure 2). In addition, chronic sympathetic activation causes numerous derangements in the vascular endothelium and platelet activation.29, 30 Sleep‐disordered breathing has been independently associated with cardiovascular diseases such as hypertension, CHF, ischemic heart disease, atrial fibrillation, and cerebrovascular disease.31, 32
OSA is also associated with sleep‐related gastroesophageal reflux, which is characterized by pain and nocturnal cough, and can induce nocturnal asthma attacks and laryngospasm.33 Green et al.29 found that OSA patients treated with continuous positive airway pressure (CPAP) had a 48% improvement in nocturnal reflux symptoms. Although the pathophysiology connecting OSA to the renal system is unknown, OSA has been found in up to 60% of patients with end‐stage renal disease and chronic renal failure.34
Patients with pulmonary disorders can be profoundly affected by the normal physiologic changes during sleep, particularly in REM sleep. During REM sleep, all respiratory muscles except the diaphragm become paralyzed. Thus, episodes of marked oxygen desaturation can occur in patients who rely on their accessory muscles for respiration. COPD patients have decreased TST, SWS, and REM sleep. Shortness of breath, nocturnal cough, and wheezing worsen sleep.35 The resulting fatigue and sleep deprivation negatively impact the work of breathing and impair gas exchange. Airflow obstruction tends to worsen in the early morning hours in patients with COPD and asthma, and may be related to the effect of REM on the accessory muscles for respiration. Although used to target CO2 retention, investigations using bilevel positive airway pressure ventilators (BiPAP) for improving sleep in COPD patients have been limited. Noninvasive positive pressure ventilation (NPPV) appears to acutely improve SE and TST in patients with hypercapnic COPD without significantly improving gas exchange. Other sleep parameters such as sleep architecture and the number of arousals during the night, remain unchanged during NPPV.36
CPAP has several side effects that could worsen sleep, which may explain its poor adherence rate among ambulatory patients.37 Side effects include nasal bridge discomfort, nasal congestion, swallowing air, dry nose, dry or red eyes, noise, ear pain, and rhinitis.38 During hospitalization, efforts should be made to improve patient comfort by resizing ill‐fitting masks, adding heated humidification or nasal steroids to alleviate nasal congestion, or adding a chin strap to reduce air leak and ingestion of air.
Endocrine disorders have also been associated with sleep disruption. Studies suggest that patients with diabetes mellitus have decreased TST and impaired sleep quality due to nocturia and neuropathic pain.39 Inadequate sleep may also affect glucose control. Inadequate quality or quantity of sleep has been shown to be a risk factor for developing Type 2 diabetes mellitus in large prospective studies.40 Sleep duration and quality were significant predictors of increased levels of glycosylated hemoglobin (HbA1c) in patients with Type 2 diabetes mellitus. Thyroid diseases often coexist with diabetes mellitus. Both hypo‐ and hyperthyroidism have been associated with sleep disruption. Hypothyroidism is associated with daytime somnolence and fatigue. Patients with hypothyroidism tend to have reduced SWS. Hyperthyroid patients often complain of insomnia, which has been attributed to a hypermetabolic state.
Approximately 50% of patients with chronic end‐stage renal disease (ESRD) have insomnia and other sleep disorders.41 Patients often complain of restless leg syndrome (RLS), periodic limb movement disorder (PLMD), bone pain, nausea, and pruritus. The etiology of sleep disorders appears to be related to metabolic derangements associated with ESRD or from coexisting diabetes mellitus.
RLS and PLMD are distinct problems that affect sleep differently. RLS is characterized by an unpleasant crampy, creeping or crawling sensation in the lower extremities that is relieved by movement of the legs.42 RLS symptoms typically occur soon after going to bed, and therefore tend to disrupt sleep onset. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems.43 Since RLS may partially be caused by disrupted iron metabolism, serum ferritin levels should be evaluated.44 Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, ESRD, and Parkinson's disease. The differential diagnosis for RLS and PLMD includes neuroleptic‐induced akathisia, peripheral neuropathy, and positional or nocturnal leg cramps. PLMD occurs in about 80% of those with RLS, and is characterized by involuntary limb movements that occur every 20 to 40 seconds during NREM sleep. Unaware of these movements, patients often experience frequent arousals throughout the night, and complain of daytime somnolence and fatigue.42
A pilot study of 35 patients with minimal hepatic encephalopathy found that nearly 50% complained of sleep difficulties.45 Hypothesizing that a dysregulation of histaminergic neurotransmission in cirrhosis alters the sleep‐wake cycle, Spahr et al.46 found that 40% of their patients reported subjective improvement in sleep when administered 25 mg of hydroxyzine, compared to none who received placebo.
Neurologic Disorders
Since the brain and its various neurotransmitter systems are critical in regulating sleep and wakefulness, patients with neurologic disorders have an increased risk of developing sleep disorders. Patients with dementia, other neurodegenerative disorders, epilepsy, and traumatic brain injury (TBI) have a higher prevalence of sleep disturbance and sleep disorders.47 Poststroke patients can develop insomnia or hypersomnia, a reduction in sleep latency, increased sleep, or excessive daytime sleepiness, and are at higher risk for OSA during the first several months after a stroke.48 Specific neurologic lesions may lead to uncommon problems such as inversion of the sleep‐wake cycle, parasomnias, and hallucinatory dream‐like states.
Both Parkinson's disease and Alzheimer's disease are associated with multiple sleep disturbances, which tend to worsen with disease progression.14 Common problems include increased sleep fragmentation and wakefulness, with increases of stage 1 sleep and reductions of SWS and REM. Patients with neurodegenerative disorders also have an increased risk of REM sleep behavior disorder, or RBD.49 RBD is characterized by vivid and unusual dreams, and physically vigorous sleep behaviors that may result in ecchymoses, lacerations, and fractures.50 Fifty percent of patients with TBI reported insomnia symptoms.51 Disorders in initiating and maintaining sleep were the most common complaints among hospitalized patients with TBI. Some patients with TBI may develop circadian rhythm disturbances.52
Pain
A majority of patients with chronic pain, 50% to 70%, complain of impaired sleep.53 Sleep disruption is so common in fibromyalgia (75%) that it is considered to be a key diagnostic symptom.54 In a study investigating the affect of pain on sleep in burn patients, pain was associated with increased intermittent awakenings and prolonged periods of wake time during the night.55 The following day, these patients had poorer pain tolerance and greater pain intensity. Pain causes sleep fragmentation by increasing cortical arousals. Recent evidence suggests that sleep deprivation can increase pain sensitivity by inhibiting opioid protein synthesis or reducing opioid receptor affinity.56
Psychiatric Disorders
Sleep problems are so common in psychiatric conditions that the Diagnostic and Statistical Manual of Mental Disorders (DMS‐IV‐TR) includes sleep disturbance as a diagnostic criterion for a manic episode, and for various depressive, anxiety, and substance abuse disorders.57 The presence of sleep disturbance in hospitalized patients may suggest the presence of an underlying psychiatric disorder that would otherwise go unrecognized. In a survey of 200 general medical patients in a Brazilian hospital, Rocha et al.58 found that 112 (56.5%) complained of insomnia, and 100 (50%) met criteria for at least 1 psychiatric disorder. However, only 3 out of the total number of 200 surveyed (1.5%) were identified as having psychiatric diagnoses in the medical record, and sleep history was not noted in the clinical evaluation. An episode of major depressive disorder was the most common psychiatric diagnosis (35%). In this study, hospitalized patients with insomnia had a 3.6 times higher risk of having major depressive disorder than inpatients without insomnia.
Insomnia has a profound effect on mental health by worsening health‐related quality of life. In a study of outpatients at family medicine, internal medicine, endocrinology, cardiology, and psychiatry clinics in 3 U.S. cities (n = 3,445), insomnia worsened health‐related quality of life nearly as much as CHF or major depressive disorder did.59 Another survey of outpatients found that those with chronic insomnia were nearly 40 times more likely to have major depression and 6 times more likely to have an anxiety disorder compared to those without insomnia.60 Longitudinal studies have found that prior insomnia was associated with 2‐ to 5‐fold increase in the odds of mood and anxiety disorders and suicide.61, 62 Examining prodromes and precursors to mental disorders, Eaton et al.63 found that 47% of those with onset of depression at the 1‐year follow‐up had sleep problems at baseline.
An estimated 65% of patients with major depression have difficulty falling asleep, frequent awakenings, or early morning awakenings.64 Three patterns of sleep architecture abnormalities have been observed in patients with major depression: 1) sleep continuity disturbances characterized by prolonged sleep‐onset, increased wake time during sleep, increased early morning wake time, and decreased TST; 2) decreased proportion and length of SWS; and 3) REM sleep abnormalities such as reduced time to REM sleep, prolonged first REM sleep episode, and increased REM sleep percentage.65 Sleep during a manic episode has been less studied than in depression, but the data suggest that abnormal sleep in mania includes disrupted sleep continuity, shortened REM latency, and increased REM density (REM eye movement activity/total REM sleep time).65
Substance use disorders are also associated with sleep problems. In a survey by Brower et al.66 of patients who were undergoing alcohol rehabilitation, 61% (n = 172) had symptoms of insomnia such as increased sleep latency during the 6 months prior to entering treatment. Approximately 45% of these patients reported using alcohol for the purpose of initiating sleep. Alcohol and illicit substance intoxication and withdrawal are known to be associated with disrupted sleep. However, sleep disturbances may persist long after withdrawal symptoms have abated. Drummond et al. found that some patients continued to have alcohol‐associated sleep problems even after 27 months of abstinence.67 Evidence also suggests that untreated insomnia and other sleep problems may increase the risk of developing substance abuse problems due to self‐medicating with alcohol and other substances to help with sleep.68
Drugs that Affect Sleep
Numerous drugs can alter sleep quantity and quality. Sedatives and opioids may initially help with sleep onset, but impair sleep architecture. Medications used to treat medical and psychiatric illnesses also disrupt sleep (Table 3). The most common agents that impair sleep include antiepileptic drugs, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, antihypertensives, antihistamines, and corticosteroids.
