User login
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
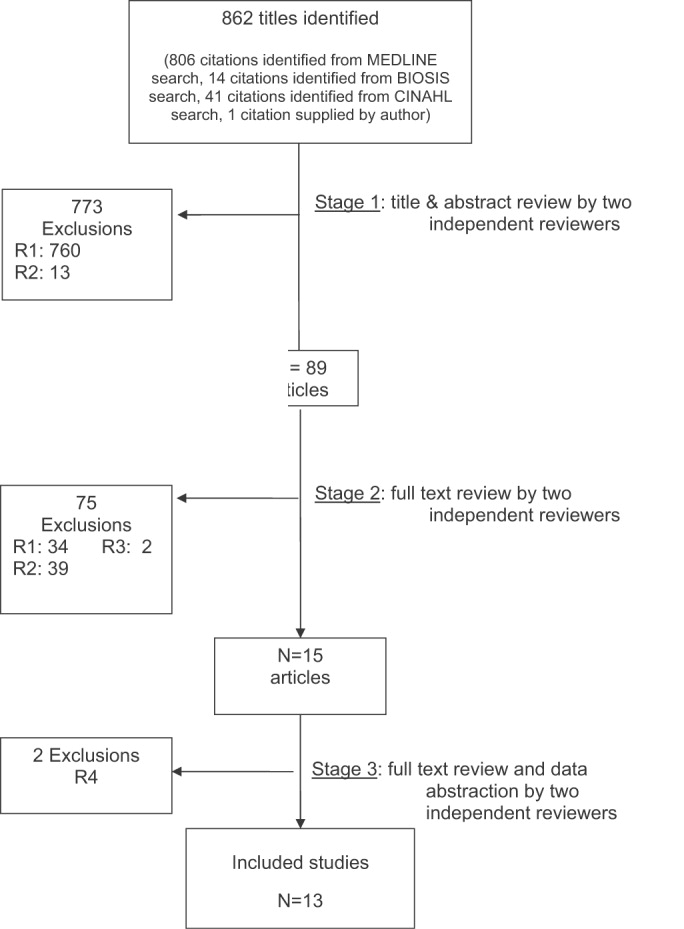
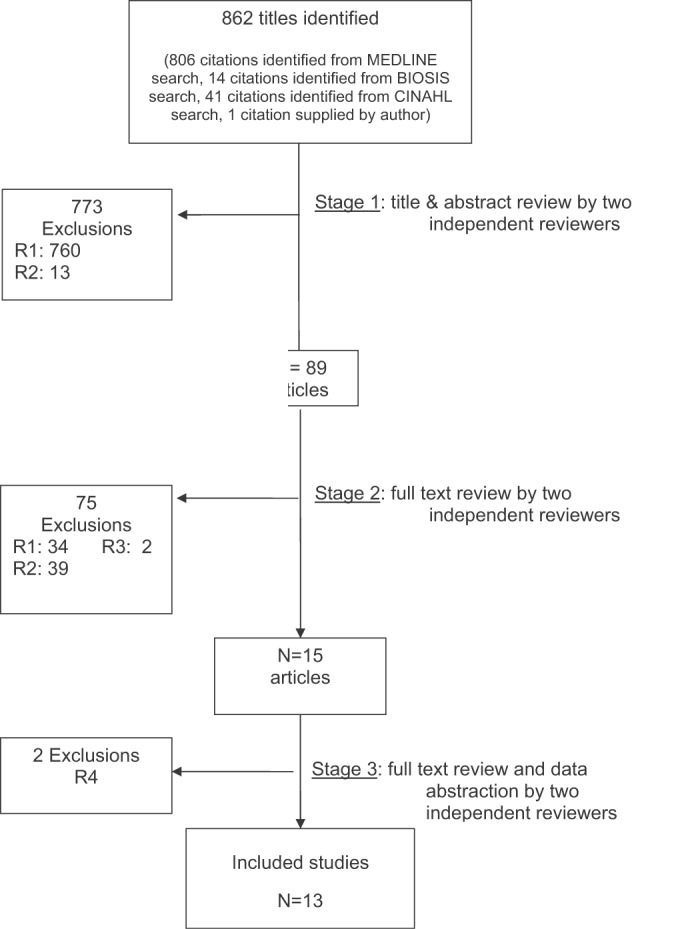
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.
Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
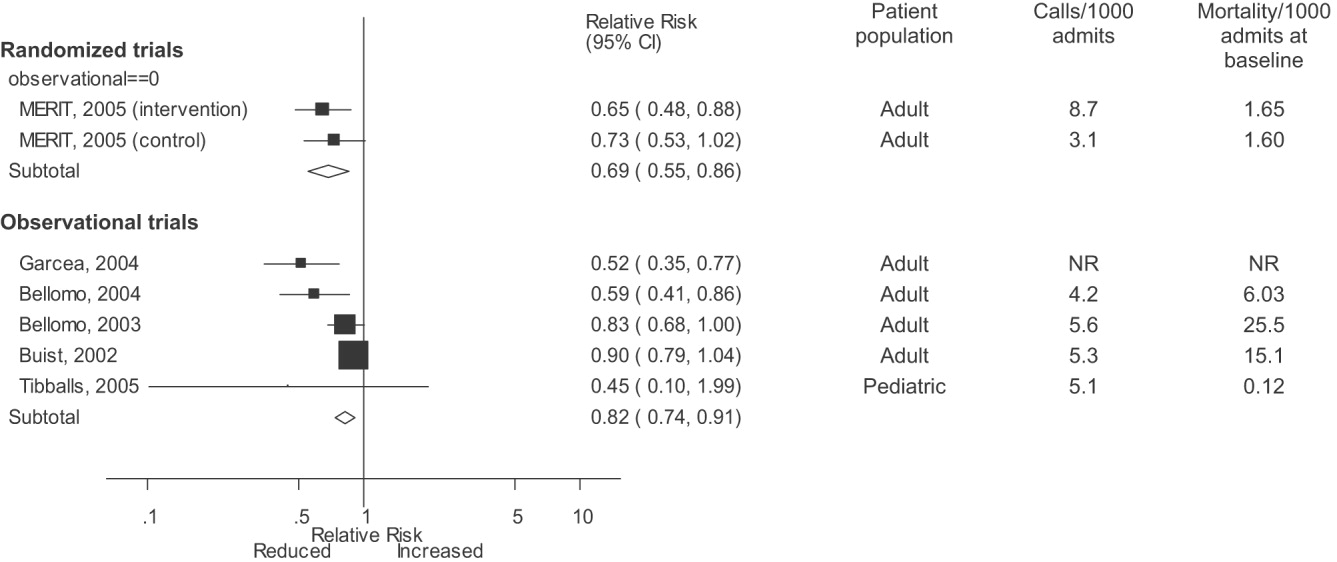
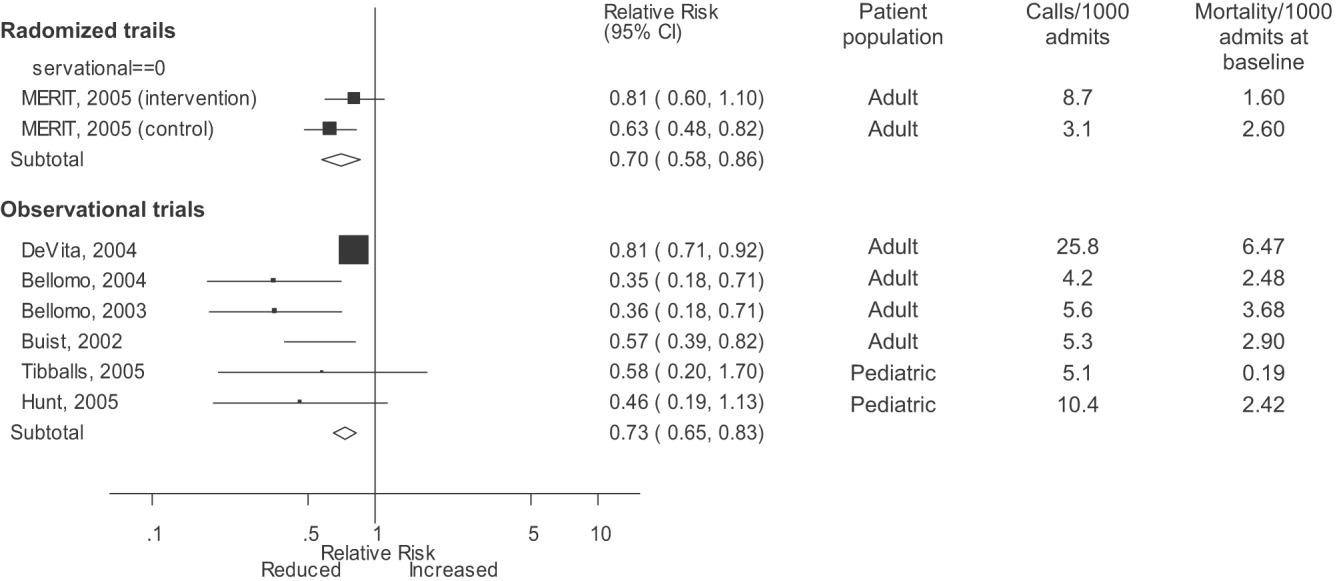
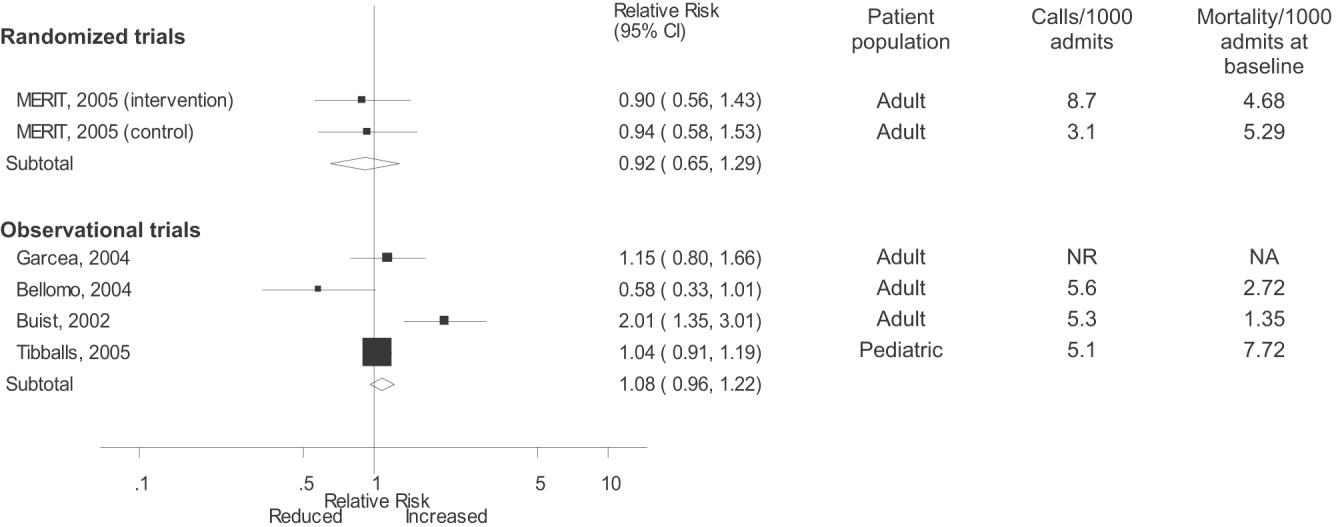
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).
DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.

Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).



DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
A medical emergency team1 is a group of clinicians trained to quickly assess and treat hospitalized patients showing acute signs of clinical deterioration. Equivalent terms used are rapid response team,2 critical care outreach team,3 and patient‐at‐risk team.4 A consensus panel5 recently endorsed use of the term rapid response system (RRS) to denote any system that uses a standard set of clinical criteria to summon caregivers to the bedside of a patient who is deemed unstable but not in cardiopulmonary arrest (in which case a standard resuscitation team would be summoned). Such teams primarily evaluate patients on general hospital wards.
RRSs have been developed in response to data indicating that patients frequently demonstrate premonitory signs or receive inadequate care prior to unanticipated intensive care unit (ICU) admission, cardiopulmonary arrest, or death outside the ICU.614 Earlier identification and treatment of such patients could prevent adverse clinical outcomes. The structure of RRSs varies but generally includes a physician and nurse and may also include other staff such as respiratory therapists.5 Teams are summoned by hospital staff to assess patients meeting specific clinical criteria (see box) about whom the bedside staff has significant concern.150
