To the Editor:
Pityriasis rubra pilaris (PRP) is a rare papulosquamous condition with an unknown pathogenesis and limited efficacy data, which can make treatment challenging. Some cases of PRP spontaneously resolve in a few months, which is most common in the pediatric population.1 Pityriasis rubra pilaris in adults is likely to persist for years, and spontaneous resolution is unpredictable. Randomized clinical trials are difficult to perform due to the rarity of PRP.
Although there is no cure and no standard protocol for treating PRP, systemic retinoids historically are considered first-line therapy for moderate to severe cases.2 Additional management approaches include symptomatic control with moisturizers and psychological support. Alternative systemic treatments for moderate to severe cases include methotrexate, phototherapy, and cyclosporine.2
Pityriasis rubra pilaris demonstrates a favorable response to methotrexate treatment, especially in type I cases; however, patients on this alternative therapy should be monitored for severe adverse effects (eg, hepatotoxicity, pancytopenia, pneumonitis).2 Phototherapy should be approached with caution. Narrowband UVB, UVA1, and psoralen plus UVA therapy have successfully treated PRP; however, the response is variable. In some cases, the opposite effect can occur, in which the condition is photoaggravated. Phototherapy is a valid alternative form of treatment when used in combination with acitretin, and a phototest should be performed prior to starting this regimen. Cyclosporine is another immunosuppressant that can be considered for PRP treatment, though there are limited data demonstrating its efficacy.2
