User login
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
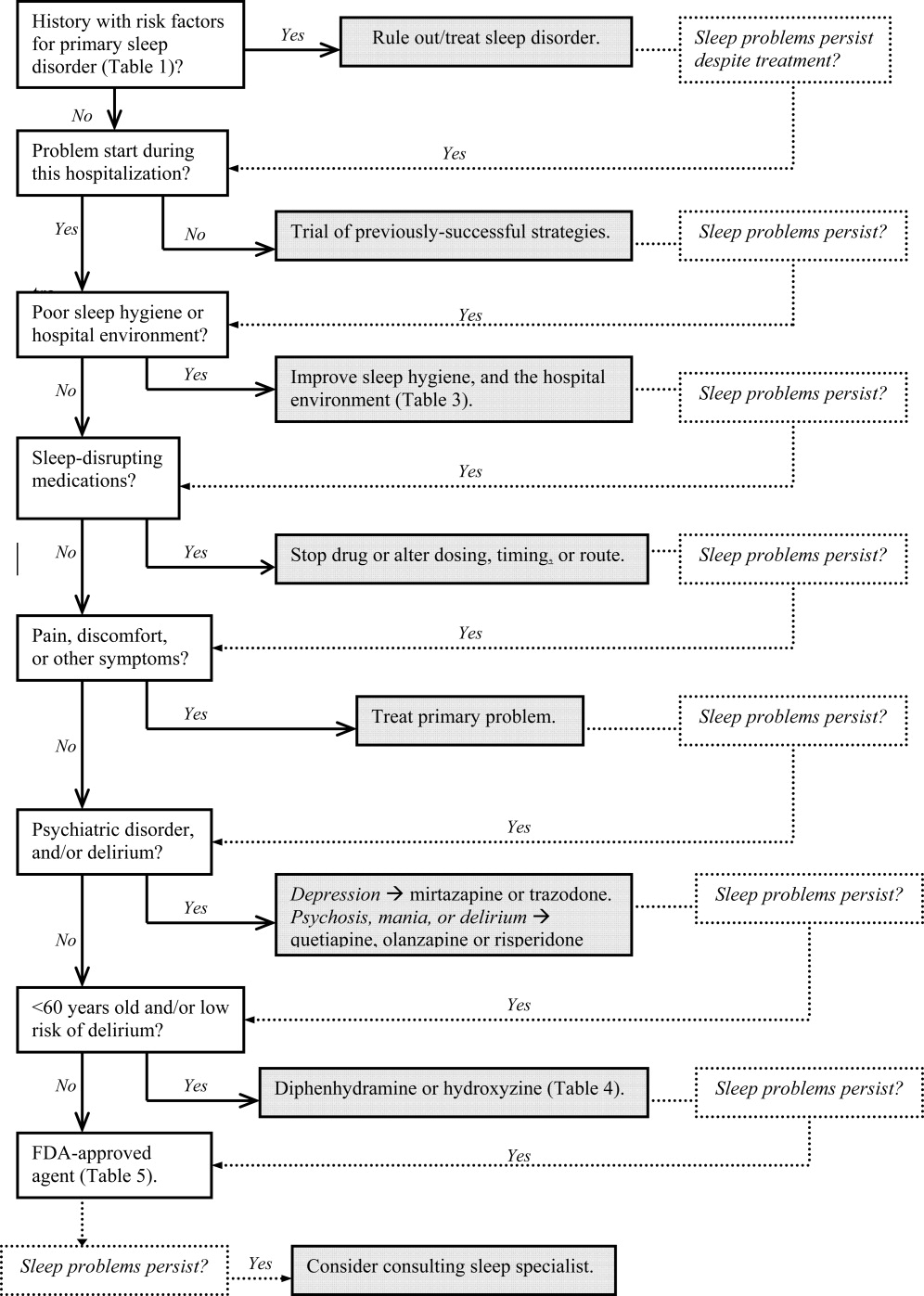
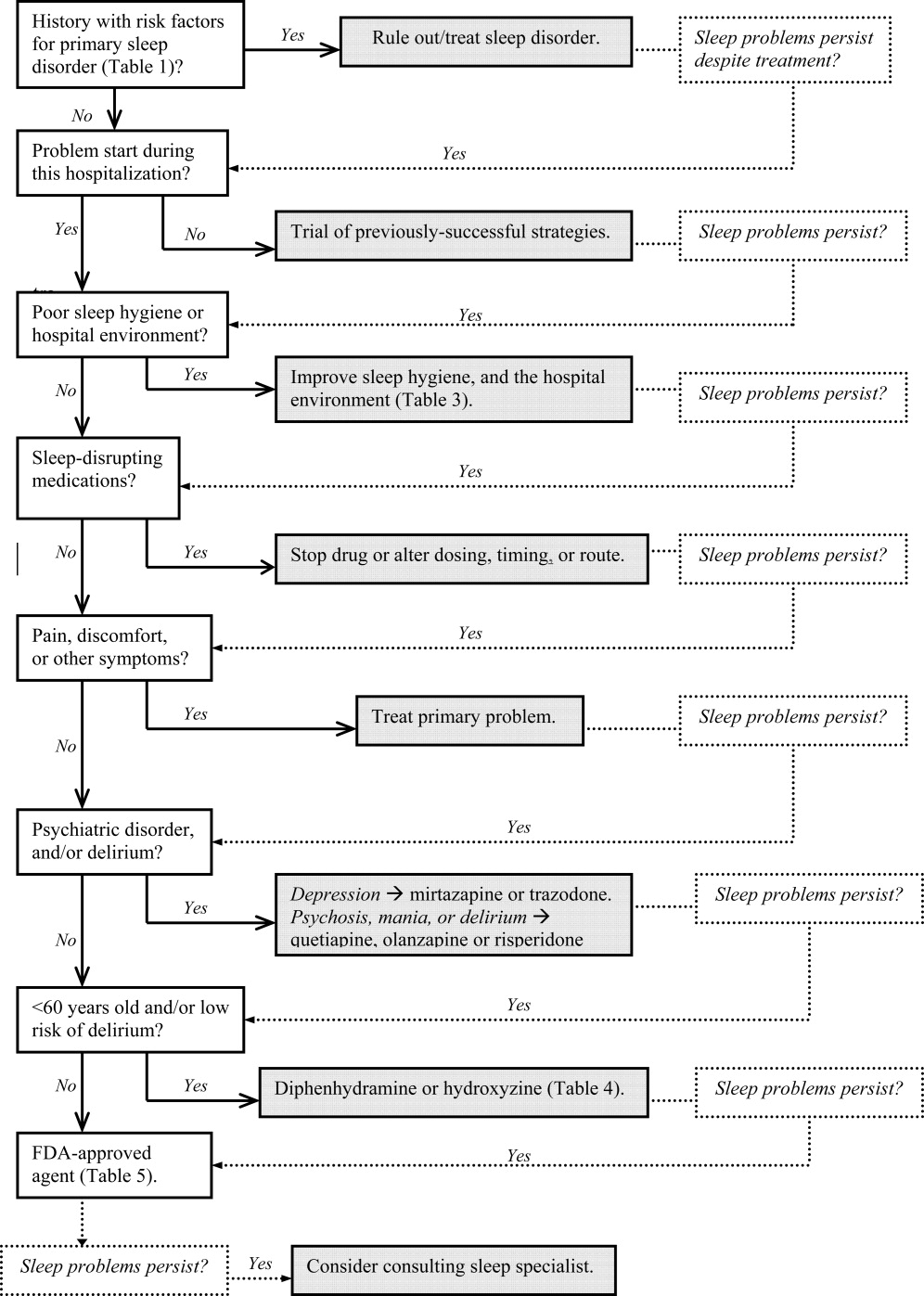
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.
| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (<2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, <45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | <2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.

| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (<2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, <45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | <2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
In Part 1, we reviewed normal sleep architecture, and discussed the numerous factors that often disrupt the sleep of hospitalized medical patients. Effective management of sleep complaints among acutely ill patients includes a thorough assessment of medical and psychiatric conditions, medications and other psychosocial factors that may be directly or indirectly impairing sleep. In Part 2, we review and introduce an algorithm for assessing and managing sleep complaints in acutely ill hospitalized patients.
ASSESSMENT AND EVALUATION OF SLEEP COMPLAINTS
Assessment and evaluation of a sleep complaint begins with (Figure 1) an initial review of the medical record for documentation of the signs and symptoms of an underlying primary sleep disorder, which may be exacerbated during an acute medical illness. Common sleep disorders that are often overlooked include obstructive sleep apnea (OSA), restless leg syndrome (RLS), and periodic limb movement disorder (PLMD). Predisposing factors, characteristic clinical features, and differential diagnoses of these disorders are described in Table 1.