| Drug Class | Examples of Drugs | Affect on Sleep Architecture | Potential Mechanism | Clinical Implications |
|---|---|---|---|---|
| ||||
| CNS | ||||
| AEDs | Phenobarbital, carbamazepine, phenytoin | Very sedating. AEDs tend to TST, sleep latency | Inhibit neuronal calcium influx, adenosine, or 5HT activity | Sedation is dose‐dependent, and tends to occur with acute use |
| TCAs | Amoxapine, amitriptyline, imipramine, nortriptyline, desipramine, doxepin, clomipramine | Very sedating. Suppresses REM sleep, TST, stage‐2 sleep | Stimulate antimuscarinic‐receptor and alpha1‐receptor | Suppressed REM sleep motor inhibition restlessness, psychomotor agitation during sleep subjectively sleep quality, daytime sleepiness |
| BzRAs | Alprazolam, lorazepam, chlordiazepoxide, diazepam, oxazepam | Very sedating. TST, sleep latency, SWS duration, REM, stage‐2 sleep | Stimulate GABA type A receptor | Minimize daytime use. Chronic BzRAs SWS long‐term sequelae unknown |
| MAOIs | Phenylzine, tranylcypromine | Very sedating. TST, REM, REM rebound if stop MAOIs | Mechanism unknown | Daytime sleepiness; dosing time does not affect daytime somnolence |
| SSRIs | Sedating: paroxetine, fluvoxamine. Activating: fluoxetine, sertraline, citalopram | TST, are less sedating than TCAs and MAOIs. May REM, TWT, TST, SE | 5HT activity | Some patients get the opposite reaction |
| SNRI | Venlafaxine, duloxetine | Activating in some patients; sedating in 12% to 31%. TST | 5HT and NE activity | If activating, switch to AM dosing. If sedating, switch to PM dosing |
| Mood stabilizer | Lithium | Sedating. TST, SWS, REM, REM latency | daytime sedation. Dose at night | |
| Stimulants | Ephedrine, pseudoephedrine, modafinil | Activating. TST, SWS, sleep latency | DOPA, NE, and 5HT activity | Avoid after 6 PM |
| Anti‐Parkinson | Bromocriptine, levodopa | Sedating. Nightmares, SWS | DOPA | Dose at night, if possible |
| Cardiac | ||||
| Lipophilic beta‐blockers | Propranolol, pindolol, metoprolol, timolol. Hydrophilic agents (atenolol and sotalol) lack these effects | Activating. awakenings, TWT, REM, nightmares | CNS beta‐blockade | Lipophilic beta‐blockers daytime sleep when dosed in AM |
| CNS agents | Norepinephrine, epinephrine | Activating. REM, SWS | Stimulate alpha1‐receptor | Minimize use at night |
| Dopamine | Activating. REM, SWS | Stimulate dopamine2‐receptor and alpha1‐receptor | Minimize use at night | |
| Ca++ channel blockers | Amlodipine, verapamil, nifedipine | Exacerbate underlying medical condition | Lower esophageal sphincter tone nocturnal GER sleep disturbance | |
| Alpha2‐receptor agonist | Clonidine | Stage 1, REM, nightmares | Stimulate alpha2‐receptor | Alpha2‐agonists daytime sleep and sleepiness directly. Dose at night |
| Alpha1‐receptor blockers | Doxazosin, prazosin, terazosin | Inhibit alpha1‐receptor | Alpha1‐receptor blockers daytime sleepiness | |
| Diuretics | HCTZ, furosemide | Sedating. | PM diuresis frequent awakenings | |
| Other | ||||
| Opioids | Codeine, morphine | Sedating. SWS, REM | Stimulate mu‐receptor | Minimize use at night |
| NSAIDs | Ibuprofen, indomethcin, celecoxib | TST, SE | Inhibit prostaglandin synthesis | Minimize use at night |
| Methylxanthine | Theophylline | Activating. stage 1, REM | Causes less restful sleep | |
| Antihistamines | Diphenhydramine, promethazine | Sedating | H1 receptor blockade | Minimize use at night |
| Corticosteroids | Dexamethasone, prednisone | Activating. REM, SWS, nightmares | Melatonin secretion | Can disrupt sleep, anxiety, induce mania or psychosis |
| H2 blockers | Cimetidine, ranitidine, famotidine | Sedating. TST | H2 receptor blockade | Sedating if >60 years old, renal impairment |
| Quinolone | Ciprofloxacin, sparfloxacin, ofloxacin, grepafloxacin, levofloxacin | Activating | Stimulate GABA type A receptor | Consider sleep agent after maximizing sleep hygiene. Linezolid rarely causes sleep disturbances |
Lipophilic beta antagonists such as propranolol and timolol can increase total wake time, decrease REM sleep, and increase the incidence of nightmares and insomnia.69 Anabolic steroids and beta‐agonist bronchodilator therapy can cause severe anxiety, sleeplessness, and even psychosis. Vasopressor agents such as dopamine can cause cortical activation, leading to increased arousal and reduced SWS.
Hospital Environment
Environmental noise and patient care activities often interfere with sleep in the hospital. They account for about 30% of patient awakenings in ICU patients.70 Noise levels in the ICU have average sound peaks of 150 to 200 dB, and evening peaks >80 dB between midnight and 6 AM.71 By comparison, the front row seats at a rock concert have sound levels of 110 dB. The high noise level in hospitals has long been implicated as a sleep disruptor,72 but studies in the past decade have found that patient care activities probably contribute more to awakenings than does environmental noise.73 An analysis of critical care nursing routines found that activities such as taking vital signs and giving baths occurred a mean 42.6 times a night per patient.74 Tamburri et al.74 found that patients experienced 2 to 3 hours without interruption on only 6% of the 147 nights studied. Routine daily baths were provided on 55 of the 147 study nights between 2 AM and 5 AM, which is unlikely to be an opportune time for most patients.
CONCLUSION
Hospitalization often prevents patients from achieving adequate sleep and can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing sedative/hypnotic agents, the treatment for sleep disruption includes addressing multiple medical, behavioral, and environmental factors, which will be discussed in Part 2 of this article.
- NIH State‐of‐the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults.NIH Consens Sci Statements.2005;22(2):1–30.
- ,,, et al.Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey.Hypertension.2006;47(5):833–839.
- ,,, et al.Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique.Sleep.2003;26(8):986–989.
- ,,,,.Postural control after a night without sleep.Neuropsychologia.2006;44(12):2520–2525.
- .Sleep deprivation decreases ventilatory responses to CO2 but not load compensation.Chest.1983;84(6):695–698.
- ,,, et al.Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation.J Appl Physiol.2005;98(6):2024–2032.
- .Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats.Am J Physiol Endocrinol Metab.2004;286(6):E1060–E1070.
- ,,,.The metabolic consequences of sleep deprivation.Sleep Med Rev.2007;11(3):163–178.
- ,,,,.Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.Arch Int Med.2006;166:1756–1752.
- ,,,.Effects of sleep and sleep deprivation on immunoglobulins and complement in humans.Brain Behav Immun.2007;21:308–310.
- ,,,,.The effects of sleep deprivation on symptoms of psychopathology in healthy adults.Sleep Med.2007;8:215–221.
- ,.Normal human sleep. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:15–16.
- .Update on the Science, Diagnosis and Management of Insomnia.International Congress and Symposium Series 262.London:Royal Society of Medicine Press Ltd;2006.
- ,,,,,.The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture.Arch Intern Med.2004;164:406–18.
- ,.Sleep disruption in older adults.Am J Nurs.2007;107(5):40–49.
- ,,,.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey.J Psychosom Res.2004;56(5):497–502.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Insomnia and its treatment: prevalence and correlates.Arch Gen Psychiatry.1985;42(3):225–232.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117:809–818.
- ,.Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill.Crit Care Med.2006;22:313–327.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,,,.Failure of physician documentation of sleep complaints in hospitalized patients.West J Med.1998;169:146–149.
- .Sleep and medical disorders.Prim Care.2005;35:511–533.
- ,,,,,.The occurrence of sleep‐disordered breathing among middle‐aged adults.N Engl J Med.1993;328:1230–1235.
- ,.Clinical features and evaluation of obstructive sleep apnea‐hypopnea syndrome. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:869–872.
- ,,.Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea.Arch Intern Med.2003;163:41–45.
- ,.Sleep and medical disorders.Med Clin North Am.2004;88:679–703.
- ,,,.Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness.Atherosclerosis.2008;197(1):125– 131.
- ,,, et al.Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med.2001;163:19–25.
- .Gastroesophageal reflux during sleep.Sleep Med Clin.2007;2:41–50.
- ,.Sleep apnea in renal failure.Adv Perit Dial.1997;13:88–92.
- .Sleep in chronic obstructive pulmonary disease.Sleep Med Clin.2007;2:1–8.
- ,,,.Effects of non‐invasive positive pressure ventilation on gas exchange and sleep in COPD patients.Chest.1997;112:623–628.
- ,,, et al.Night‐to‐night variability in CPAP use over the first three months of treatment.Sleep.1997;20(4):278–283.
- ,.Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS).Sleep Med Rev.2003;7(1):81–99.
- ,,.Factors predicting sleep disruption in type II diabetes.Sleep.2000;23:415–416.
- ,,.Sleep duration as a risk factor for the development of type 2 diabetes.Diabetes Care.2006;29:657–661.
- .Sleep disorders and end‐stage renal disease.Sleep Med Clin.2007;2:59–66.
- ,,,.Sleep and aging: 1. Sleep disorders commonly found in older people.CMAJ.2007;176(9):1299–1304.
- ,,,,,.Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS Epidemiology, Symptoms, and Treatment) primary care study.Sleep Med.2004;5(3):237–246.
- ,.Epidemiology and clinical findings of restless leg syndrome.Sleep Med.2004;5(3):293–299.
- ,,,,,.High prevalence of sleep disturbance in cirrhosis.Hepatology.1998;27:339–345.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Obstructive sleep apnea: implications for cardiac and vascular disease.JAMA.2003;290(14):1906–1914.
- ,,, et al.Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.Brain.2007;130(Pt 11):2770–2788.
- ,.REM sleep parasomnias. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:724–725.
- ,,.Insomnia in patients with traumatic brain injury.J Head Trauma Rehabil.2006;21(3):199–212.
- ,,,,.Circadian rhythm sleep disorders following mild traumatic brain injury.Neurology.2007;68(14):1136–1140.
- ,.Comorbidities: psychiatric, medical, medications, and substances.Sleep Med Clin.2006;231–245.
- ,,.Sleep disturbance and fibromyalgia.Sleep Med Clin.2007;2:31–39.
- ,,.Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries.Sleep Med.2004;5:551–559.
- ,,.Sleep deprivation and pain perception.Sleep Med Rev.2006;10:357–369.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision.Washington, DC:American Psychiatric Association;2000.
- ,,, et al.Is insomnia a marker for psychiatric disorders in general hospitals?Sleep Med.2005;6:549–553.
- ,.The relationship between insomnia and health‐related quality of life in patients with chronic illness.J Fam Pract.2002;51(3):229–235.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,.Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults.Biol Psychiatry.1996;39:411–418.
- ,,,.The morbidity of insomnia uncomplicated by psychiatric disorders.Gen Hosp Psychiatry.1997;19:245–250.
- ,,.Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset.Am J Psychiatry.1995;152:967–972.
- ,,,,,.Which depressive symptoms are related to which sleep electroencephalographic variables?Biol Psychol.1997;42:904–913.
- ,.Sleep in mood disorders.Psychiatr Clin North Am.2006;29:1009–1032.
- ,,,,.Insomnia, self‐medication, and relapse to alcoholism.Am J Psychiatry.2001;158:399–404.
- ,,,.The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse.Alcohol Clin Exp Res.1998;22:1796–1802.
- ,,,,.Screening for substance use patterns among patients referred for a variety of sleep complaints.Am J Drug Alcohol Abuse.2006;32:111–120.
- .Drugs that disturb sleep and wakefulness. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:441–462.
- ,,, et al.Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects.Am J Respir Crit Care Med.2003;167(5):708–715.
- ,,.Adverse environmental conditions in the respiratory and medical ICU settings.Chest.1994;105:1211–1216.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118(6):3629–3645.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,,.Nocturnal care interactions with patients in critical care units.Am J Crit Care.2004;13(2):102–115.