| Any staff member may call the team if 1 of the following criteria is met: |
| Heart rate > 140/min or < 40/min |
| Respiratory rate > 28/min or < 8/min |
| Systolic blood pressure > 180 mmHg or < 90 mm Hg |
| Oxygen saturation < 90% despite supplementation |
| Acute change in mental status |
| Urine output < 50 cc over 4 hours |
| Staff member has significant concern about patient's condition |
| Additional criteria used at some institutions: |
| Chest pain unrelieved by nitroglycerin |
| Threatened airway |
| Seizure |
| Uncontrolled pain |
Initial studies of RRSs, performed primarily in Australia and the United Kingdom, showed promising reductions in unanticipated ICU admissions, cardiac arrests, and even overall inpatient mortality.1, 16, 17 The considerable enthusiasm generated by these studies18, 19 resulted in the Institute for Healthcare Improvement (IHI) incorporating RRSs into its 100,000 Lives campaign,2 and RRSs are now being implemented in the more than 3000 U.S. hospitals that joined the campaign. However, a recent commentary on rapid response teams20 and a systematic review of critical care outreach teams21 have raised concerns that this widespread implementation may not be justified by the available evidence. We performed a systematic review of studies of all variations of RRSs in order to determine their effect on patient outcomes and to characterize variations in their organization and implementation.