| Sleep Disorder | Predisposing Factors | Clinical Features | Differential Diagnosis |
|---|---|---|---|
| |||
| Obstructive sleep apnea (OSA) | Nasopharyngeal abnormalities, craniofacial abnormalities, obesity, >40 years old, men > women (2:1), neurologic disorder (eg, recent stroke) | Repetitive episodes of upper airway obstruction that occur during sleep, usually associated with oxygen desaturation. Episodes include loud snoring or gasps lasting 2030 seconds. Associated with morning headaches and dry mouth. | Sleep‐related laryngospasm, nocturnal gastroesophageal reflux, narcolepsy, hypersomnia, PLMD, central alveolar hypoventilation, paroxysmal nocturnal dyspnea, primary snoring, Cheyne‐Stokes ventilation, nocturnal asthma |
| Periodic limb movement disorder (PLMD) | OSA. RLS, or narcolepsy; aging; chronic uremia; TCAs or MAOIs; withdrawal from antiepileptic agents, or other sedating agents | Periodic episodes of repetitive and stereotyped limb movements: extension of the big toe with partial flexion of the ankles, knees, or hips. Muscle contractions last 0.5 to 5 seconds, with 20‐second to 40‐second intervals between them. | Sleep starts (occur just prior to, not during, sleep, and do not have a regular periodicity like PLMD), nocturnal epileptic seizures, myoclonic epilepsy |
| Restless leg syndrome (RLS) | Pregnancy (>20 weeks gestation), uremia, anemia, rheumatoid arthritis, peak onset is middle age | Uncomfortable leg sensations that occur prior to sleep onset that leads to an irresistible urge to move the legs. Described as achy, crawling, pulling, prickling, or tingling, and disrupts sleep onset. | Chronic myelopathy, peripheral neuropathy, akathisia, fasciculation syndromes, anemia |
| Sleep starts | Can worsen with anxiety, caffeine or other stimulants, daytime physical exertion | Sudden, brief contraction of the legs that occurs at sleep onset. Usually benign, but may worsen during hospitalization, and interfere with sleep. | PLMD, RLS, hyperekplexia syndrome, in which generalized myoclonus is readily elicited by stimuli |
Obtain a focused history by using questions listed in Table 2 to characterize the onset, duration, frequency, and specific characteristics of the patient's current sleep patterns. Next, establish whether the onset of the patient's sleep complaint began with the time of hospitalization. Subsequent questions can then focus on factors that may be impairing sleep such as the hospital environment and sleep hygiene behaviors by comparing the patient's home sleep habits with those during hospitalization. Inquire about the use or abuse of substances such as sedatives, antidepressants, sedatives, antiepileptic drugs (AEDs), and opioids. Ask questions about the presence of pain syndromes and other comorbidities that often impact sleep.
| Focus | Examples of Questions |
|---|---|
| |
| Sleep pattern | Do you have problems falling asleep or staying asleep? How often do you wake up during the night? How long does it take you to fall back asleep? When did the problem start? What can we do to help you sleep? What time do you try to go to sleep, and what time do you wake up? |
| Behavioral factors | Compare your bedtime routine at home, and in the hospital. |
| Environment | Does the lighting or noise level in the hospital disrupt your sleep? How so? Are you awoken from sleep for laboratory work, monitoring, bathing, or other nursing/medical procedures? |
| Patient comfort | Is your pain adequately controlled at night? If not, are you on a scheduled analgesic regimen, or do you have to ask for pain medications? Do you have breathing problems, gastroesophageal reflux, or other type of discomfort that keeps you from sleeping well? |
| Substances | Do you drink alcohol? How much, and how often? When was your last alcoholic beverage? Inquire about cocaine, methamphetamine, marijuana, and medically‐unsupervised use of opioids. |
| Psychosocial | How was your mood just prior to being hospitalized? How has your mood been since you were admitted? Have you experienced any emotionally or physically traumatic event prior to, or during, this hospitalization that continues to bother you (eg, intubation, resuscitation, surgery, blood draws, MRI scanning)? |
MANAGEMENT OF SLEEP COMPLAINTS
Management of sleep disturbance is multifactorial and consists of nonpharmacologic as well as pharmacologic therapies. A stepwise approach is suggested and begins with nonpharmacologic strategies.
Nonpharmacologic Interventions
Before using sedative/hypnotic agents, address sleep hygiene and other factors that disrupt sleep during a hospitalization such as those listed in Table 3.
| Barriers to Sleep | Strategies To Optimize Sleep in the Hospital |
|---|---|
| |
| Noise | Limit the volume level of television sets, and do not allow patients or visitors to increase the volume. |
| Promptly respond to alarm monitors, and consider liberalizing the monitor alarm setting, if appropriate. | |
| Keep patients' doors closed, if possible. | |
| Post signs to remind staff and visitors to minimize conversations at or near the bedside. | |
| Adhere strictly to visiting hours. | |
| Encourage staff to switch their beepers and other electronic devices to vibrate at night. | |
| Limit the number of visitors at a time and/or if appropriate, have the patient meet with visitors in another location (eg, conference room, cafeteria). | |
| Offer earplugs. | |
| Ask patients to turn their phone ringers off when visiting hours are over. | |