Hospitalized patients often have difficulty initiating and maintaining sleep, or complain of early awakening and nonrestorative sleep.1 The etiology of sleep disruption is multifactorial and includes the patient's underlying illness(es), medical treatments, and the hospital environment. Often unrecognized and untreated during hospitalization, sleep disruption may lead to sleep deprivation, or a chronic lack of restorative sleep.
Even in healthy individuals, sleep deprivation can result in numerous physical and psychological consequences. Sleep deprivation is associated with hypertension,2, 3 impaired postural control,4 decreased ventilatory drive,5 increased sympathetic cardiovascular activation,6 blunted hypothalamic‐pituitary‐adrenal axis,7 impaired host defenses, and possibly diabetes mellitus and obesity.810 The lack of restorative sleep increases the risk of developing anxiety and mood disorders and delirium, especially in acutely ill older patients.11 In the presence of acute physical infirmity, inadequate sleep may further compound illness and impair recovery. We provide an overview of normal sleep architecture and discuss factors that lead to sleep disruption in hospitalized medical patients.
NORMAL SLEEP ARCHITECTURE AND REGULATION
Normal sleep architecture refers to a characteristic pattern of sleep, and consists of two major stages: nonrapid eye movement (NREM, pronounced non‐rem) and rapid eye movement (REM). (For a table of pertinent abbreviations and terms, see Table 1) Sleep is quantified by polysomnography (PSG), which includes an electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). A PSG also includes an electrocardiogram (ECG), and measures of airflow, oxygen saturation, and body position. NREM sleep comprises 75% to 80% of total sleep time (TST), and is characterized by relatively quiescent brain activity and decreased metabolic rate.12 NREM sleep consists of four stages (S1‐S4), with each stage leading to a progressively deeper sleep (Figure 1). REM sleep follows slow wave sleep (SWS), or deep sleep, and increases over the night, comprising 20% to 25% of TST. REM sleep is characterized by an activated EEG pattern, muscle atonia, and episodic bursts of rapid eye movements.

| Acronym | Term |
|---|---|
| BiPAP | Bilevel positive airway pressure |
| CHF | Congestive heart failure |
| CPAP | Continuous positive airway pressure |
| COPD | Chronic obstructive pulmonary disease |
| EEG | Electroencephalogram |
| EOG | Electroculogram |
| EMG | Electromyogram |
| ESRD | End‐stage renal disease |
| NPPV | Noninvasive positive pressure ventilation |
| NREM | Nonrapid eye movement |
| OSA | Obstructive sleep apnea |
| PLMD | Periodic limb movement disorder |
| PSG | Polysomnography |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement |
| RLS | Restless leg syndrome |
| S1‐S4 | 4 Stages of sleep in NREM |
| SE | Sleep efficiency; TST divided by total time in bed |
| SWS | Slow wave sleep |
| TBI | Traumatic brain injury |
| TST | Total sleep time |
Normal sleep provides a period of physiologic and mental rest. During sleep, sympathetic tone decreases and parasympathetic tone increases, leading to a reduction in heart rate, arterial blood pressure, and cardiac output.13 Deep sleep is theorized to be necessary for physiologic restoration. REM sleep is associated with dreaming, and is essential for maintaining emotional and cognitive well‐being. Sleep architecture undergoes characteristic changes as people age.14 The duration of SWS peaks in childhood and decreases with age. Consequently, people >60 years old tend to have lower arousal thresholds and to have more frequent awakenings. The results of the Sleep Heart Health Study found that increased age was associated with decreased percentage of REM sleep, worse sleep efficiency (SE, which is TST divided by total time in bed), and lower arousal thresholds.14 With the reduction of SE, older people need to spend more hours in bed to achieve the same amount of restorative sleep as when they were younger. Although sleep tends to become more disrupted as people age, insomnia should not be considered a normal part of aging, and needs to be addressed clinically.15 The results of a National Sleep Foundation telephone survey of subjects between the ages of 55 and 84 years old (n = 1,506) suggested that sleep complaints in older adults are frequently secondary to comorbid medical conditions.16
Multiple anatomic structures, pathways, and neurotransmitter systems are involved in controlling wakefulness and sleep. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Sleep‐promoting neurotransmitters include gamma aminobutyric acid (GABA), adenosine, and melatonin. Specific stages of sleep are regulated by the turning on and off of various neurons. REM on cells use GABA, acetylcholine, and glutamine, whereas REM off cells use norepinephrine and serotonin. SWS is promoted by GABA and serotonin.17
Sleep regulation is a balance between a homeostatic sleep need and an intrinsic body clock, or circadian pacemaker. Located in the suprachiasmic nucleus, the circadian pacemaker determines the onset and termination of sleep, and is partially regulated by environmental cues such as light and ambient temperature. Melatonin, a physiologic sleep promoter, is inhibited by ambient light, and its circulation is decreased during daylight hours. The adrenal secretion of cortisol, which is associated with wakefulness, follows a circadian pattern. Regulated by the hypothalamic‐pituitary axis, cortisol levels peak in the early morning hours in preparation for the increased metabolic demands during wakefulness.
SLEEP PROBLEMS IN HOSPITALIZED PATIENTS
Insomnia, which is characterized by difficulty initiating or maintaining sleep, is the most common sleep disorder in the United States. About one‐third of the adult population in the United States experiences insomnia at some point in their lives,18 and it is a persistent problem in approximately 10% of U.S. adults.19 Insomnia can be exacerbated during hospitalization.
Studies investigating sleep in hospitalized patients using PSG have been limited primarily to the setting of the intensive care unit (ICU). Critically ill patients, particularly those requiring mechanical ventilation, are prone to sleep disturbances and an associated delirium.2022 Critically ill patients have fragmented sleep, with decreased SE and SWS, and increased S1 and S2.23 Physician awareness of the impact of sleep disturbance in hospitalized patients is vital. Surveys reveal that approximately one‐half of patients admitted on general medical wards complain of sleep disruption.24, 25 Meissner et al.25 examined the prevalence of sleep complaints and physician recognition of these complaints among general medical patients admitted to a Veterans Affairs tertiary care center. Results showed that 47% (n = 222) of patients had complaints of either insomnia and/or excessive daytime sleepiness.
FACTORS AFFECTING SLEEP DURING HOSPITALIZATION
Many medical and neurologic illnesses, psychiatric disorders, pain, medication therapy and the hospital environment may impair sleep, and hinder recovery from illness.
General Medical Disorders
Primary sleep disorders, such as obstructive sleep apnea (OSA) and numerous other medical illnesses, can directly impair sleep physiology, leading to a cyclical interaction (Figure 2). Other conditions that disrupt sleep include congestive heart failure (CHF), diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux, cardiovascular disease, thyroid disorders, renal disease, and severe liver disease.26 Table 2 lists selected medical and neurological conditions, their associated sleep‐related problems, and suggestions on how to ameliorate these problems.

| Disease | Problem | Clinical Implications and Strategies to Improve Sleep |
|---|---|---|
| ||
| Asthma | Nocturnal exacerbation, nocturnal GER | Inhaled corticosteroids and/or long‐acting inhaled beta‐adrenergic agents |
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep‐disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne‐Stokes respiration | Keep the head of bed elevated 30 degrees. Nocturnal O2 to keep O2 saturation >88%. Daytime diuresis. Optimize cardiac function to treat Cheyne‐Stokes respiration. Consider CPAP for CHF |
| COPD | Persistent nocturnal hypoxemia with complications (e.g., cor pulmonale, polycythemia) | O2 for COPD and persistent hypoxemia (PaO2 55‐60 mm Hg) |
| Sporadic nighttime desaturations | PaO2 55 mm Hg monitor O2 saturation by pulse oximetry. If patient desaturates to 88% at night consistently, start nocturnal O2. For hypercapnia, adjust O2 to maintain O2 saturation at 88% to 90% | |
| Early‐morning airflow obstruction | Consider bedtime tiotropium and inhaled long‐acting beta‐adrenergic agonist agents | |
| Inhibition of respiratory muscles in REM | Avoid sedative‐hypnotics that cause respiratory depression | |
| Decreased functional residual capacity from recumbent position during sleep | Minimize recumbancy by keeping the head of bed up at 30 degrees | |
| End‐stage renal disease | Pruritus, nausea; increased risk of RLS and PLMD | Ambulation may help with RLS. Consider ropinirole and pramipexole. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents |
| Nocturnal GER | Nocturnal GER decreased sleep, heartburn, coughing, asthma | Avoid eating or drinking 2 hours before bedtime, especially those that delay gastric emptying, increase acid secretion, or decrease lower esophageal sphincter pressure; e.g., high‐fat foods, ethanol, chocolate, peppers, peppermint. Keep head of bed 30 degrees. Minimize medications that could worsen nocturnal GER; e.g., theophylline, calcium channel blockers, prostaglandins, bisphosphonates |
| OSA | Snoring with upper airway obstruction | No ethanol 2 hours before bedtime. Minimize CNS depressants. Avoid supine position. Consider CPAP, oral mandibular advancement device, and/or surgical correction. Long‐term plan should include weight loss |
| Stroke | Focal neurologic deficits (e.g., dysphagia, weakness or paralysis) | Keep head of bed 30 degrees. Regularly suction secretions. Post‐stroke patients have an increased risk of hypersomnia, insomnia, and/or OSA |
Affecting approximately 24% of men and 9% of women in the United States, OSA is the most common primary sleep disorder,27, 28 and causes significant mental and physical morbidity. Risk factors for OSA include obesity, hypothyroidism‐induced muscle weakness, and structural abnormalities in the oropharynx region such as acromegaly, micrognathia, or retrognathia. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness.28 These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment (Figure 2). In addition, chronic sympathetic activation causes numerous derangements in the vascular endothelium and platelet activation.29, 30 Sleep‐disordered breathing has been independently associated with cardiovascular diseases such as hypertension, CHF, ischemic heart disease, atrial fibrillation, and cerebrovascular disease.31, 32
OSA is also associated with sleep‐related gastroesophageal reflux, which is characterized by pain and nocturnal cough, and can induce nocturnal asthma attacks and laryngospasm.33 Green et al.29 found that OSA patients treated with continuous positive airway pressure (CPAP) had a 48% improvement in nocturnal reflux symptoms. Although the pathophysiology connecting OSA to the renal system is unknown, OSA has been found in up to 60% of patients with end‐stage renal disease and chronic renal failure.34
Patients with pulmonary disorders can be profoundly affected by the normal physiologic changes during sleep, particularly in REM sleep. During REM sleep, all respiratory muscles except the diaphragm become paralyzed. Thus, episodes of marked oxygen desaturation can occur in patients who rely on their accessory muscles for respiration. COPD patients have decreased TST, SWS, and REM sleep. Shortness of breath, nocturnal cough, and wheezing worsen sleep.35 The resulting fatigue and sleep deprivation negatively impact the work of breathing and impair gas exchange. Airflow obstruction tends to worsen in the early morning hours in patients with COPD and asthma, and may be related to the effect of REM on the accessory muscles for respiration. Although used to target CO2 retention, investigations using bilevel positive airway pressure ventilators (BiPAP) for improving sleep in COPD patients have been limited. Noninvasive positive pressure ventilation (NPPV) appears to acutely improve SE and TST in patients with hypercapnic COPD without significantly improving gas exchange. Other sleep parameters such as sleep architecture and the number of arousals during the night, remain unchanged during NPPV.36
CPAP has several side effects that could worsen sleep, which may explain its poor adherence rate among ambulatory patients.37 Side effects include nasal bridge discomfort, nasal congestion, swallowing air, dry nose, dry or red eyes, noise, ear pain, and rhinitis.38 During hospitalization, efforts should be made to improve patient comfort by resizing ill‐fitting masks, adding heated humidification or nasal steroids to alleviate nasal congestion, or adding a chin strap to reduce air leak and ingestion of air.