METHODS
Literature Search and Inclusion and Exclusion Criteria
We systematically searched MEDLINE, CINAHL, and BIOSIS through August 2006 for relevant studies using the various terms for RRSs (eg, medical emergency team, rapid response team, critical care outreach) and medical subject headings relevant to inpatient care and critical illness (eg, patient care team and resuscitation; the full search strategy is given in the Appendix). We also reviewed the abstract lists from the 2004 and 2005 American Thoracic Society and Society of Critical Care Medicine annual meetings and scanned reference lists from key articles.
We screened the abstracts of the articles identified by the search, and 2 independent reviewers abstracted potentially relevant articles using a standardized data abstraction form. Disagreements between the reviewers were resolved by consensus and, if necessary, discussion with a third reviewer. We included randomized controlled trials (RCTs), controlled before‐after studies, and interrupted time series, including simple before‐after studies with no contemporaneous control group, though we planned to separately analyze data from controlled studies if possible. We included only English‐language articles.
On the basis of RRS features in widely cited articles2224 and the recommendations of a recent consensus statement,5 we defined an RRS as having the following characteristics: (1) its primary responsibility is to intervene whenever hospitalized patients become unstable before cardiopulmonary arrest occurs; (2) it must primarily provide care outside the ICU and emergency department; (3) specific clinical criteria must be in place that define instability and trigger a call to the team; and (4) it must be expected to respond within a specified time. We defined these criteria in order to distinguish studies of RRSs from studies of cardiac arrest (code blue) teams or traditional consulting services.
To be included in the analysis, articles had to report the effects of a rapid response system on at least 1 of these outcomes: inpatient mortality, inpatient cardiac arrest, or unscheduled ICU transfer. We used the definitions of cardiac arrest and unscheduled ICU transfer given in the primary studies. In addition to these outcomes, we abstracted information on the number of admissions and the number of RRS calls during the study period. To maximize the comparability of study outcomes, we calculated the rates of mortality, cardiac arrest, unscheduled ICU transfer, and RRS calls per 1000 admissions for studies that did not supply data in this fashion.
Assessment of Study Quality
Quality scoring instruments for studies included in systematic reviews generally focus on randomized controlled trials, which we anticipated would account for a minority of included studies. On the basis of recommendations for the assessment of methodology for nonrandomized study designs,25, 26 we identified and abstracted 4 important determinants of internal validity (Table 1). The consensus statement5 recommends monitoring the effectiveness of RRSs by measuring the rate of unscheduled ICU admissions (defined as an unplanned admission to the ICU from a general ward27) and cardiac arrests of patients who were not listed as do not resuscitate (DNR). As the definition of unscheduled ICU admission allows room for subjectivity, we considered the blinding of assessment of this outcome to study group assignment to be important, especially for retrospective studies. Measurement of cardiac arrests should be less susceptible to blinding issues, but one of the functions of an RRS can be to initiate discussions that result in changes in the goals of care and code status.22 Thus, excluding patients made DNR by the team from cardiac arrest calculations could falsely lower the cardiac arrest rate.
| Quality measures |
|---|
|
| A. Internal validity |
| 1. Did the study have a contemporaneous control group? |
| 2. If there was no contemporaneous control group, did the study report data for more than 1 time point before and after the intervention? |
| 3. Were nonobjective primary outcomes (eg, unplanned ICU transfer) measured in a blinded fashion? |
| 4. Were patients made DNR by the RRS included in calculations of the cardiac arrest and mortality rates? |
| B. Generalizability |
| 5. Was the intervention performed independent of other quality improvement interventions targeting the care of critically ill patients? |
| 6. Did the study report the number of admissions and RRS calls during the study period? |
| 7. Did the study report the availability of intensivists before and after the intervention? |
We also abstracted 3 separate elements of study quality pertaining to the external validity or generalizability of included studies (Table 1). These elements were defined a priori by consensus reached by discussion among the reviewers. These elements were intended to provide a framework for interpreting the included studies and to guide subgroup analyses. They were not used to form a composite quality score.
Statistical Analysis
We performed a random‐effects meta‐analysis to calculate summary risk ratios with 95% confidence intervals for the effects of RRSs on inpatient mortality, cardiopulmonary arrest, and unscheduled ICU admission. Included in the meta‐analysis were the studies that reported total number of admissions and incidence of each outcome before and after institution of the RRS. For randomized trials that reported pre‐ and postintervention data, we treated the intervention and control groups as separate trials in order to be able to compare their effects with the before‐after trials. For studies that reported results adjusted for clustering (ie, by hospital), we back‐calculated the unadjusted results by multiplying the standard error by the square of the design factor coefficient.28, 29 We calculated the I2 statistic to assess heterogeneity.30 All analyses were performed using Stata version 8.2 (Stata Corporation, College Station, TX).