| Anxiety | Encourage visitors to minimize discussing emotionally difficult topics with patients near bedtime. |
| Lighting | Offer eye masks. |
| Encourage exposure to brighter light during the day (turn on the lights, open the curtains), and turn off the lights by 9 PM. | |
| Poor sleep hygiene | Encourage regular nocturnal sleep time, and discourage lengthy naps during the day. |
| Medications and substances | Minimize BzRAs for sleep. Try to wean patients off BzRAs prior to discharge. At discharge, provide the minimum number of pills until they are scheduled to see their primary care clinician posthospitalization, and do not provide refills. |
|
Avoid starting multiple medications at one time. Minimize use of sleep‐disrupting medications (see Part 1, Table 3). |
|
| Change medication regimens to promote sleep; eg, avoid night‐time diuretics if possible. | |
| No caffeine or cigarette smoking after 6 PM. | |
| Effects of treatments | Minimize bathing, dressing changes, room switches, and other activities at night. |
| Regularly review nighttime orders to see if you could decrease the frequency of overnight monitoring (eg, fingersticks, labdraws, checking vitals). | |
| Delirium | Provide an updated calendar to facilitate cognitive orientation. |
| Discontinue nonessential medications. Minimize use of BzRAs, barbiturates, opiates, antihistamines, and anticholinergic agents. | |
| Regularly provide verbal and other cues to orient patients to the date, time, location, and circumstances. | |
| Nocturnal discomfort | Optimize nighttime glycemic control, and maximize pain management. |
| For patients with reflux: No oral intake after 8 PM, and keep head of bed elevated 30 degrees. | |
| Provide nocturnal O2, CPAP, and/or other medications, as appropriate. If patient is on CPAP, assess the mask's fit and comfort. | |
Pharmacologic (Sedative/Hypnotic) Interventions
Pharmacologic therapy may be necessary to treat disordered sleep. The ideal sleep aid would reduce sleep latency or time to fall asleep, increase total sleep time (TST), not cause next‐day sedation, improve daytime functioning, and minimize the development of tolerance. Unfortunately, no single agent meets all these independent criteria. In the past 10 years, newer benzodiazepines (BzRAs) with shorter half‐lives have been shown to be efficacious in reducing sleep latency, but the problem of sleep maintenance without next‐day sedation persists.1 To choose an appropriate sleep agent, evaluate the drug's efficacy, mechanism of action, and side‐effect profile. Then, match these characteristics with the patient's clinical condition(s). In patients with comorbid sleep and psychiatric problems, consider using a sedating psychotropic at bedtime to promote sleep.
Non‐Food and Drug AdministrationApproved (Off‐Label) Sleep Aids: Psychotropic Medications
Limited data exist on the efficacy of non‐Food and Drug Administration (FDA)approved medications for insomnia,2 such as antidepressants and atypical antipsychotics (AAPs), and antihistamines; examples of which are listed in Table 4. The administration of antihistamines, barbiturates, chloral hydrate, and alternative/herbal therapies has been discouraged, because the benefits rarely outweigh the risks associated with their use. Currently, trazodone is the most commonly prescribed antidepressant for the treatment of insomnia, despite the relative lack of data regarding its use for insomnia.3 Prescription data suggest that trazodoneat hypnotic doses, which are lower than the full antidepressant doseis more commonly prescribed for insomnia rather than for its FDA‐approved use for depression.4 In general, sleep specialists refrain from recommending sedating antidepressants for primary insomnia due to insufficient data regarding efficacy and safety. In addition, trazodone has been associated with arrhythmias in patients with preexisting cardiac conduction system disease. Curry et al.3 speculated that trazodone is popular among prescribers because, unlike most BzRAs, trazodone does not have a recommended limited duration of use and is perceived as being safer than BzRAs. Walsh et al.5 conducted a randomized double‐blind, placebo‐controlled trial (n = 589) that compared the hypnotic efficacy and other sleep‐associated variables of trazodone (50 mg) and zolpidem (10 mg). During the first week of treatment, the subjects on trazodone or zolpidem decreased their time to fall asleep, or sleep latency, by 22% and 35%, respectively, compared to placebo. Sleep latency was significantly shorter on zolpidem (57.75 2.7 minutes) than for trazodone (57.7 + 4.0 minutes). By the second week, subjects on zolpidem continued to have a reduction in the time to fall asleep, but there was no significant difference between subjects on trazodone and placebo.5 Trazodone may be an acceptable short‐term alternative to BzRAs for patients with hypercapnia or hypoxemia, and in those with a history of drug abuse or dependence. At doses of 150 to 450 mg, trazodone may be an appropriate medication in patients with major depressive disorder and problems with sleep maintenance.6 Tolerance to trazodone's sedating property tends to develop after 2 weeks of treatment, however, so other treatments may need to be considered if sleep problems persist. The available data address relatively short‐term use of trazodone, so questions of safety and efficacy for chronic insomnia remain unanswered.