Endocrine disorders have also been associated with sleep disruption. Studies suggest that patients with diabetes mellitus have decreased TST and impaired sleep quality due to nocturia and neuropathic pain.39 Inadequate sleep may also affect glucose control. Inadequate quality or quantity of sleep has been shown to be a risk factor for developing Type 2 diabetes mellitus in large prospective studies.40 Sleep duration and quality were significant predictors of increased levels of glycosylated hemoglobin (HbA1c) in patients with Type 2 diabetes mellitus. Thyroid diseases often coexist with diabetes mellitus. Both hypo‐ and hyperthyroidism have been associated with sleep disruption. Hypothyroidism is associated with daytime somnolence and fatigue. Patients with hypothyroidism tend to have reduced SWS. Hyperthyroid patients often complain of insomnia, which has been attributed to a hypermetabolic state.
Approximately 50% of patients with chronic end‐stage renal disease (ESRD) have insomnia and other sleep disorders.41 Patients often complain of restless leg syndrome (RLS), periodic limb movement disorder (PLMD), bone pain, nausea, and pruritus. The etiology of sleep disorders appears to be related to metabolic derangements associated with ESRD or from coexisting diabetes mellitus.
RLS and PLMD are distinct problems that affect sleep differently. RLS is characterized by an unpleasant crampy, creeping or crawling sensation in the lower extremities that is relieved by movement of the legs.42 RLS symptoms typically occur soon after going to bed, and therefore tend to disrupt sleep onset. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems.43 Since RLS may partially be caused by disrupted iron metabolism, serum ferritin levels should be evaluated.44 Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, ESRD, and Parkinson's disease. The differential diagnosis for RLS and PLMD includes neuroleptic‐induced akathisia, peripheral neuropathy, and positional or nocturnal leg cramps. PLMD occurs in about 80% of those with RLS, and is characterized by involuntary limb movements that occur every 20 to 40 seconds during NREM sleep. Unaware of these movements, patients often experience frequent arousals throughout the night, and complain of daytime somnolence and fatigue.42
A pilot study of 35 patients with minimal hepatic encephalopathy found that nearly 50% complained of sleep difficulties.45 Hypothesizing that a dysregulation of histaminergic neurotransmission in cirrhosis alters the sleep‐wake cycle, Spahr et al.46 found that 40% of their patients reported subjective improvement in sleep when administered 25 mg of hydroxyzine, compared to none who received placebo.
Neurologic Disorders
Since the brain and its various neurotransmitter systems are critical in regulating sleep and wakefulness, patients with neurologic disorders have an increased risk of developing sleep disorders. Patients with dementia, other neurodegenerative disorders, epilepsy, and traumatic brain injury (TBI) have a higher prevalence of sleep disturbance and sleep disorders.47 Poststroke patients can develop insomnia or hypersomnia, a reduction in sleep latency, increased sleep, or excessive daytime sleepiness, and are at higher risk for OSA during the first several months after a stroke.48 Specific neurologic lesions may lead to uncommon problems such as inversion of the sleep‐wake cycle, parasomnias, and hallucinatory dream‐like states.
Both Parkinson's disease and Alzheimer's disease are associated with multiple sleep disturbances, which tend to worsen with disease progression.14 Common problems include increased sleep fragmentation and wakefulness, with increases of stage 1 sleep and reductions of SWS and REM. Patients with neurodegenerative disorders also have an increased risk of REM sleep behavior disorder, or RBD.49 RBD is characterized by vivid and unusual dreams, and physically vigorous sleep behaviors that may result in ecchymoses, lacerations, and fractures.50 Fifty percent of patients with TBI reported insomnia symptoms.51 Disorders in initiating and maintaining sleep were the most common complaints among hospitalized patients with TBI. Some patients with TBI may develop circadian rhythm disturbances.52
Pain
A majority of patients with chronic pain, 50% to 70%, complain of impaired sleep.53 Sleep disruption is so common in fibromyalgia (75%) that it is considered to be a key diagnostic symptom.54 In a study investigating the affect of pain on sleep in burn patients, pain was associated with increased intermittent awakenings and prolonged periods of wake time during the night.55 The following day, these patients had poorer pain tolerance and greater pain intensity. Pain causes sleep fragmentation by increasing cortical arousals. Recent evidence suggests that sleep deprivation can increase pain sensitivity by inhibiting opioid protein synthesis or reducing opioid receptor affinity.56
Psychiatric Disorders
Sleep problems are so common in psychiatric conditions that the Diagnostic and Statistical Manual of Mental Disorders (DMS‐IV‐TR) includes sleep disturbance as a diagnostic criterion for a manic episode, and for various depressive, anxiety, and substance abuse disorders.57 The presence of sleep disturbance in hospitalized patients may suggest the presence of an underlying psychiatric disorder that would otherwise go unrecognized. In a survey of 200 general medical patients in a Brazilian hospital, Rocha et al.58 found that 112 (56.5%) complained of insomnia, and 100 (50%) met criteria for at least 1 psychiatric disorder. However, only 3 out of the total number of 200 surveyed (1.5%) were identified as having psychiatric diagnoses in the medical record, and sleep history was not noted in the clinical evaluation. An episode of major depressive disorder was the most common psychiatric diagnosis (35%). In this study, hospitalized patients with insomnia had a 3.6 times higher risk of having major depressive disorder than inpatients without insomnia.
Insomnia has a profound effect on mental health by worsening health‐related quality of life. In a study of outpatients at family medicine, internal medicine, endocrinology, cardiology, and psychiatry clinics in 3 U.S. cities (n = 3,445), insomnia worsened health‐related quality of life nearly as much as CHF or major depressive disorder did.59 Another survey of outpatients found that those with chronic insomnia were nearly 40 times more likely to have major depression and 6 times more likely to have an anxiety disorder compared to those without insomnia.60 Longitudinal studies have found that prior insomnia was associated with 2‐ to 5‐fold increase in the odds of mood and anxiety disorders and suicide.61, 62 Examining prodromes and precursors to mental disorders, Eaton et al.63 found that 47% of those with onset of depression at the 1‐year follow‐up had sleep problems at baseline.
An estimated 65% of patients with major depression have difficulty falling asleep, frequent awakenings, or early morning awakenings.64 Three patterns of sleep architecture abnormalities have been observed in patients with major depression: 1) sleep continuity disturbances characterized by prolonged sleep‐onset, increased wake time during sleep, increased early morning wake time, and decreased TST; 2) decreased proportion and length of SWS; and 3) REM sleep abnormalities such as reduced time to REM sleep, prolonged first REM sleep episode, and increased REM sleep percentage.65 Sleep during a manic episode has been less studied than in depression, but the data suggest that abnormal sleep in mania includes disrupted sleep continuity, shortened REM latency, and increased REM density (REM eye movement activity/total REM sleep time).65
Substance use disorders are also associated with sleep problems. In a survey by Brower et al.66 of patients who were undergoing alcohol rehabilitation, 61% (n = 172) had symptoms of insomnia such as increased sleep latency during the 6 months prior to entering treatment. Approximately 45% of these patients reported using alcohol for the purpose of initiating sleep. Alcohol and illicit substance intoxication and withdrawal are known to be associated with disrupted sleep. However, sleep disturbances may persist long after withdrawal symptoms have abated. Drummond et al. found that some patients continued to have alcohol‐associated sleep problems even after 27 months of abstinence.67 Evidence also suggests that untreated insomnia and other sleep problems may increase the risk of developing substance abuse problems due to self‐medicating with alcohol and other substances to help with sleep.68
Drugs that Affect Sleep
Numerous drugs can alter sleep quantity and quality. Sedatives and opioids may initially help with sleep onset, but impair sleep architecture. Medications used to treat medical and psychiatric illnesses also disrupt sleep (Table 3). The most common agents that impair sleep include antiepileptic drugs, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, antihypertensives, antihistamines, and corticosteroids.
| Drug Class | Examples of Drugs | Affect on Sleep Architecture | Potential Mechanism | Clinical Implications |
|---|---|---|---|---|
| ||||
| CNS | ||||
| AEDs | Phenobarbital, carbamazepine, phenytoin | Very sedating. AEDs tend to TST, sleep latency | Inhibit neuronal calcium influx, adenosine, or 5HT activity | Sedation is dose‐dependent, and tends to occur with acute use |
| TCAs | Amoxapine, amitriptyline, imipramine, nortriptyline, desipramine, doxepin, clomipramine | Very sedating. Suppresses REM sleep, TST, stage‐2 sleep | Stimulate antimuscarinic‐receptor and alpha1‐receptor | Suppressed REM sleep motor inhibition restlessness, psychomotor agitation during sleep subjectively sleep quality, daytime sleepiness |
| BzRAs | Alprazolam, lorazepam, chlordiazepoxide, diazepam, oxazepam | Very sedating. TST, sleep latency, SWS duration, REM, stage‐2 sleep | Stimulate GABA type A receptor | Minimize daytime use. Chronic BzRAs SWS long‐term sequelae unknown |
| MAOIs | Phenylzine, tranylcypromine | Very sedating. TST, REM, REM rebound if stop MAOIs | Mechanism unknown | Daytime sleepiness; dosing time does not affect daytime somnolence |
| SSRIs | Sedating: paroxetine, fluvoxamine. Activating: fluoxetine, sertraline, citalopram | TST, are less sedating than TCAs and MAOIs. May REM, TWT, TST, SE | 5HT activity | Some patients get the opposite reaction |
| SNRI | Venlafaxine, duloxetine | Activating in some patients; sedating in 12% to 31%. TST | 5HT and NE activity | If activating, switch to AM dosing. If sedating, switch to PM dosing |
| Mood stabilizer | Lithium | Sedating. TST, SWS, REM, REM latency | daytime sedation. Dose at night | |
| Stimulants | Ephedrine, pseudoephedrine, modafinil | Activating. TST, SWS, sleep latency | DOPA, NE, and 5HT activity | Avoid after 6 PM |
| Anti‐Parkinson | Bromocriptine, levodopa | Sedating. Nightmares, SWS | DOPA | Dose at night, if possible |
| Cardiac | ||||
| Lipophilic beta‐blockers | Propranolol, pindolol, metoprolol, timolol. Hydrophilic agents (atenolol and sotalol) lack these effects | Activating. awakenings, TWT, REM, nightmares | CNS beta‐blockade | Lipophilic beta‐blockers daytime sleep when dosed in AM |
| CNS agents | Norepinephrine, epinephrine | Activating. REM, SWS | Stimulate alpha1‐receptor | Minimize use at night |
| Dopamine | Activating. REM, SWS | Stimulate dopamine2‐receptor and alpha1‐receptor | Minimize use at night | |
| Ca++ channel blockers | Amlodipine, verapamil, nifedipine | Exacerbate underlying medical condition | Lower esophageal sphincter tone nocturnal GER sleep disturbance | |
| Alpha2‐receptor agonist | Clonidine | Stage 1, REM, nightmares | Stimulate alpha2‐receptor | Alpha2‐agonists daytime sleep and sleepiness directly. Dose at night |
| Alpha1‐receptor blockers | Doxazosin, prazosin, terazosin | Inhibit alpha1‐receptor | Alpha1‐receptor blockers daytime sleepiness | |
| Diuretics | HCTZ, furosemide | Sedating. | PM diuresis frequent awakenings | |
| Other | ||||
| Opioids | Codeine, morphine | Sedating. SWS, REM | Stimulate mu‐receptor | Minimize use at night |
| NSAIDs | Ibuprofen, indomethcin, celecoxib | TST, SE | Inhibit prostaglandin synthesis | Minimize use at night |
| Methylxanthine | Theophylline | Activating. stage 1, REM | Causes less restful sleep | |
| Antihistamines | Diphenhydramine, promethazine | Sedating | H1 receptor blockade | Minimize use at night |
| Corticosteroids | Dexamethasone, prednisone | Activating. REM, SWS, nightmares | Melatonin secretion | Can disrupt sleep, anxiety, induce mania or psychosis |
| H2 blockers | Cimetidine, ranitidine, famotidine | Sedating. TST | H2 receptor blockade | Sedating if >60 years old, renal impairment |
| Quinolone | Ciprofloxacin, sparfloxacin, ofloxacin, grepafloxacin, levofloxacin | Activating | Stimulate GABA type A receptor | Consider sleep agent after maximizing sleep hygiene. Linezolid rarely causes sleep disturbances |
Lipophilic beta antagonists such as propranolol and timolol can increase total wake time, decrease REM sleep, and increase the incidence of nightmares and insomnia.69 Anabolic steroids and beta‐agonist bronchodilator therapy can cause severe anxiety, sleeplessness, and even psychosis. Vasopressor agents such as dopamine can cause cortical activation, leading to increased arousal and reduced SWS.