RESULTS
The database searches identified 861 citations, and 1 additional prepublication study was supplied by the study's first author31; 89 articles underwent full‐text review (Fig. 1). Most studies excluded during the full‐text review did not meet our criteria for a study of an RRS or were observational studies or review articles. For instance, Sebat et al.32 published a study of a shock team at a community hospital that intervened when any patient suffered nontraumatic shock; the study did not meet our inclusion criteria as all patients were admitted to the ICU, most directly from the emergency department. Another frequently cited study, by Bristow et al.,16 was excluded as it was a case‐control study. Thirteen studies,3, 2224, 31, 334011 full‐length studies and 2 abstractsmet all criteria for inclusion.

Characteristics of Included Trials
The characteristics of included studies are outlined in Table 2. Five studies were performed in Australia, 4 in the United States, and 4 in the United Kingdom. All were conducted in academic teaching hospitals. Two studies37, 38 focused on pediatric inpatients, and the remainder involved hospitalized adults. The RRS intervened for all hospitalized patients in all but 2 studies (1 of which focused on surgical inpatients33 and the other in which the RRS evaluated only patients discharged from the ICU3). In 2 studies,31, 39 the RRS was available to evaluate outpatients, such as hospital visitors, in addition to inpatients.0
| Study | Location | Hospital type | Patient population | RRS composition | Measured outcomes | Definition of unscheduled ICU admission | Definition of cardiac arrest |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU registrar | DNR status | Not supplied | Any code blue team activation |
| Medical registrar | Cardiac arrest | ||||||
| ICU nurse | Unscheduled ICU admission | ||||||
| Mortality | |||||||
| Bellomo et al., 200321 | Australia | Tertiary‐care academic hospital | Adult inpatients | ICU nurse and ICU fellow attended all calls; ICU attending (8 AM‐8 PM) and medical registrar attended if requested | Mortality | NA | Unresponsive, with no pulse or blood pressure, and basic life support initiated |
| Cardiac arrest | |||||||
| DNR status | |||||||
| Pittard et al., 200322 | United Kingdom | Tertiary‐care academic hospital | Adult inpatients on surgical wards | Senior critical care nurses and medical staff | Unscheduled ICU admission | Any patient not admitted directly from operating theater after elective surgery | NA |
| Bellomo et al., 200431 | Australia | Tertiary‐care academic hospital | Adult postoperative patients | ICU attending | Mortality Unscheduled ICU admission ICU length of stay DNR status | Postoperative admission to ICU because of a clinical complication | NA |
| ICU fellow | Unscheduled ICU admission | ||||||
| ICU registered nurse | ICU length of stay | ||||||
| Medical fellow | DNR status | ||||||
| DeVita et al., 200432 | United States | Tertiary‐care academic hospital | Adult inpatients | ICU physician | Cardiac arrest | NA | Any code blue team activation |
| Anesthesiologist | |||||||
| 2 other physicians | |||||||
| 2 ICU nurses | |||||||
| Floor nurse | |||||||
| Respiratory therapist | |||||||
| Garcea et al., 20043 | United Kingdom | Teaching hospital | Adult patients discharged from ICU | 2 ICU nurses | Mortality | Emergency readmissions to ICU | NA |
| 1 ICU nurse specialist | Unscheduled ICU admission | ||||||
| ICU consultant physician (if needed) | |||||||
| Kenward et al., 200438 | United Kingdom | Teaching hospital | Adult inpatients | Not stated | Mortality | NA | Loss of spontaneous circulation |
| Cardiac arrest | |||||||
| Priestley et al., 200433 | United Kingdom | Teaching hospital | Adult inpatients | ICU nurse consultant | Mortality | NA | NA |
| ICU physician available if needed | Overall length of stay | ||||||
| Hillman et al., 200534 | Australia | 23 hospitals (17 academic, 6 nonacademic) | Adult inpatients | Varied between study hospitals; required to have at least 1 MD and 1 RN from ED or ICU | Mortality | Any unscheduled admission to ICU from general ward | No palpable pulse |
| Cardiac arrest | |||||||
| Unscheduled ICU admission | |||||||
| DNR status | |||||||
| Hunt et al., 2005 (abstract)36 | United States | Pediatric tertiary‐care academic hospital | Pediatric inpatients | Not provided | Cardiac arrest | NA | Not provided |
| Meredith et al., 2005 (abstract)37 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | ICU registered nurse | Mortality | NA | Not provided |
| Respiratory therapist | |||||||
| Tibballs et al., 200535 | Australia | Pediatric tertiary‐care academic hospital | Pediatric inpatients | ICU physician | Cardiac arrest | Not supplied | Not provided |
| Medical registrar | Unscheduled ICU admission | ||||||
| ED physician | |||||||
| ICU nurse | |||||||
| King et al., 200629 | United States | Tertiary‐care academic hospital | Adult inpatients, outpatients, and visitors | Hospitalist | Cardiac arrest | NA | Not provided |
| Internal medicine resident | |||||||
| ICU nurse | |||||||
| Ward nurse | |||||||
| Pharmacist | |||||||
RRS Structure, Calling Criteria, and Responsibilities
Seven studies22, 23, 31, 33, 34, 36, 37 that described the team composition used variants of the medical emergency team model, a physician‐led team (Table 2). In 6 of these 7 studies, the team included a critical care physician (attending or fellow) and an ICU nurse; in the sole RCT (the MERIT study36), the team structure varied between hospitals, consisting of a nurse and physician from either the emergency department or ICU. Hospitalists, who are involved in RRS responses at many U.S. hospitals, were primary team leaders of the RRS in only 1 study.31 In 2 studies34, 37 the RRS also responded to code blue calls, and in 4 studies23, 31, 33, 39 the RRS and the code blue team had separate personnel; the remaining studies did not define the distinction between RRS and code blue team.