| Drug | Pertinent Side Effects | Comments |
|---|---|---|
| ||
| Antidepressants | ||
| Mirtazapine (Remeron) | Somnolence, appetite, weight, dry mouth | May be beneficial for comorbid depression and insomnia. Lower doses (15 mg) increase sedation. |
| Trazodone | Residual daytime sedation, headache, orthostatic hypotension, priapism, cardiac arrhythmias | May be beneficial for comorbid depression and insomnia. Not recommended as first‐line agent for insomnia.3 May be an alternative if BzRAs are contraindicated (severe hypercapnia or hypoxemia or history of substance abuse). Tolerance usually develops within 2 weeks. Lower doses (50100 mg) than when used for depression (400 mg). |
| TCAs | Delirium, cognition, seizure threshold, orthostatic hypotension, tachycardia, acquired prolonged QT syndrome, heart block, acute hepatitis | Avoid in hospitalized patients due to their anticholinergic, antihistaminic, and cardiovascular side effects. May be beneficial for comorbid depression and insomnia. |
| Antihistamines | ||
| Diphenhydramine (Benadryl) | Residual daytime sedation, delirium, orthostatic hypotension, psychomotor function, prolonged QT syndrome, blurred vision, urinary retention | Better than placebo to treat insomnia,12 but data is lacking to definitively endorse diphenhydramine for insomnia.13 Tolerance to antihistamines develops within a few days. Avoid in patients >60 years old.18 |
| Hydroxyzine | Drowsiness, dry mouth, dizziness, agitation, cognitive function | Efficacy as anxiolytic for >4 months use not established. Not FDA‐approved for insomnia. Avoid in patients >60 years old, closed‐angle glaucoma, prostatic hypertrophy, severe asthma, and COPD. |
| Antipsychotics | ||
| Quetiapine (Seroquel) | Sedation, orthostatic hypotension, hyperglycemia, appetite, weight, hyperlipidemia | The most sedating of the atypical antipsychotics, it is frequently used as a sleep aid. Not recommended for insomnia or other sleep problems unless there is a comorbid psychiatric disorder. Dosed lower (25100 mg) when used for insomnia versus for FDA‐approved indications (600 mg). |
| Olanzapine (Zyprexa) | Sedation, hyperglycemia, appetite, weight, hyperlipidemia | Of atypical antipsychotics, olanzapine is the most likely to cause metabolic complications. Should not be used solely for insomnia. |
| Barbiturate | ||
| Chloral hydrate | Oversedation, respiratory depression, nausea, vomiting, diarrhea, drowsiness, cognitive function, psychotic symptoms (paranoia, hallucinations), vertigo, dizziness, headache | Chloral hydrate has been used for the short‐term (<2 weeks) treatment of insomnia, but is currently not FDA‐approved for that indication. Additive CNS depression may occur if given with other sedative‐hypnotics. Caution in patients with severe cardiac disease. Contraindicated in marked hepatic or renal impairment. Highly lethal in overdose, and should be avoided in patients with risk of suicide. |
Mirtazapine (Remeron), which promotes both sleep and appetite, may be particularly helpful for patients with cancer, acquired immunodeficiency syndrome (AIDS), and other conditions in which the triad of poor sleep, anorexia, and depression are common. Mirtazapine is a noradrenergic and specific serotonergic agent that causes inverse, dose‐dependent sedation (doses 15 mg are less sedating).7 To target sleeplessness, start with a dose between 7.5 and 15 mg. If ineffective at this dose, it is unlikely that increasing the dose will be of benefit for sleep. A small randomized, double‐blind, placebo‐controlled trial found that low‐dose mirtazapine reduced the apnea‐hypopnea index (API) by half in newly‐diagnosed subjects with OSA (n = 12).8 The results were promising in terms of the use of mixed‐profile serotonergic drugs in treating OSA. However, as pointed out by the researchers, mirtazapine's tendency to cause weight gain, is problematic in this patient population.
Although sedating, tricyclic antidepressants (TCAs) should not be used to promote sleep in hospitalized patients. TCAs increase the risk of cardiac conduction abnormalities, decrease seizure threshold, and have significant anticholinergic and anti‐alpha‐adrenergic effects. In dementia patients, the anticholinergic effect of TCAs may precipitate delirium.
AAPs should not be used routinely as first‐line agents for insomnia, except in patients who are in the midst of acute manic or psychotic episodes.9 With chronic use of AAPs, the risks of hyperglycemia, hyperlipidemia, and weight gain outweigh the potential sleep benefits of these agents. AAPs, especially risperidone, may cause extrapyramidal syndrome (EPS). Risperidone, ziprasidone and quetiapine have been associated with prolonged QTc interval, but the relatively low doses of AAPs that are used purely for sedative purposes makes this risk relatively low. If a patient has a history of Parkinsonism or other EPS, risperidone should generally be avoided. If a patient treated with risperidone develops EPS, another AAP should be considered. A reasonable precaution is to obtain a pretreatment 12‐lead electrocardiogram. If the QTc is greater than 450 msec, consider using olanzapine rather than ziprasidone, risperidone, or quetiapine. Sedating AAPs include risperidone (Risperdal), olanzapine (Zyprexa), and quetiapine (Seroquel), with the latter 2 being especially sedating. Quetiapine may also cause orthostatic hypotension. The recent practice of using AAPs for delirium has not been reported to be associated with significant safety risks, probably because delirium treatment is typically of short duration under a period of close clinical observation. These agents should not be used indefinitely for insomnia without close monitoring of metabolic, psychiatric, and neurologic status. However, recent data suggest that the risk of serious adverse effects of AAPs may outweigh the potential benefits for the treatment of aggression or agitation in patients with Alzheimer's disease.10
A meta‐analysis of randomized placebo‐controlled trials of AAP use among dementia patients showed that overall, the use of AAP drugs for periods of less than 8 to 12 weeks was associated with a small increased risk for death compared with placebo.11 Data indicated that most patients' behaviors improved substantially during the first 1 to 4 weeks of treatment. In a double‐blind, placebo‐controlled trial, 421 patients with Alzheimer's disease and psychosis, aggression or agitation were randomly assigned to receive olanzapine (mean dose, 5.5 mg per day), quetiapine (mean dose, 56.5 mg per day), risperidone (mean dose, 1.0 mg per day), or placebo. Improvement was observed in 32% of patients assigned to olanzapine, 26% of patients assigned to quetiapine, 29% of patients assigned to risperidone, and 21% of patients assigned to placebo. A lower, but significant, proportion of the patients (24%, 16%, 18%, and 5%, respectively) discontinued these medications due to intolerable side effects. Thus, if minimal improvement is observed even after 8 weeks of treatment, prescribers should consider discontinuing the AAP. The management of agitation in dementia, particularly in the elderly, calls for an integrative and creative psychopharmacological approach, including the use of antidepressants, nonbenzodiazepine anxiolytics such as buspirone, and mood stabilizers such as divalproex sodium (Depakote) before exposing patients to the risks of AAPs.