Hospital Environment
Environmental noise and patient care activities often interfere with sleep in the hospital. They account for about 30% of patient awakenings in ICU patients.70 Noise levels in the ICU have average sound peaks of 150 to 200 dB, and evening peaks >80 dB between midnight and 6 AM.71 By comparison, the front row seats at a rock concert have sound levels of 110 dB. The high noise level in hospitals has long been implicated as a sleep disruptor,72 but studies in the past decade have found that patient care activities probably contribute more to awakenings than does environmental noise.73 An analysis of critical care nursing routines found that activities such as taking vital signs and giving baths occurred a mean 42.6 times a night per patient.74 Tamburri et al.74 found that patients experienced 2 to 3 hours without interruption on only 6% of the 147 nights studied. Routine daily baths were provided on 55 of the 147 study nights between 2 AM and 5 AM, which is unlikely to be an opportune time for most patients.
CONCLUSION
Hospitalization often prevents patients from achieving adequate sleep and can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing sedative/hypnotic agents, the treatment for sleep disruption includes addressing multiple medical, behavioral, and environmental factors, which will be discussed in Part 2 of this article.
Hospitalized patients often have difficulty initiating and maintaining sleep, or complain of early awakening and nonrestorative sleep.1 The etiology of sleep disruption is multifactorial and includes the patient's underlying illness(es), medical treatments, and the hospital environment. Often unrecognized and untreated during hospitalization, sleep disruption may lead to sleep deprivation, or a chronic lack of restorative sleep.
Even in healthy individuals, sleep deprivation can result in numerous physical and psychological consequences. Sleep deprivation is associated with hypertension,2, 3 impaired postural control,4 decreased ventilatory drive,5 increased sympathetic cardiovascular activation,6 blunted hypothalamic‐pituitary‐adrenal axis,7 impaired host defenses, and possibly diabetes mellitus and obesity.810 The lack of restorative sleep increases the risk of developing anxiety and mood disorders and delirium, especially in acutely ill older patients.11 In the presence of acute physical infirmity, inadequate sleep may further compound illness and impair recovery. We provide an overview of normal sleep architecture and discuss factors that lead to sleep disruption in hospitalized medical patients.
NORMAL SLEEP ARCHITECTURE AND REGULATION
Normal sleep architecture refers to a characteristic pattern of sleep, and consists of two major stages: nonrapid eye movement (NREM, pronounced non‐rem) and rapid eye movement (REM). (For a table of pertinent abbreviations and terms, see Table 1) Sleep is quantified by polysomnography (PSG), which includes an electroencephalogram (EEG), electromyogram (EMG), and electrooculogram (EOG). A PSG also includes an electrocardiogram (ECG), and measures of airflow, oxygen saturation, and body position. NREM sleep comprises 75% to 80% of total sleep time (TST), and is characterized by relatively quiescent brain activity and decreased metabolic rate.12 NREM sleep consists of four stages (S1‐S4), with each stage leading to a progressively deeper sleep (Figure 1). REM sleep follows slow wave sleep (SWS), or deep sleep, and increases over the night, comprising 20% to 25% of TST. REM sleep is characterized by an activated EEG pattern, muscle atonia, and episodic bursts of rapid eye movements.

| Acronym | Term |
|---|---|
| BiPAP | Bilevel positive airway pressure |
| CHF | Congestive heart failure |
| CPAP | Continuous positive airway pressure |
| COPD | Chronic obstructive pulmonary disease |
| EEG | Electroencephalogram |
| EOG | Electroculogram |
| EMG | Electromyogram |
| ESRD | End‐stage renal disease |
| NPPV | Noninvasive positive pressure ventilation |
| NREM | Nonrapid eye movement |
| OSA | Obstructive sleep apnea |
| PLMD | Periodic limb movement disorder |
| PSG | Polysomnography |
| RBD | REM sleep behavior disorder |
| REM | Rapid eye movement |
| RLS | Restless leg syndrome |
| S1‐S4 | 4 Stages of sleep in NREM |
| SE | Sleep efficiency; TST divided by total time in bed |
| SWS | Slow wave sleep |
| TBI | Traumatic brain injury |
| TST | Total sleep time |
Normal sleep provides a period of physiologic and mental rest. During sleep, sympathetic tone decreases and parasympathetic tone increases, leading to a reduction in heart rate, arterial blood pressure, and cardiac output.13 Deep sleep is theorized to be necessary for physiologic restoration. REM sleep is associated with dreaming, and is essential for maintaining emotional and cognitive well‐being. Sleep architecture undergoes characteristic changes as people age.14 The duration of SWS peaks in childhood and decreases with age. Consequently, people >60 years old tend to have lower arousal thresholds and to have more frequent awakenings. The results of the Sleep Heart Health Study found that increased age was associated with decreased percentage of REM sleep, worse sleep efficiency (SE, which is TST divided by total time in bed), and lower arousal thresholds.14 With the reduction of SE, older people need to spend more hours in bed to achieve the same amount of restorative sleep as when they were younger. Although sleep tends to become more disrupted as people age, insomnia should not be considered a normal part of aging, and needs to be addressed clinically.15 The results of a National Sleep Foundation telephone survey of subjects between the ages of 55 and 84 years old (n = 1,506) suggested that sleep complaints in older adults are frequently secondary to comorbid medical conditions.16
Multiple anatomic structures, pathways, and neurotransmitter systems are involved in controlling wakefulness and sleep. Neurotransmitters that promote wakefulness include acetylcholine, histamine, noradrenaline (norepinephrine), serotonin, dopamine, and hypocretin (orexin). Sleep‐promoting neurotransmitters include gamma aminobutyric acid (GABA), adenosine, and melatonin. Specific stages of sleep are regulated by the turning on and off of various neurons. REM on cells use GABA, acetylcholine, and glutamine, whereas REM off cells use norepinephrine and serotonin. SWS is promoted by GABA and serotonin.17
Sleep regulation is a balance between a homeostatic sleep need and an intrinsic body clock, or circadian pacemaker. Located in the suprachiasmic nucleus, the circadian pacemaker determines the onset and termination of sleep, and is partially regulated by environmental cues such as light and ambient temperature. Melatonin, a physiologic sleep promoter, is inhibited by ambient light, and its circulation is decreased during daylight hours. The adrenal secretion of cortisol, which is associated with wakefulness, follows a circadian pattern. Regulated by the hypothalamic‐pituitary axis, cortisol levels peak in the early morning hours in preparation for the increased metabolic demands during wakefulness.
SLEEP PROBLEMS IN HOSPITALIZED PATIENTS
Insomnia, which is characterized by difficulty initiating or maintaining sleep, is the most common sleep disorder in the United States. About one‐third of the adult population in the United States experiences insomnia at some point in their lives,18 and it is a persistent problem in approximately 10% of U.S. adults.19 Insomnia can be exacerbated during hospitalization.
Studies investigating sleep in hospitalized patients using PSG have been limited primarily to the setting of the intensive care unit (ICU). Critically ill patients, particularly those requiring mechanical ventilation, are prone to sleep disturbances and an associated delirium.2022 Critically ill patients have fragmented sleep, with decreased SE and SWS, and increased S1 and S2.23 Physician awareness of the impact of sleep disturbance in hospitalized patients is vital. Surveys reveal that approximately one‐half of patients admitted on general medical wards complain of sleep disruption.24, 25 Meissner et al.25 examined the prevalence of sleep complaints and physician recognition of these complaints among general medical patients admitted to a Veterans Affairs tertiary care center. Results showed that 47% (n = 222) of patients had complaints of either insomnia and/or excessive daytime sleepiness.
FACTORS AFFECTING SLEEP DURING HOSPITALIZATION
Many medical and neurologic illnesses, psychiatric disorders, pain, medication therapy and the hospital environment may impair sleep, and hinder recovery from illness.
General Medical Disorders
Primary sleep disorders, such as obstructive sleep apnea (OSA) and numerous other medical illnesses, can directly impair sleep physiology, leading to a cyclical interaction (Figure 2). Other conditions that disrupt sleep include congestive heart failure (CHF), diabetes mellitus, chronic obstructive pulmonary disease (COPD), gastroesophageal reflux, cardiovascular disease, thyroid disorders, renal disease, and severe liver disease.26 Table 2 lists selected medical and neurological conditions, their associated sleep‐related problems, and suggestions on how to ameliorate these problems.