| Study | Contemporaneous control group | Data reported at more than 1 time before/after intervention | RRS calling rate reported | Outcomes analysis included patients made DNR by team | Blind measurement of nonobjective outcomes | Intensivist always available | Other QI efforts during study |
|---|---|---|---|---|---|---|---|
| |||||||
| Buist et al., 200220 | No | No | Yes | No (mortality) | No | NR | NR |
| Bellomo et al., 200321 | No | No | Yes | Yes (mortality) | NA | Yes (ICU fellow) | No |
| Pittard et al., 200322 | No | No | Yes | NA | No | NR | NR |
| Bellomo et al., 200431 | No | No | Yes | Yes (mortality) | No | Yes (ICU fellow) | No |
| DeVita et al., 200432 | No | Yes | Yes | NA | No | Yes (critical care attending physician) | NR |
| Garcea et al., 200433 | No | No | No | Unclear | No | NR | NR |
| Kenward et al., 200438 | No | No | Yes | Unclear | No | NR | NR |
| Priestley et al., 200433 | No (interrupted time series) | Yes | No | NA | No | NR | NR |
| Hillman et al., 200534 | Yes | No | Yes | Unclear | Yes | NR | No |
| Hunt et al., 2005 (abstract)36 | No | No | Yes | NA | NR | NR | NR |
| Meredith et al., 2005 (abstract)37 | No | No | Yes | No | NA | No | No |
| Tibballs et al., 200535 | No | No | Yes | Unclear | No | NR | Yes (educational workshops/more training in APLS) |
| King et al., 200629 | No | Yes | Yes | NA | No | Yes | No |
In 4 studies the RRSs were led by nurses. One study published in abstract form39 used the rapid response team model, consisting of a critical care nurse and a respiratory therapist, with assistance as needed from the primary medical staff and a critical care physician. Three studies3, 24, 35 from UK hospitals used the critical care outreach (CCO) model, in which ICU‐trained nurses respond initially with assistance from intensivists. The CCO model also involves follow‐up on patients discharged from the ICU and proactive rounding on unstable ward patients.
The hospitals used broadly similar approaches to determining when to summon the RRS, relying on combinations of objective clinical criteria (eg, vital sign abnormalities) and subjective criteria (eg, acute mental status change, staff member concerned about patient's condition). Three studies3, 24, 35 used a formal clinical score (the Patient‐At‐Risk score or the Modified Early Warning score) to trigger calls to the RRS. Three studies, 2 of them from the same institution,23, 33 reported the frequency of specific triggers for RRS activation. Concern by bedside staff and respiratory distress were the most frequent activators of the RRS.
Study Internal Validity and Generalizability
One study,36 the MERIT trial, conducted in Australia, was a cluster‐randomized RCT (randomized by hospital) that adhered to recommended elements of design and reporting for studies of this type.41 In this study, hospitals in the control group received an educational intervention on caring for deteriorating patients only; hospitals in the intervention group received the educational module and started an RRS. An additional study35 identified itself as a randomized trial, but randomization occurred at the hospital ward level, with introduction of the intervention (critical care outreach) staggered so that at different points an individual ward could have been in either the control or intervention group; therefore, this study was considered an interrupted time series. All other trials included were before‐after studies with no contemporaneous control group
Most studies did not meet criteria for internal validity or generalizability (Table 2). Two studies3, 35 did not report the number of RRS calls during the study period. One study22 omitted patients whose resuscitation status was changed after RRS evaluation from the calculation of inpatient mortality; thus, the patients who had been made do not resuscitate by the RRS did not contribute to the calculated mortality rate. The disposition of these patients was unclear in another study.36 All studies measured clinical outcomes retrospectively, and no studies reported blinding of outcomes assessors for nonobjective outcomes (eg, unplanned ICU admission). Studies generally did not report on the availability of intensivists or if other quality improvement interventions targeting critically ill patients were implemented along with the RRS.