Antihistamines are the most commonly used over‐the‐counter agents for chronic insomnia.1 Diphenhydramine (Benadryl) has been shown to be better than placebo to treat insomnia,12 but data is lacking to definitively endorse its use to promote sleep.13 Diphenhydramine is also limited by the development of tolerance within a few days of daily use. The anticholinergic action of antihistamines may lead to orthostatic hypotension, urinary retention, and may induce delirium in vulnerable patients. Therefore, diphenhydramine should be avoided in hospitalized patients.
Recent data suggest that hydroxyzine, an antihistamine, may be an appropriate sleep aid for patients with hepatic encephalopathy in whom BzRAs are contraindicated.14 Subjective improvement in sleep was observed in 40% of hydroxyzine‐treated patients with hepatic encephalopathy compared to placebo.
Chloral hydrate is one of the Western world's oldest known sedative‐hypnotics and was commonly used as a sleep aid through the 1970s.15 Chloral hydrate was eventually supplanted by BzRAs,16 and fell out of favor as a sleep aid due to its relatively high tolerance rate, drug‐drug interaction profile, and the high risk of death in an overdose. Doses of 500 to 1000 mg sufficed to promote sleep in most of the hospitalized subjects. More recent data regarding its use for treating insomnia are not available, but chloral hydrate may be an alternative short‐term treatment for insomnia in selected hospitalized patients. Because of its high‐risk profile, chloral hydrate would be used as a last‐resort medication, preferably with input from critical care and/or sleep medicine specialists.
FDA‐Approved Sleep Aids
As shown in Table 5, the FDA has approved 3 classes of medications for the treatment of insomnia: benzodiazepine gamma‐aminobutyric acid (GABA)A receptor agonists (BzRAs), nonbenzodiazepine GABAA receptor agonists (non‐BzRAs), and melatonin‐receptor agonists.17 BzRAs include estazolam (ProSom), flurazepam (Dalmane), quazepam (Doral), temazepam (Restoril), and triazolam (Halcion). Though BzRAs decrease sleep latency, increase TST, and decrease slow wave or deep sleep, they also have adverse side effects such as daytime sedation, anterograde amnesia, cognitive impairment, motor incoordination, dependence, tolerance, and rebound insomnia.18 Because of these side effects, BzRAs should be limited to generally healthy, young (ie, <45 years old) patients who are expected to have brief hospital stays.