| Disease | Problem | Clinical Implications and Strategies to Improve Sleep |
|---|---|---|
| ||
| Asthma | Nocturnal exacerbation, nocturnal GER | Inhaled corticosteroids and/or long‐acting inhaled beta‐adrenergic agents |
| CHF | Orthopnea, paroxysmal nocturnal dyspnea, sleep‐disordered breathing, increased sympathetic tone, nighttime diuresis, Cheyne‐Stokes respiration | Keep the head of bed elevated 30 degrees. Nocturnal O2 to keep O2 saturation >88%. Daytime diuresis. Optimize cardiac function to treat Cheyne‐Stokes respiration. Consider CPAP for CHF |
| COPD | Persistent nocturnal hypoxemia with complications (e.g., cor pulmonale, polycythemia) | O2 for COPD and persistent hypoxemia (PaO2 55‐60 mm Hg) |
| Sporadic nighttime desaturations | PaO2 55 mm Hg monitor O2 saturation by pulse oximetry. If patient desaturates to 88% at night consistently, start nocturnal O2. For hypercapnia, adjust O2 to maintain O2 saturation at 88% to 90% | |
| Early‐morning airflow obstruction | Consider bedtime tiotropium and inhaled long‐acting beta‐adrenergic agonist agents | |
| Inhibition of respiratory muscles in REM | Avoid sedative‐hypnotics that cause respiratory depression | |
| Decreased functional residual capacity from recumbent position during sleep | Minimize recumbancy by keeping the head of bed up at 30 degrees | |
| End‐stage renal disease | Pruritus, nausea; increased risk of RLS and PLMD | Ambulation may help with RLS. Consider ropinirole and pramipexole. Correct hyperphosphatemia and uremia. Consider antipruritic and antiemetic agents |
| Nocturnal GER | Nocturnal GER decreased sleep, heartburn, coughing, asthma | Avoid eating or drinking 2 hours before bedtime, especially those that delay gastric emptying, increase acid secretion, or decrease lower esophageal sphincter pressure; e.g., high‐fat foods, ethanol, chocolate, peppers, peppermint. Keep head of bed 30 degrees. Minimize medications that could worsen nocturnal GER; e.g., theophylline, calcium channel blockers, prostaglandins, bisphosphonates |
| OSA | Snoring with upper airway obstruction | No ethanol 2 hours before bedtime. Minimize CNS depressants. Avoid supine position. Consider CPAP, oral mandibular advancement device, and/or surgical correction. Long‐term plan should include weight loss |
| Stroke | Focal neurologic deficits (e.g., dysphagia, weakness or paralysis) | Keep head of bed 30 degrees. Regularly suction secretions. Post‐stroke patients have an increased risk of hypersomnia, insomnia, and/or OSA |
Affecting approximately 24% of men and 9% of women in the United States, OSA is the most common primary sleep disorder,27, 28 and causes significant mental and physical morbidity. Risk factors for OSA include obesity, hypothyroidism‐induced muscle weakness, and structural abnormalities in the oropharynx region such as acromegaly, micrognathia, or retrognathia. OSA is characterized by episodes of complete or partial pharyngeal obstruction during sleep that cause snoring, apneic episodes, choking, dyspnea, and restlessness.28 These episodes are associated with intermittent nocturnal sympathetic activation leading to nocturnal awakenings and cortical arousals, all of which lead to daytime symptoms of fatigue, sleepiness, and cognitive impairment (Figure 2). In addition, chronic sympathetic activation causes numerous derangements in the vascular endothelium and platelet activation.29, 30 Sleep‐disordered breathing has been independently associated with cardiovascular diseases such as hypertension, CHF, ischemic heart disease, atrial fibrillation, and cerebrovascular disease.31, 32
OSA is also associated with sleep‐related gastroesophageal reflux, which is characterized by pain and nocturnal cough, and can induce nocturnal asthma attacks and laryngospasm.33 Green et al.29 found that OSA patients treated with continuous positive airway pressure (CPAP) had a 48% improvement in nocturnal reflux symptoms. Although the pathophysiology connecting OSA to the renal system is unknown, OSA has been found in up to 60% of patients with end‐stage renal disease and chronic renal failure.34
Patients with pulmonary disorders can be profoundly affected by the normal physiologic changes during sleep, particularly in REM sleep. During REM sleep, all respiratory muscles except the diaphragm become paralyzed. Thus, episodes of marked oxygen desaturation can occur in patients who rely on their accessory muscles for respiration. COPD patients have decreased TST, SWS, and REM sleep. Shortness of breath, nocturnal cough, and wheezing worsen sleep.35 The resulting fatigue and sleep deprivation negatively impact the work of breathing and impair gas exchange. Airflow obstruction tends to worsen in the early morning hours in patients with COPD and asthma, and may be related to the effect of REM on the accessory muscles for respiration. Although used to target CO2 retention, investigations using bilevel positive airway pressure ventilators (BiPAP) for improving sleep in COPD patients have been limited. Noninvasive positive pressure ventilation (NPPV) appears to acutely improve SE and TST in patients with hypercapnic COPD without significantly improving gas exchange. Other sleep parameters such as sleep architecture and the number of arousals during the night, remain unchanged during NPPV.36
CPAP has several side effects that could worsen sleep, which may explain its poor adherence rate among ambulatory patients.37 Side effects include nasal bridge discomfort, nasal congestion, swallowing air, dry nose, dry or red eyes, noise, ear pain, and rhinitis.38 During hospitalization, efforts should be made to improve patient comfort by resizing ill‐fitting masks, adding heated humidification or nasal steroids to alleviate nasal congestion, or adding a chin strap to reduce air leak and ingestion of air.
Endocrine disorders have also been associated with sleep disruption. Studies suggest that patients with diabetes mellitus have decreased TST and impaired sleep quality due to nocturia and neuropathic pain.39 Inadequate sleep may also affect glucose control. Inadequate quality or quantity of sleep has been shown to be a risk factor for developing Type 2 diabetes mellitus in large prospective studies.40 Sleep duration and quality were significant predictors of increased levels of glycosylated hemoglobin (HbA1c) in patients with Type 2 diabetes mellitus. Thyroid diseases often coexist with diabetes mellitus. Both hypo‐ and hyperthyroidism have been associated with sleep disruption. Hypothyroidism is associated with daytime somnolence and fatigue. Patients with hypothyroidism tend to have reduced SWS. Hyperthyroid patients often complain of insomnia, which has been attributed to a hypermetabolic state.
Approximately 50% of patients with chronic end‐stage renal disease (ESRD) have insomnia and other sleep disorders.41 Patients often complain of restless leg syndrome (RLS), periodic limb movement disorder (PLMD), bone pain, nausea, and pruritus. The etiology of sleep disorders appears to be related to metabolic derangements associated with ESRD or from coexisting diabetes mellitus.
RLS and PLMD are distinct problems that affect sleep differently. RLS is characterized by an unpleasant crampy, creeping or crawling sensation in the lower extremities that is relieved by movement of the legs.42 RLS symptoms typically occur soon after going to bed, and therefore tend to disrupt sleep onset. The requisite bed rest during hospitalization can worsen RLS, further exacerbating sleep problems.43 Since RLS may partially be caused by disrupted iron metabolism, serum ferritin levels should be evaluated.44 Other conditions associated with RLS include pregnancy, rheumatoid arthritis, fibromyalgia, multiple sclerosis, ESRD, and Parkinson's disease. The differential diagnosis for RLS and PLMD includes neuroleptic‐induced akathisia, peripheral neuropathy, and positional or nocturnal leg cramps. PLMD occurs in about 80% of those with RLS, and is characterized by involuntary limb movements that occur every 20 to 40 seconds during NREM sleep. Unaware of these movements, patients often experience frequent arousals throughout the night, and complain of daytime somnolence and fatigue.42
A pilot study of 35 patients with minimal hepatic encephalopathy found that nearly 50% complained of sleep difficulties.45 Hypothesizing that a dysregulation of histaminergic neurotransmission in cirrhosis alters the sleep‐wake cycle, Spahr et al.46 found that 40% of their patients reported subjective improvement in sleep when administered 25 mg of hydroxyzine, compared to none who received placebo.
Neurologic Disorders
Since the brain and its various neurotransmitter systems are critical in regulating sleep and wakefulness, patients with neurologic disorders have an increased risk of developing sleep disorders. Patients with dementia, other neurodegenerative disorders, epilepsy, and traumatic brain injury (TBI) have a higher prevalence of sleep disturbance and sleep disorders.47 Poststroke patients can develop insomnia or hypersomnia, a reduction in sleep latency, increased sleep, or excessive daytime sleepiness, and are at higher risk for OSA during the first several months after a stroke.48 Specific neurologic lesions may lead to uncommon problems such as inversion of the sleep‐wake cycle, parasomnias, and hallucinatory dream‐like states.
Both Parkinson's disease and Alzheimer's disease are associated with multiple sleep disturbances, which tend to worsen with disease progression.14 Common problems include increased sleep fragmentation and wakefulness, with increases of stage 1 sleep and reductions of SWS and REM. Patients with neurodegenerative disorders also have an increased risk of REM sleep behavior disorder, or RBD.49 RBD is characterized by vivid and unusual dreams, and physically vigorous sleep behaviors that may result in ecchymoses, lacerations, and fractures.50 Fifty percent of patients with TBI reported insomnia symptoms.51 Disorders in initiating and maintaining sleep were the most common complaints among hospitalized patients with TBI. Some patients with TBI may develop circadian rhythm disturbances.52
Pain
A majority of patients with chronic pain, 50% to 70%, complain of impaired sleep.53 Sleep disruption is so common in fibromyalgia (75%) that it is considered to be a key diagnostic symptom.54 In a study investigating the affect of pain on sleep in burn patients, pain was associated with increased intermittent awakenings and prolonged periods of wake time during the night.55 The following day, these patients had poorer pain tolerance and greater pain intensity. Pain causes sleep fragmentation by increasing cortical arousals. Recent evidence suggests that sleep deprivation can increase pain sensitivity by inhibiting opioid protein synthesis or reducing opioid receptor affinity.56
Psychiatric Disorders
Sleep problems are so common in psychiatric conditions that the Diagnostic and Statistical Manual of Mental Disorders (DMS‐IV‐TR) includes sleep disturbance as a diagnostic criterion for a manic episode, and for various depressive, anxiety, and substance abuse disorders.57 The presence of sleep disturbance in hospitalized patients may suggest the presence of an underlying psychiatric disorder that would otherwise go unrecognized. In a survey of 200 general medical patients in a Brazilian hospital, Rocha et al.58 found that 112 (56.5%) complained of insomnia, and 100 (50%) met criteria for at least 1 psychiatric disorder. However, only 3 out of the total number of 200 surveyed (1.5%) were identified as having psychiatric diagnoses in the medical record, and sleep history was not noted in the clinical evaluation. An episode of major depressive disorder was the most common psychiatric diagnosis (35%). In this study, hospitalized patients with insomnia had a 3.6 times higher risk of having major depressive disorder than inpatients without insomnia.
Insomnia has a profound effect on mental health by worsening health‐related quality of life. In a study of outpatients at family medicine, internal medicine, endocrinology, cardiology, and psychiatry clinics in 3 U.S. cities (n = 3,445), insomnia worsened health‐related quality of life nearly as much as CHF or major depressive disorder did.59 Another survey of outpatients found that those with chronic insomnia were nearly 40 times more likely to have major depression and 6 times more likely to have an anxiety disorder compared to those without insomnia.60 Longitudinal studies have found that prior insomnia was associated with 2‐ to 5‐fold increase in the odds of mood and anxiety disorders and suicide.61, 62 Examining prodromes and precursors to mental disorders, Eaton et al.63 found that 47% of those with onset of depression at the 1‐year follow‐up had sleep problems at baseline.