RRS Usage and Effects on Patient Outcomes
Seven studies2224, 34, 3638 reported enough information to calculate the RRS calling rate (4 studies24, 31, 39, 40 reported the total number of calls but not the number of admissions, and 2 studies3, 35 did not report either). In these 7 studies, the calling rate varied from 4.5 to 25.8 calls per 1000 admissions. Three studies documented the calling rate before and after the intervention: a study at a hospital with a preexisting RRS34 reported that the calling rate increased from 13.7 to 25.8 calls per 1000 admissions after an intensive education and publicity program; in a pediatric trial,38 the overall emergency calling rate (for cardiac arrests and medical emergencies) was reported to increase from 6.6 to 10.4 per 1000 admissions; and in the MERIT trial,36 calls increased from 3.1 to 8.7 per 1000 admissions.
Effects of RRS on Clinical Outcomes
Nine studies3, 22, 23, 33, 3537, 39, 40 reported the effect of an RRS on inpatient mortality, 9 studies22, 23, 31, 33, 34, 3638, 40 reported its effect on cardiopulmonary arrests, and 6 studies3, 22, 24, 33, 36, 37 reported its effect on unscheduled ICU admissions. Of these, 7 trials that reported mortality and cardiopulmonary arrests and 6 studies that reported unscheduled ICU admissions supplied sufficient data for meta‐analysis.
Observational studies demonstrated improvement in inpatient mortality, with a summary risk ratio of 0.82 (95% CI: 0.74‐0.91, heterogeneity I2 62.1%; Fig. 2). However, the magnitude of these improvements was very similar to that seen in the control group of the MERIT trial (RR 0.73, 95% CI: 0.53‐1.02). The intervention group of the MERIT trial also demonstrated a reduction in mortality that was not significantly different from that of the control group (RR 0.65, 95% CI: 0.48‐0.87). We found a similar pattern in studies reporting RRS effects on cardiopulmonary arrests (Fig. 3). The observational studies did not show any effect on the risk of unscheduled ICU admissions (summary RR 1.08, 95% CI: 0.96‐1.22, heterogeneity I2 79.1%) nor did the MERIT trial (Fig. 4).



DISCUSSION
Despite the strong face validity of the RRS concept, the current literature on medical emergency teams, rapid response teams, and critical care outreach suffers from substantial flaws that make it difficult to determine the effect of an RRS on patient outcomes. These flaws include the use of suboptimal study designs, failure to report important cointerventions, the methods in which outcomes were defined, and lack of verification of the validity of the outcomes measured. As a result, very little empiric data are available to define the effectiveness of RRSs or to provide guidance for hospitals planning to implement an RRS.
Though early studies reported that RRSs appeared to reduce mortality and cardiac arrest rates, the sole randomized trial of an RRS (the MERIT trial36) showed no differences between intervention and control hospitals for any clinical outcome. Both inpatient mortality and cardiac arrest rates declined in the intervention and control groups of the MERIT trial, and the reductions in these outcomes in observational trials were similar to those seen in the MERIT control group. This strongly implies that other factors besides the RRS were responsible for the results of previous before‐after studies. These studies, which have been widely cited by proponents of the RRS, suffer from methodological limitations intrinsic to the study design and issues with outcome measurement that may have introduced systematic bias; these factors likely explain the contrast between the generally positive results of the before‐after studies and the negative results of the MERIT trial.
Most early RRS trials used an uncontrolled before‐after study design, as is common in quality improvement studies.42 This study design cannot account for secular trends or other factors, including other QI interventions, that could influence the effect of an intervention.26 The statistically significant reduction in impatient mortality in the control arm of the MERIT trial is an instructive example; this decline could have been a result of the educational intervention on caring for deteriorating patients, other ongoing QI projects at the individual hospitals, or simply random variation during the relatively short (6‐month) follow‐up period. Such factors could also entirely account for the impressive results seen in the initial uncontrolled RRS studies. Nearly all the studies we reviewed also did not discuss any aspects of the hospital context that could influence outcomes for critically ill patients, such as the nurse‐staffing ratio,43 ICU bed availability,4446 overall hospital census,47 or availability of intensivists48 or hospitalists.49 Failure to control foror at least report important aspects ofthe environment in which the intervention was performed is akin to failing to report baseline patient comorbidities or concurrent therapies in a study of a drug's effectiveness.