| Drugs | Adult Dose (mg) | Half‐Life (hours)* | Onset (minutes) | Peak Effect (hours) | Major Effects/Clinical Comments |
|---|---|---|---|---|---|
| |||||
| BzRAs | Caution in elderly patients. Tolerance to BzRAs develop to the sedative, hypnotic, and anticonvulsant effects. | ||||
| Estazolam (ProSom) | 12 | 1024 | 60 | 0.51.5 | Short‐term (710 days) treatment for frequent arousals, early morning awakening. Not as useful for sleep onset. Avoid in patients with OSA. Caution in elderly patients, liver disease. High doses can cause respiratory depression. |
| Flurazepam (Dalmane) | 1530 | 47100 | 1520 | 36 | In general, avoid in hospitalized medical patients, especially elderly patients. |
| Quazepam (Doral) | 7.515 | 25114 | 1.5 | In general, avoid in hospitalized medical patients, especially elderly patients. | |
| Temazepam (Restoril) | 1530 | 616 | 23 | Short‐term (710 days) treatment for sleep onset and maintenance. Doses 30 mg/day: morning grogginess, nausea, headache, and vivid dreaming. | |
| Triazolam (Halcion) | 0.1250.25 | 1.55.5 | 1530 | 1.75 | Maximum dose is 0.5 mg. Short‐term (710 days) treatment. Rapid onset; should be in bed when taking medication. Contraindicated with atazanavir, ketoconazole, itraconazole, nefazodone, ritonavir. |
| Non‐BzRAs | |||||
| Eszopiclone (Lunesta) | 23 | 69 | 1 | In elderly: difficulty falling asleep, then initial: 1 mg; maximum 2 mg. Difficulty staying asleep: 2 mg. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with high‐fat foods. No tolerance after 6 months. | |
| Zaleplon (Sonata) | 520 | 1 | Rapid | 1 | Short‐term (710 days) treatment for falling asleep and/or next‐day wakefulness is crucial (eg, shift workers). |
| Zopiclone (Imovane) | 515 | 3.86.5 (510 in elderly) | 30 | <2 | Transient and short‐term (710 days) treatment. Contraindicated in severe respiratory impairment. Caution in liver disease and depression; elderly prone to side effects. Anticholinergic agents may plasma level. |
| Zolpidem (Ambien) | 520 | 1.44.5 | 30 | 2 | Short‐term (710 days) treatment for sleep onset and maintenance. Rapid onset; should be in bed when taking medication. For faster sleep onset, do not ingest with food. No tolerance after 50 weeks. |
| Melatonin agonist | |||||
| Ramelton (Rozerem) | 8 | 12 | 30 | 11.5 | For sleep onset. For faster sleep onset, do not ingest with high‐fat foods. No tolerance. Contraindicated with fluvoxamine. |
Efficacy and safety studies have generally been limited to healthy, younger individuals without a history of primary sleep disorder. Potential adverse effects of BzRAs may become even more pronounced in hospitalized medical patients due to older age, acute illness, cointeraction drugs, and multidrug regimens. Although BzRAs are FDA‐approved for the treatment of insomnia, flurazepam and quazepam should generally be avoided in hospitalized patients. These agents' long half‐lives increase the risk of drug‐drug interactions and adverse events such as respiratory depression, cognitive decline, and delirium in acutely ill patients. For similar reasons, other long‐acting BzRAs such as clonazepam (Klonopin) and diazepam (Valium) should also not be used to treat insomnia in hospitalized patients. An exception to this is a patient with RLS, in which clonazepam is an approved treatment. However, now that ropinirole HCl (Requip) is FDA‐approved for RLS, BzARs may be able to be avoided. Lorazepam (Ativan), due to its relatively short half‐life and its anxiolytic property, is frequently used to treat insomnia in hospitalized medical patients.18 Start with the lowest dose possible (eg, 0.5 mg) as a one‐time‐only order, or on a as needed basis for 3 days. Alprazolam (Xanax), a potent, fast‐acting BzRA with a relatively short half‐life, has developed a reputation as being notoriously addictive, and experts feel alprazolam has similar potential for withdrawal and rebound.19, 20
The use of BzRAs should be minimized in all patients, and avoided in the elderly or those with a particularly high risk for delirium (eg, traumatic brain injury, stroke, multiple new medications). All BzRAs should be avoided in patients with a prior history of sedative‐hypnotic and/or alcohol dependence unless medically indicated, such as in alcohol withdrawal. Refrain from ordering nightly scheduled BzRAs without a specific time limit to ensure that sedative‐hypnotic use is closely monitored.
For the past 2 decades, physicians have been advised against using long‐acting BzRAs in the elderly (>65 years old) due to the increased risks of hip fractures, falls, motor vehicle accidents, daytime sedation, and adverse cognitive events such as delirium.2124 A large 5‐year prospective study in Quebec found that the risk of injury varied by the BzRA, and was independent of half‐life.25 Importantly, the risk of injury was dose‐dependent: the higher the dose of oxazepam, flurazepam, or chlordiazepoxide, the higher the risk of injury in the elderly.
Non‐BzRAs seem to have a superior side‐effect profile when compared to BzRAs, but should also be used with caution in the elderly. Non‐BzRAs include eszopiclone (Lunesta), zaleplon (Sonata), zolpidem (Ambien), and zolpidem extended‐release. The number of comparison studies is limited, but the available data reveal that: (1) zolpidem (Ambien) may be better than temazepam (Restoril) in terms of sleep latency and quality; and (2) zaleplon (Sonata) may lead to a shorter sleep latency than zolpidem (Ambien), but the latter is associated with longer sleep duration.26 Non‐BzRAs have less next‐day sedation, psychomotor dysfunction, tolerance/withdrawal, and rapid‐eye‐movement (REM) sleep rebound; and lower abuse potential than BzRAs.27
The most commonly prescribed hypnotic, zolpidem has a short half‐life, and seems to reduce sleep latency with minimal residual side effects when compared to BzRAs. The results of a recent multicenter, randomized, double‐blind, placebo‐controlled trial indicated that zolpidem extended‐release may be efficacious for up to 6 months in outpatients with chronic insomnia.28
The sole melatonin‐receptor agonist, ramelteon (Rozerem), also reduces time to fall asleep without next‐day psychomotor and memory effects.29 Ramelteon is believed to target receptors melatonin 1 and 2 receptors located in the brain's suprachiasmatic nucleus to stabilize circadian rhythms and stabilize the sleep‐wake cycle.30
CONCLUSION
Hospitalization is often associated with disrupted sleep, which can affect recovery from illness. Understanding the major factors that impair sleep during hospitalization allows clinicians to systemically evaluate and treat sleep problems. More than just prescribing a sedative/hypnotic, the treatment for sleep disruption includes addressing sleep hygiene and hospital environment issues, identifying medications that could disrupt sleep, and treating specific syndromes that impair sleep. We suggest a practical algorithm to guide clinical assessment, treatment options, and selection of appropriate sleeping medications. Critical to optimizing recovery from illness, sleep may be considered as the sixth vital sign, and should be part of the routine evaluation of every hospitalized patient.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.