An estimated 65% of patients with major depression have difficulty falling asleep, frequent awakenings, or early morning awakenings.64 Three patterns of sleep architecture abnormalities have been observed in patients with major depression: 1) sleep continuity disturbances characterized by prolonged sleep‐onset, increased wake time during sleep, increased early morning wake time, and decreased TST; 2) decreased proportion and length of SWS; and 3) REM sleep abnormalities such as reduced time to REM sleep, prolonged first REM sleep episode, and increased REM sleep percentage.65 Sleep during a manic episode has been less studied than in depression, but the data suggest that abnormal sleep in mania includes disrupted sleep continuity, shortened REM latency, and increased REM density (REM eye movement activity/total REM sleep time).65
Substance use disorders are also associated with sleep problems. In a survey by Brower et al.66 of patients who were undergoing alcohol rehabilitation, 61% (n = 172) had symptoms of insomnia such as increased sleep latency during the 6 months prior to entering treatment. Approximately 45% of these patients reported using alcohol for the purpose of initiating sleep. Alcohol and illicit substance intoxication and withdrawal are known to be associated with disrupted sleep. However, sleep disturbances may persist long after withdrawal symptoms have abated. Drummond et al. found that some patients continued to have alcohol‐associated sleep problems even after 27 months of abstinence.67 Evidence also suggests that untreated insomnia and other sleep problems may increase the risk of developing substance abuse problems due to self‐medicating with alcohol and other substances to help with sleep.68
Drugs that Affect Sleep
Numerous drugs can alter sleep quantity and quality. Sedatives and opioids may initially help with sleep onset, but impair sleep architecture. Medications used to treat medical and psychiatric illnesses also disrupt sleep (Table 3). The most common agents that impair sleep include antiepileptic drugs, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, tricyclic antidepressants, antihypertensives, antihistamines, and corticosteroids.
| Drug Class | Examples of Drugs | Affect on Sleep Architecture | Potential Mechanism | Clinical Implications |
|---|---|---|---|---|
| ||||
| CNS | ||||
| AEDs | Phenobarbital, carbamazepine, phenytoin | Very sedating. AEDs tend to TST, sleep latency | Inhibit neuronal calcium influx, adenosine, or 5HT activity | Sedation is dose‐dependent, and tends to occur with acute use |
| TCAs | Amoxapine, amitriptyline, imipramine, nortriptyline, desipramine, doxepin, clomipramine | Very sedating. Suppresses REM sleep, TST, stage‐2 sleep | Stimulate antimuscarinic‐receptor and alpha1‐receptor | Suppressed REM sleep motor inhibition restlessness, psychomotor agitation during sleep subjectively sleep quality, daytime sleepiness |
| BzRAs | Alprazolam, lorazepam, chlordiazepoxide, diazepam, oxazepam | Very sedating. TST, sleep latency, SWS duration, REM, stage‐2 sleep | Stimulate GABA type A receptor | Minimize daytime use. Chronic BzRAs SWS long‐term sequelae unknown |
| MAOIs | Phenylzine, tranylcypromine | Very sedating. TST, REM, REM rebound if stop MAOIs | Mechanism unknown | Daytime sleepiness; dosing time does not affect daytime somnolence |
| SSRIs | Sedating: paroxetine, fluvoxamine. Activating: fluoxetine, sertraline, citalopram | TST, are less sedating than TCAs and MAOIs. May REM, TWT, TST, SE | 5HT activity | Some patients get the opposite reaction |
| SNRI | Venlafaxine, duloxetine | Activating in some patients; sedating in 12% to 31%. TST | 5HT and NE activity | If activating, switch to AM dosing. If sedating, switch to PM dosing |
| Mood stabilizer | Lithium | Sedating. TST, SWS, REM, REM latency | daytime sedation. Dose at night | |
| Stimulants | Ephedrine, pseudoephedrine, modafinil | Activating. TST, SWS, sleep latency | DOPA, NE, and 5HT activity | Avoid after 6 PM |
| Anti‐Parkinson | Bromocriptine, levodopa | Sedating. Nightmares, SWS | DOPA | Dose at night, if possible |
| Cardiac | ||||
| Lipophilic beta‐blockers | Propranolol, pindolol, metoprolol, timolol. Hydrophilic agents (atenolol and sotalol) lack these effects | Activating. awakenings, TWT, REM, nightmares | CNS beta‐blockade | Lipophilic beta‐blockers daytime sleep when dosed in AM |
| CNS agents | Norepinephrine, epinephrine | Activating. REM, SWS | Stimulate alpha1‐receptor | Minimize use at night |
| Dopamine | Activating. REM, SWS | Stimulate dopamine2‐receptor and alpha1‐receptor | Minimize use at night | |
| Ca++ channel blockers | Amlodipine, verapamil, nifedipine | Exacerbate underlying medical condition | Lower esophageal sphincter tone nocturnal GER sleep disturbance | |
| Alpha2‐receptor agonist | Clonidine | Stage 1, REM, nightmares | Stimulate alpha2‐receptor | Alpha2‐agonists daytime sleep and sleepiness directly. Dose at night |
| Alpha1‐receptor blockers | Doxazosin, prazosin, terazosin | Inhibit alpha1‐receptor | Alpha1‐receptor blockers daytime sleepiness | |
| Diuretics | HCTZ, furosemide | Sedating. | PM diuresis frequent awakenings | |
| Other | ||||
| Opioids | Codeine, morphine | Sedating. SWS, REM | Stimulate mu‐receptor | Minimize use at night |
| NSAIDs | Ibuprofen, indomethcin, celecoxib | TST, SE | Inhibit prostaglandin synthesis | Minimize use at night |
| Methylxanthine | Theophylline | Activating. stage 1, REM | Causes less restful sleep | |
| Antihistamines | Diphenhydramine, promethazine | Sedating | H1 receptor blockade | Minimize use at night |
| Corticosteroids | Dexamethasone, prednisone | Activating. REM, SWS, nightmares | Melatonin secretion | Can disrupt sleep, anxiety, induce mania or psychosis |
| H2 blockers | Cimetidine, ranitidine, famotidine | Sedating. TST | H2 receptor blockade | Sedating if >60 years old, renal impairment |
| Quinolone | Ciprofloxacin, sparfloxacin, ofloxacin, grepafloxacin, levofloxacin | Activating | Stimulate GABA type A receptor | Consider sleep agent after maximizing sleep hygiene. Linezolid rarely causes sleep disturbances |
Lipophilic beta antagonists such as propranolol and timolol can increase total wake time, decrease REM sleep, and increase the incidence of nightmares and insomnia.69 Anabolic steroids and beta‐agonist bronchodilator therapy can cause severe anxiety, sleeplessness, and even psychosis. Vasopressor agents such as dopamine can cause cortical activation, leading to increased arousal and reduced SWS.
Hospital Environment
Environmental noise and patient care activities often interfere with sleep in the hospital. They account for about 30% of patient awakenings in ICU patients.70 Noise levels in the ICU have average sound peaks of 150 to 200 dB, and evening peaks >80 dB between midnight and 6 AM.71 By comparison, the front row seats at a rock concert have sound levels of 110 dB. The high noise level in hospitals has long been implicated as a sleep disruptor,72 but studies in the past decade have found that patient care activities probably contribute more to awakenings than does environmental noise.73 An analysis of critical care nursing routines found that activities such as taking vital signs and giving baths occurred a mean 42.6 times a night per patient.74 Tamburri et al.74 found that patients experienced 2 to 3 hours without interruption on only 6% of the 147 nights studied. Routine daily baths were provided on 55 of the 147 study nights between 2 AM and 5 AM, which is unlikely to be an opportune time for most patients.
CONCLUSION
Hospitalization often prevents patients from achieving adequate sleep and can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing sedative/hypnotic agents, the treatment for sleep disruption includes addressing multiple medical, behavioral, and environmental factors, which will be discussed in Part 2 of this article.
- NIH State‐of‐the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults.NIH Consens Sci Statements.2005;22(2):1–30.
- ,,, et al.Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey.Hypertension.2006;47(5):833–839.
- ,,, et al.Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique.Sleep.2003;26(8):986–989.
- ,,,,.Postural control after a night without sleep.Neuropsychologia.2006;44(12):2520–2525.
- .Sleep deprivation decreases ventilatory responses to CO2 but not load compensation.Chest.1983;84(6):695–698.
- ,,, et al.Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation.J Appl Physiol.2005;98(6):2024–2032.
- .Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats.Am J Physiol Endocrinol Metab.2004;286(6):E1060–E1070.
- ,,,.The metabolic consequences of sleep deprivation.Sleep Med Rev.2007;11(3):163–178.
- ,,,,.Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.Arch Int Med.2006;166:1756–1752.
- ,,,.Effects of sleep and sleep deprivation on immunoglobulins and complement in humans.Brain Behav Immun.2007;21:308–310.
- ,,,,.The effects of sleep deprivation on symptoms of psychopathology in healthy adults.Sleep Med.2007;8:215–221.
- ,.Normal human sleep. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:15–16.
- .Update on the Science, Diagnosis and Management of Insomnia.International Congress and Symposium Series 262.London:Royal Society of Medicine Press Ltd;2006.
- ,,,,,.The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture.Arch Intern Med.2004;164:406–18.
- ,.Sleep disruption in older adults.Am J Nurs.2007;107(5):40–49.
- ,,,.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey.J Psychosom Res.2004;56(5):497–502.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Insomnia and its treatment: prevalence and correlates.Arch Gen Psychiatry.1985;42(3):225–232.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117:809–818.
- ,.Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill.Crit Care Med.2006;22:313–327.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,,,.Failure of physician documentation of sleep complaints in hospitalized patients.West J Med.1998;169:146–149.
- .Sleep and medical disorders.Prim Care.2005;35:511–533.
- ,,,,,.The occurrence of sleep‐disordered breathing among middle‐aged adults.N Engl J Med.1993;328:1230–1235.
- ,.Clinical features and evaluation of obstructive sleep apnea‐hypopnea syndrome. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:869–872.
- ,,.Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea.Arch Intern Med.2003;163:41–45.
- ,.Sleep and medical disorders.Med Clin North Am.2004;88:679–703.
- ,,,.Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness.Atherosclerosis.2008;197(1):125– 131.
- ,,, et al.Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med.2001;163:19–25.
- .Gastroesophageal reflux during sleep.Sleep Med Clin.2007;2:41–50.
- ,.Sleep apnea in renal failure.Adv Perit Dial.1997;13:88–92.
- .Sleep in chronic obstructive pulmonary disease.Sleep Med Clin.2007;2:1–8.
- ,,,.Effects of non‐invasive positive pressure ventilation on gas exchange and sleep in COPD patients.Chest.1997;112:623–628.
- ,,, et al.Night‐to‐night variability in CPAP use over the first three months of treatment.Sleep.1997;20(4):278–283.
- ,.Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS).Sleep Med Rev.2003;7(1):81–99.
- ,,.Factors predicting sleep disruption in type II diabetes.Sleep.2000;23:415–416.
- ,,.Sleep duration as a risk factor for the development of type 2 diabetes.Diabetes Care.2006;29:657–661.