The introduction of biologic agents has changed the treatment approach for many dermatologic diseases, including PRP. Given the similar features between psoriasis and PRP, the biologics prescribed for psoriasis therapy also are used for patients with PRP that is challenging to treat, such as anti–tumor necrosis factor α inhibitors and IL inhibitors—specifically IL-17 and IL-23. Remission has been achieved with the use of biologics in combination with retinoid therapy.2
Biologic therapies used for PRP effectively inhibit cytokines and reduce the overall inflammatory processes involved in the development of the scaly patches and plaques seen in this condition. However, most reported clinical experiences are case studies, and more research in the form of randomized clinical trials is needed to understand the efficacy and long-term effects of this form of treatment in PRP. We present a case of a patient with refractory adult subtype I PRP that was successfully treated with the IL-23 inhibitor risankizumab.
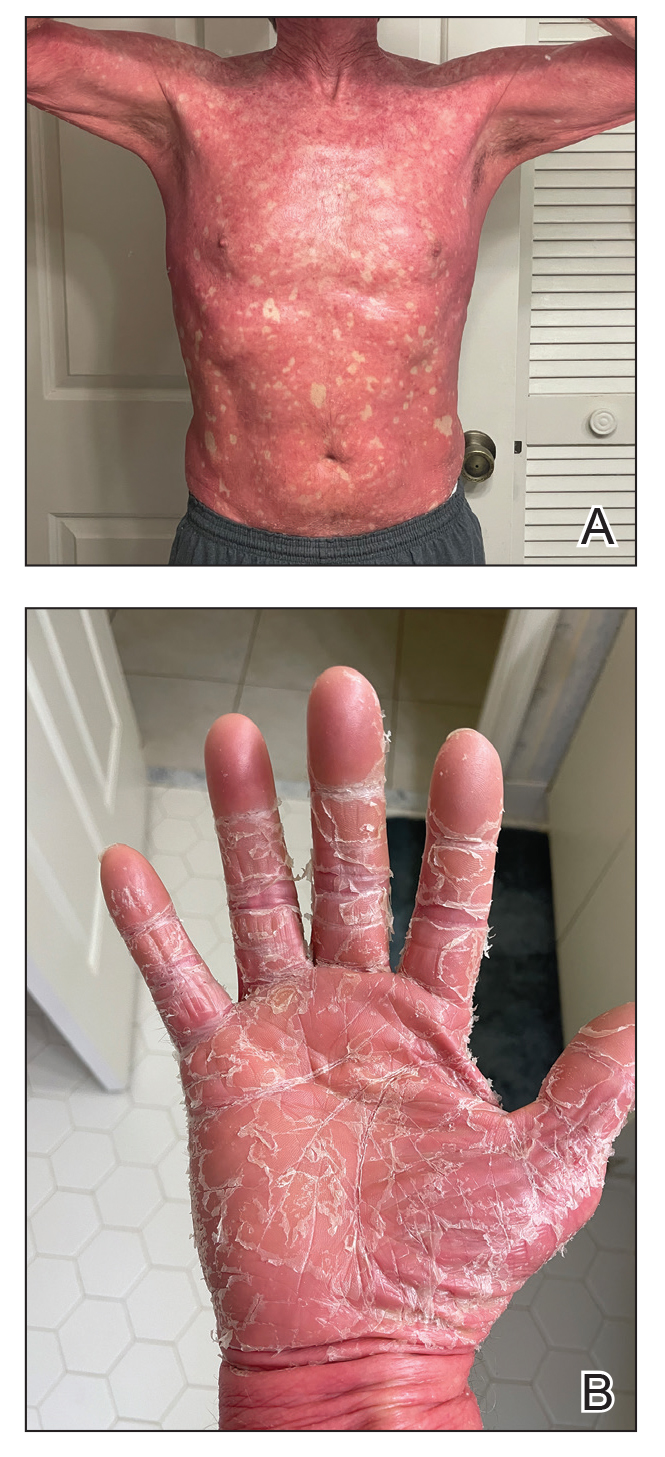
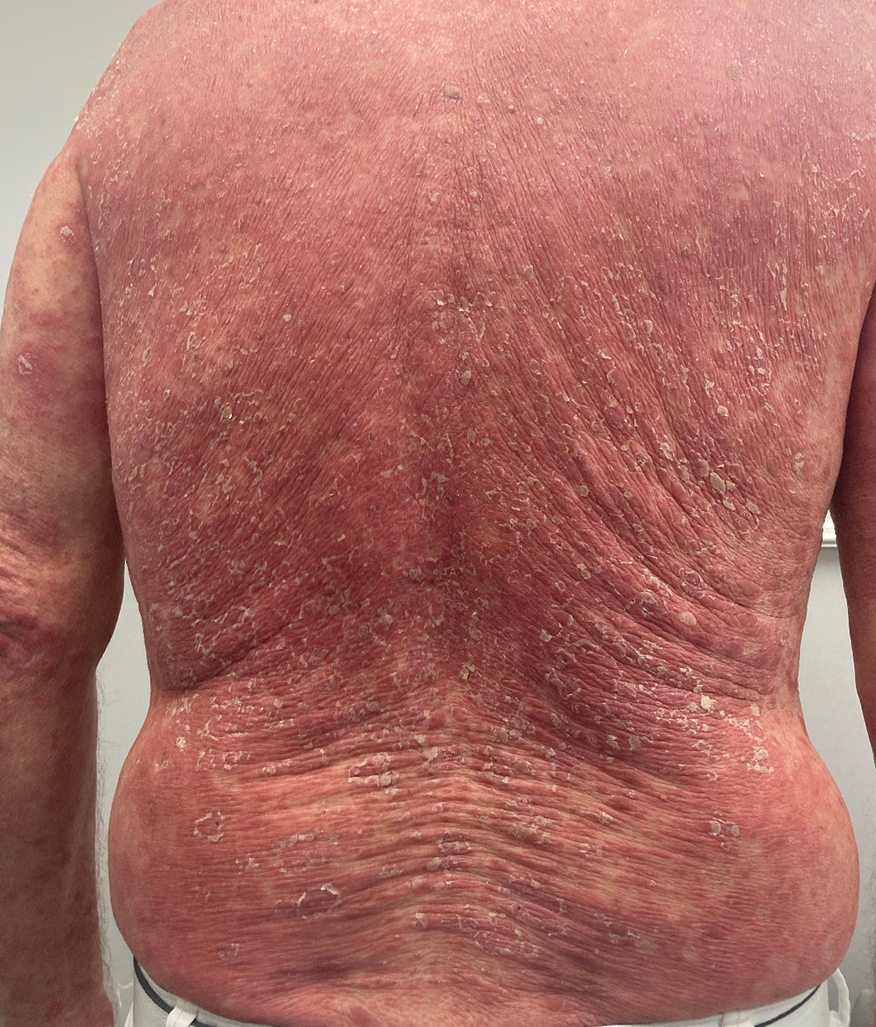
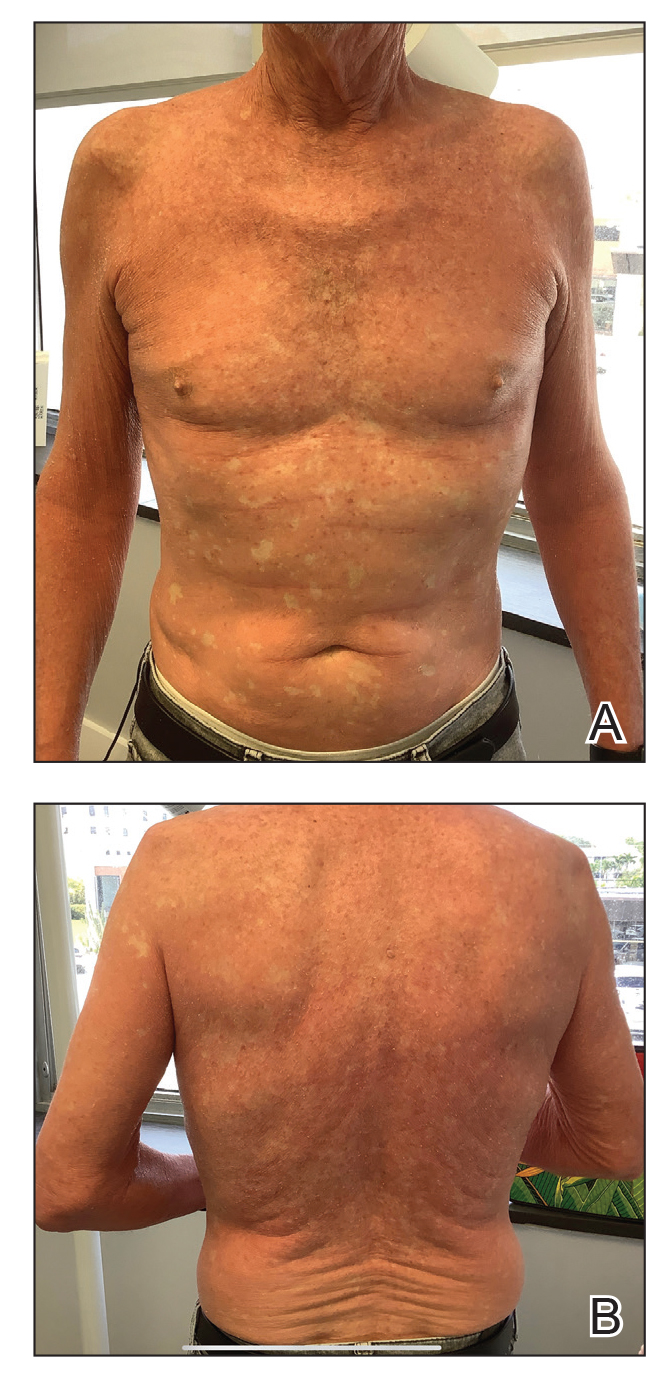
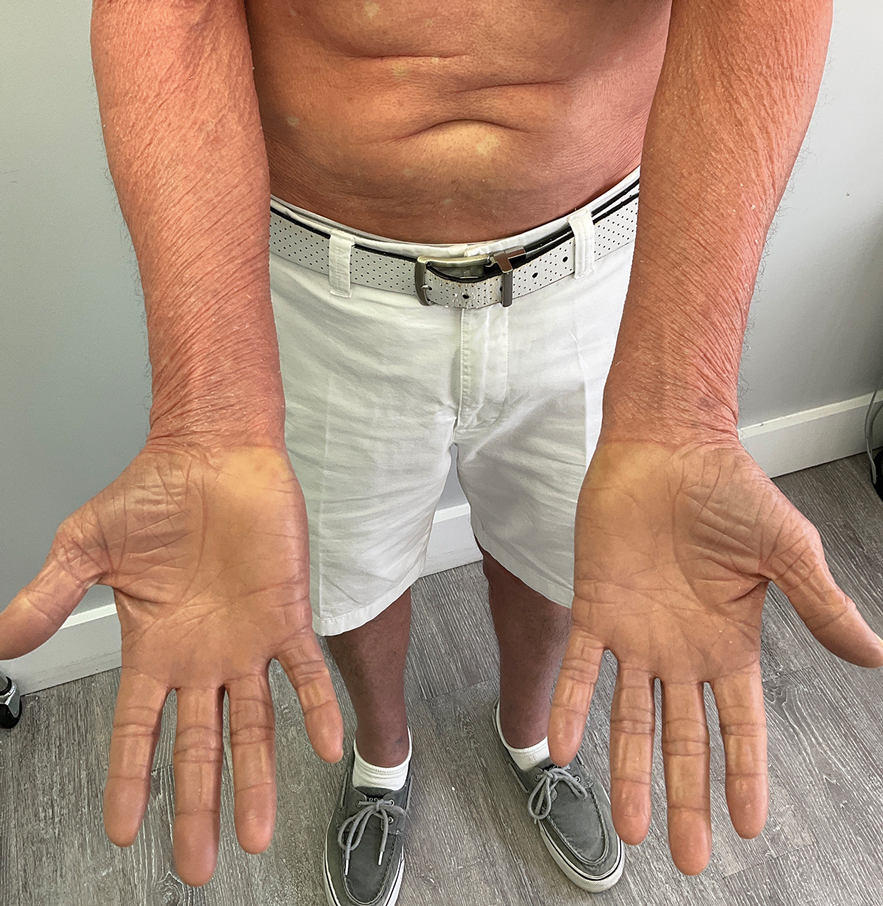
A 65-year-old man was referred to Florida Academic Dermatology Center (Coral Gables, Florida) with biopsy-proven PRP diagnosed 1 year prior. The patient reported experiencing a debilitating quality of life in the year since diagnosis (Figure 1). Treatment attempts