- .Sleep maintenance insomnia: strengths and weaknesses of current pharmacologic therapies.Ann Clin Psychiatry.2006;18(1):49–56.
- ,.Treatment of insomnia.Prim Psychiatry.2005;12(8):47–56.
- ,,.Pharmacologic management of insomnia: past, present, and future.Psychiatr Clin North Am.2006;29:871–893.
- ,.“Hypnotic” prescription patterns in a large managed‐care population.Sleep Med.2004;5(5):463–466.
- ,,, et al.Subjective hypnotic efficacy of trazodone and zolpidem in DSM III‐R primary insomnia.Hum Psychopharmacol.1998;13:191–198.
- ,,, et al.Mirtazapine is more effective than trazodone: a double‐blind controlled study in hospitalized patients with major depression.Int Clin Psychopharmacol.1995;10:3–9.
- ,,.Mirtazapine: an antidepressant with noradrenergic and specific serotonergic effects.Pharmacotherapy.1997;17:10–21.
- ,,,.Efficacy of mirtazapine in obstructive sleep apnea syndrome.Sleep.2007;30(1):35–41.
- ,.Atypical antipsychotics in bipolar disorder: systematic review of randomised trials.BMC Psychiatry.2007;7:40:1–17.
- ,,, et al.Effectiveness of atypical antipsychotic drugs in patients with Alzheimer's Disease.N Engl J Med.2006;355:1525–1538.
- ,,.Risk of death with atypical antipsychotic drug treatment for dementia: meta‐analysis of randomized placebo‐controlled trials.JAMA.2005;294(15):1934–1943.
- ,.Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double‐blind study.J Clin Pharmacol.1983;23:234–242.
- .Diagnosis and treatment of chronic insomnia: a review.Psychiatr Serv.2005;56:332–343.
- ,,,,.Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial.Am J Gastroenterol.2007;102:744–753.
- ,.Clinical effects of chloral hydrate in hospitalized medical patients.J Clin Pharmacol.1979;19(10):669–674.
- Miller RD, editor.Miller's Anesthesia.6th ed.Philadelphia, PA:Elsevier;2005.
- .State‐of‐the‐art sleep management. Awakening insomnia management. Proceedings from a satellite symposium at SLEEP 2006: 20th Anniversary Meeting of the Associated Professional Sleep Societies, Salt Lake City, UT.2006:6–12.
- ,,.Use of a computer‐based reminder to improve sedative‐hypnotic prescribing in older hospitalized patients.J Am Geriatr Soc.2007;55:43–47.
- ,,,.Topiramate use in alprazolam addiction.World J Biol Psychiatry.2006;7(4):265–267.
- ,,,.Trends in recommendations for the pharmacotherapy of anxiety disorders by an international expert panel, 1992–1997.Eur Neuropsychopharmacol.1999;9(Suppl 6):S393–S398.
- ,,,,.Benzodiazepine use and the risk of motor vehicle crash in the elderly.JAMA.1997;278:27–31.
- ,,,,.Psychotropic drug use and the risk of hip fracture.NEngl J Med.1987;316:363–369.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169–1175.
- ,,,,,.Delirium in hospitalized older persons: outcomes and predictors.J Am Geriatr Soc.1994;42:809–815.
- ,,,,.A 5‐year prospective assessment of the risk associated with individual benzodiazdepines and doses in new elderly users.J Am Geriatr Soc.2005;53:233–241.
- ,,, et al.Newer hypnotic drugs for the short‐term management of insomnia: a systematic review and economic evaluation.Health Technol Assess.2004;19:305–322.
- .Medications and their effect on sleep.Prim Care Clin Off Pract.2005;32:401–509.
- ,,,,.Long‐term efficacy and safety of zolpidem extended‐release 12.5 mg, administered 3 to 7 nights per week for 24 weeks, in patients with chronic primary insomnia: a 6‐month, randomized, double‐blind, placebo‐controlled, parallel‐group, multicenter study.Sleep.2008;31(1):79–90.
- ,,,.An efficacy, safety, and dose‐response study of Ramelteon in patients with chronic primary insomnia.Sleep Med.2006;7(1):17–24.
- ,.Melatonin, sleep, and circadian rhythms: rationale for development of specific melatonin agonists.Sleep Med.2004;5(6):523–532.