- .Sleep disorders and end‐stage renal disease.Sleep Med Clin.2007;2:59–66.
- ,,,.Sleep and aging: 1. Sleep disorders commonly found in older people.CMAJ.2007;176(9):1299–1304.
- ,,,,,.Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS Epidemiology, Symptoms, and Treatment) primary care study.Sleep Med.2004;5(3):237–246.
- ,.Epidemiology and clinical findings of restless leg syndrome.Sleep Med.2004;5(3):293–299.
- ,,,,,.High prevalence of sleep disturbance in cirrhosis.Hepatology.1998;27:339–345.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Obstructive sleep apnea: implications for cardiac and vascular disease.JAMA.2003;290(14):1906–1914.
- ,,, et al.Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.Brain.2007;130(Pt 11):2770–2788.
- ,.REM sleep parasomnias. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:724–725.
- ,,.Insomnia in patients with traumatic brain injury.J Head Trauma Rehabil.2006;21(3):199–212.
- ,,,,.Circadian rhythm sleep disorders following mild traumatic brain injury.Neurology.2007;68(14):1136–1140.
- ,.Comorbidities: psychiatric, medical, medications, and substances.Sleep Med Clin.2006;231–245.
- ,,.Sleep disturbance and fibromyalgia.Sleep Med Clin.2007;2:31–39.
- ,,.Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries.Sleep Med.2004;5:551–559.
- ,,.Sleep deprivation and pain perception.Sleep Med Rev.2006;10:357–369.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision.Washington, DC:American Psychiatric Association;2000.
- ,,, et al.Is insomnia a marker for psychiatric disorders in general hospitals?Sleep Med.2005;6:549–553.
- ,.The relationship between insomnia and health‐related quality of life in patients with chronic illness.J Fam Pract.2002;51(3):229–235.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,.Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults.Biol Psychiatry.1996;39:411–418.
- ,,,.The morbidity of insomnia uncomplicated by psychiatric disorders.Gen Hosp Psychiatry.1997;19:245–250.
- ,,.Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset.Am J Psychiatry.1995;152:967–972.
- ,,,,,.Which depressive symptoms are related to which sleep electroencephalographic variables?Biol Psychol.1997;42:904–913.
- ,.Sleep in mood disorders.Psychiatr Clin North Am.2006;29:1009–1032.
- ,,,,.Insomnia, self‐medication, and relapse to alcoholism.Am J Psychiatry.2001;158:399–404.
- ,,,.The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse.Alcohol Clin Exp Res.1998;22:1796–1802.
- ,,,,.Screening for substance use patterns among patients referred for a variety of sleep complaints.Am J Drug Alcohol Abuse.2006;32:111–120.
- .Drugs that disturb sleep and wakefulness. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:441–462.
- ,,, et al.Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects.Am J Respir Crit Care Med.2003;167(5):708–715.
- ,,.Adverse environmental conditions in the respiratory and medical ICU settings.Chest.1994;105:1211–1216.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118(6):3629–3645.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,,.Nocturnal care interactions with patients in critical care units.Am J Crit Care.2004;13(2):102–115.
- NIH State‐of‐the Science Conference Statement on Manifestations and Management of Chronic Insomnia in Adults.NIH Consens Sci Statements.2005;22(2):1–30.
- ,,, et al.Short sleep duration as a risk factor for hypertension: analyses of the first National Health and Nutrition Examination Survey.Hypertension.2006;47(5):833–839.
- ,,, et al.Total sleep deprivation elevates blood pressure through arterial baroreflex resetting: a study with microneurographic technique.Sleep.2003;26(8):986–989.
- ,,,,.Postural control after a night without sleep.Neuropsychologia.2006;44(12):2520–2525.
- .Sleep deprivation decreases ventilatory responses to CO2 but not load compensation.Chest.1983;84(6):695–698.
- ,,, et al.Increased sympathetic and decreased parasympathetic cardiovascular modulation in normal humans with acute sleep deprivation.J Appl Physiol.2005;98(6):2024–2032.
- .Reductions in circulating anabolic hormones induced by sustained sleep deprivation in rats.Am J Physiol Endocrinol Metab.2004;286(6):E1060–E1070.
- ,,,.The metabolic consequences of sleep deprivation.Sleep Med Rev.2007;11(3):163–178.
- ,,,,.Sleep deprivation and activation of morning levels of cellular and genomic markers of inflammation.Arch Int Med.2006;166:1756–1752.
- ,,,.Effects of sleep and sleep deprivation on immunoglobulins and complement in humans.Brain Behav Immun.2007;21:308–310.
- ,,,,.The effects of sleep deprivation on symptoms of psychopathology in healthy adults.Sleep Med.2007;8:215–221.
- ,.Normal human sleep. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:15–16.
- .Update on the Science, Diagnosis and Management of Insomnia.International Congress and Symposium Series 262.London:Royal Society of Medicine Press Ltd;2006.
- ,,,,,.The effects of age, sex, ethnicity, and sleep‐disordered breathing on sleep architecture.Arch Intern Med.2004;164:406–18.
- ,.Sleep disruption in older adults.Am J Nurs.2007;107(5):40–49.
- ,,,.Sleep disturbances and chronic disease in older adults: results of the 2003 National Sleep Foundation Sleep in America Survey.J Psychosom Res.2004;56(5):497–502.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Insomnia and its treatment: prevalence and correlates.Arch Gen Psychiatry.1985;42(3):225–232.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,,,.Sleep in critically ill patients requiring mechanical ventilation.Chest.2000;117:809–818.
- ,.Sedative and analgesic medications: risk factors for delirium and sleep disturbances in the critically ill.Crit Care Med.2006;22:313–327.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,,,.Failure of physician documentation of sleep complaints in hospitalized patients.West J Med.1998;169:146–149.
- .Sleep and medical disorders.Prim Care.2005;35:511–533.
- ,,,,,.The occurrence of sleep‐disordered breathing among middle‐aged adults.N Engl J Med.1993;328:1230–1235.
- ,.Clinical features and evaluation of obstructive sleep apnea‐hypopnea syndrome. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:869–872.
- ,,.Marked improvement in nocturnal gastroesophageal reflux in a large cohort of patients with obstructive sleep apnea.Arch Intern Med.2003;163:41–45.
- ,.Sleep and medical disorders.Med Clin North Am.2004;88:679–703.
- ,,,.Relation of sleep‐disordered breathing to carotid plaque and intima‐media thickness.Atherosclerosis.2008;197(1):125– 131.
- ,,, et al.Sleep‐disordered breathing and cardiovascular disease: cross‐sectional results of the Sleep Heart Health Study.Am J Respir Crit Care Med.2001;163:19–25.
- .Gastroesophageal reflux during sleep.Sleep Med Clin.2007;2:41–50.
- ,.Sleep apnea in renal failure.Adv Perit Dial.1997;13:88–92.
- .Sleep in chronic obstructive pulmonary disease.Sleep Med Clin.2007;2:1–8.
- ,,,.Effects of non‐invasive positive pressure ventilation on gas exchange and sleep in COPD patients.Chest.1997;112:623–628.
- ,,, et al.Night‐to‐night variability in CPAP use over the first three months of treatment.Sleep.1997;20(4):278–283.
- ,.Improving CPAP use by patients with the sleep apnoea/hypopnoea syndrome (SAHS).Sleep Med Rev.2003;7(1):81–99.
- ,,.Factors predicting sleep disruption in type II diabetes.Sleep.2000;23:415–416.
- ,,.Sleep duration as a risk factor for the development of type 2 diabetes.Diabetes Care.2006;29:657–661.
- .Sleep disorders and end‐stage renal disease.Sleep Med Clin.2007;2:59–66.
- ,,,.Sleep and aging: 1. Sleep disorders commonly found in older people.CMAJ.2007;176(9):1299–1304.
- ,,,,,.Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: The REST (RLS Epidemiology, Symptoms, and Treatment) primary care study.Sleep Med.2004;5(3):237–246.
- ,.Epidemiology and clinical findings of restless leg syndrome.Sleep Med.2004;5(3):293–299.
- ,,,,,.High prevalence of sleep disturbance in cirrhosis.Hepatology.1998;27:339–345.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- .Sleep in patients with neurologic and psychiatric disorders.Prim Care.2005;32:535–548.
- ,,.Obstructive sleep apnea: implications for cardiac and vascular disease.JAMA.2003;290(14):1906–1914.
- ,,, et al.Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.Brain.2007;130(Pt 11):2770–2788.
- ,.REM sleep parasomnias. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:724–725.
- ,,.Insomnia in patients with traumatic brain injury.J Head Trauma Rehabil.2006;21(3):199–212.
- ,,,,.Circadian rhythm sleep disorders following mild traumatic brain injury.Neurology.2007;68(14):1136–1140.
- ,.Comorbidities: psychiatric, medical, medications, and substances.Sleep Med Clin.2006;231–245.
- ,,.Sleep disturbance and fibromyalgia.Sleep Med Clin.2007;2:31–39.
- ,,.Sleep disturbances, pain and analgesia in adults hospitalized for burn injuries.Sleep Med.2004;5:551–559.
- ,,.Sleep deprivation and pain perception.Sleep Med Rev.2006;10:357–369.
- American Psychiatric Association.Diagnostic and Statistical Manual of Mental Disorders.4th ed. Text Revision.Washington, DC:American Psychiatric Association;2000.
- ,,, et al.Is insomnia a marker for psychiatric disorders in general hospitals?Sleep Med.2005;6:549–553.
- ,.The relationship between insomnia and health‐related quality of life in patients with chronic illness.J Fam Pract.2002;51(3):229–235.
- ,.Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention?JAMA.1989;262:1479–1484.
- ,,,.Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults.Biol Psychiatry.1996;39:411–418.
- ,,,.The morbidity of insomnia uncomplicated by psychiatric disorders.Gen Hosp Psychiatry.1997;19:245–250.
- ,,.Prodromes and precursors: epidemiologic data for primary prevention of disorders with slow onset.Am J Psychiatry.1995;152:967–972.
- ,,,,,.Which depressive symptoms are related to which sleep electroencephalographic variables?Biol Psychol.1997;42:904–913.
- ,.Sleep in mood disorders.Psychiatr Clin North Am.2006;29:1009–1032.
- ,,,,.Insomnia, self‐medication, and relapse to alcoholism.Am J Psychiatry.2001;158:399–404.
- ,,,.The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse.Alcohol Clin Exp Res.1998;22:1796–1802.
- ,,,,.Screening for substance use patterns among patients referred for a variety of sleep complaints.Am J Drug Alcohol Abuse.2006;32:111–120.
- .Drugs that disturb sleep and wakefulness. In:Kryger MH,Roth T,Dement WC, editors.Principles and Practice of Sleep Medicine.3rd ed.Philadelphia:W.B. Saunders;2000:441–462.
- ,,, et al.Contribution of the intensive care unit environment to sleep disruption in mechanically ventilated patients and healthy subjects.Am J Respir Crit Care Med.2003;167(5):708–715.
- ,,.Adverse environmental conditions in the respiratory and medical ICU settings.Chest.1994;105:1211–1216.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118(6):3629–3645.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,,.Nocturnal care interactions with patients in critical care units.Am J Crit Care.2004;13(2):102–115.