with dupilumab, tralokinumab, intramuscular steroid injections, and topical corticosteroids had failed (Figure 2). Following evaluation at Florida Academic Dermatology Center, the patient was started on acitretin 25 mg every other day and received an initial subcutaneous injection of ixekizumab 160 mg (an IL-17 inhibitor) followed 2 weeks later by a second injection of 80 mg. After the 2 doses of ixekizumab, the patient’s condition worsened with the development of pinpoint hemorrhagic lesions. The medication was discontinued, and he was started on risankizumab 150 mg at the approved dosing regimen for plaque psoriasis in combination with the acitretin therapy. Prior to starting risankizumab, the affected body surface area (BSA) was 80%. At 1-month follow-up, he showed improvement with reduction in scaling and erythema and an affected BSA of 30% (Figure 3). At 4-month follow-up, he continued showing improvement with an affected BSA of 10% (Figure 4). Acitretin was discontinued, and the patient has been successfully maintained on risankizumab 150 mg/mL subcutaneous injections every 12 weeks since.
Oral retinoid therapy historically was considered first-line therapy for moderate to severe PRP. A systematic review (N=105) of retinoid therapies showed 83% of patients with PRP who were treated with acitretin plus biologic therapy had a favorable response, whereas only 36% of patients treated with acitretin as monotherapy had the same response, highlighting the importance of dual therapy.3 The use of ustekinumab, ixekizumab, and secukinumab (IL-17 inhibitors) for refractory PRP has been well documented, but a PubMed search of articles indexed for MEDLINE using the search terms risankizumab and pityriasis rubra pilaris yielded only 8 published cases of risankizumab for treatment of PRP.4-8 All patients were diagnosed with refractory PRP, and multiple treatment modalities failed.
Ustekinumab has been shown to create a rapid response and maintain it long term, especially in patients with type 1 PRP who did not respond to systemic therapies or anti–tumor necrosis factor α agents.2 An open-label, single-arm clinical trial found secukinumab was an effective therapy for PRP and demonstrated transcription heterogeneity of this dermatologic condition.9 The researchers proposed that some patients may respond to IL-17 inhibitors but others may not due to the differences in RNA molecules transcribed.9 Our patient demonstrated worsening of his condition with an IL-17 inhibitor but experienced remarkable improvement with risankizumab, an IL-23 inhibitor.
Risankizumab is indicated for the treatment of adults with moderate to severe plaque psoriasis. This humanized IgG1 monoclonal antibody targets the p19 subunit of IL-23, inhibiting its role in the pathogenic helper T cell (TH17) pathway. Research has shown that it is an efficacious and well-tolerated treatment modality for psoriatic conditions.10 It is well known that PRP and psoriasis have similar cytokine activations; therefore, we propose that combination therapy with risankizumab and acitretin may show promise for refractory PRP.