Our review also suggests how bias in the measurement of clinical outcomes may have contributed to the apparent effect of RRSs. In 1 before‐after study, patients for whom RRS activation resulted in a change code status to do not resuscitate (DNR) were excluded from calculations of mortality,22, 50 resulting in underreporting of mortality after RRS implementation. Disposition of such patients was unclear in 3 other studies.3, 36, 40 Some studies22, 34 defined cardiopulmonary arrest as any activation of the code blue team, regardless of whether the patient was actually in cardiac arrest. This almost inevitably would result in fewer arrests after implementation of the RRS, as the indications for calling the code blue team would be narrower. Finally, nearly all studies used trends in nonobjective primary outcomes (eg, unplanned ICU transfer) to support RRS effects but did not validate any of these outcomes (eg, how often did reviewers agree an ICU transfer was preventable), and none of the assessors of these outcomes were blinded.
Some have attributed the MERIT trial not finding the RRS beneficial to inadequate implementation, as the RRS calling rate of 8.7 calls per 1000 admissions was less than the 15 calls per 1000 admissions cited as optimal in a mature RRS.51 However, published studies generally reported a calling rate of 4‐5 calls per 1000 admissions,22, 23, 37 with only 1 trial reporting a higher calling rate.34
A recent commentary20 and a systematic review of critical care outreach teams21 both addressed the effectiveness of RRSs. We sought to examine the effects of all RRS subtypes and using quantitative analysis and analysis of methodological quality, to determine the overall effect of RRSs. The results of our analysis (which included data from several newer studies31, 38, 39) support and extend the conclusion of prior reviews that RRSs, although a potentially promising intervention, do not unequivocally benefit patients and are not worthy of more widespread use until more evidence becomes available. Our analysis also demonstrates that many studies widely cited as supporting wide implementation of RRSs are flawed and probably not generalizable.
Despite these caveats, RRSs remain an intuitively attractive concept and may be of benefit at some hospitals. Further studies in this area should focus on identifying which patient populations are at high risk for clinical decompensation, identifying the role of clinical structures of care (eg, nurse‐staffing ratio, presence of hospitalists) in preventing adverse outcomes and determining which specific RRS model is most effective. As well, more information is needed about educating bedside staff and RRS team members, as this is likely critical to success of the team. Unfortunately, only the article by King et al.31 provided sufficient detail about the implementation process to assist hospitals in planning an RRS. The remaining articles had only scant details about the intervention and its implementation, a common problem noted in the quality improvement literature.42, 52, 53
Our analysis had several limitations. We attempted to identify as many RRS trials as possible by searching multiple databases and reviewing abstract proceedings, but as the RRS literature is in its infancy, we may not have located other unpublished studies or gray literature. There is no validated system for evaluating the methodological strength of nonrandomized studies; therefore, we assessed study quality on the basis of prespecified criteria for internal and external validity. Finally, we found significant statistical heterogeneity in our quantitative analyses, indicating that the variability between individual studies in treatment effects was greater than that expected by chance. As the primary reasons we conducted a meta‐analysis was to compare the results of before‐after trials with those of the randomized MERIT trial, we did not further explore the reasons for this heterogeneity, although variation in patient populations and RRS structure likely accounts for a significant proportion of the heterogeneity.
Although there is a theoretical basis for implementing a rapid response system, the published literature shows inconsistent benefits to patients and suffers from serious methodological flaws. Future studies of RRSs should attempt to define which patient populations are at risk, the essential characteristics of RRSs, effective implementation strategies, andmost importantwhether any RRS improves clinical outcomes. Until such evidence is available, hospitals should not be mandated to establish an RRS and should consider prioritizing quality improvement resources for interventions with a stronger evidence base.
Acknowledgements
The authors thank Emmanuel King, MD, for graciously providing a copy of his manuscript prior to publication and Alexis Meredith, MD, for providing additional information regarding his study. Dr. Shojania holds a Government of Canada Research Chair in Patient Safety and Quality Improvement.
APPENDIX
| Search terms | Citations | |
|---|---|---|
| 1 | {(rapid [ti] AND (response [ti] OR resuscitation [ti]) OR (patient at risk [ti])} AND (program [ti] OR team* [ti] OR service* [ti]) | 23 |
| 2 | medical emergency team* [ti] OR medical crisis team* [ti] OR {(critical [ti] OR intensive [ti]) AND care [ti] AND outreach [ti]} | 87 |
| 3 | hospital [ti] AND resuscitation [ti] AND team* [ti] | 11 |
| 4 | medical emergency team* [ab] OR rapid response team [ab] OR medical crisis team* [ab] | 89 |
| 5 | #1 OR #2 OR #3 OR #4 | 158 |
| 6 | Resuscitation [mh] OR heart arrest [mh] OR hospital mortality [mh] | 72,488 |
| 7 | (patient care team [mh] OR critical care [mh] OR intensive care units [mh]) AND (patient readmission [mh] OR organization and administration [mh]) | 20,321 |
| 8 | #6 AND #7 | 1,419 |
| 9 | {(randomised[ti] OR randomized[ti] OR controlled[ti] OR intervention[ti] OR evaluation[ti] OR comparative[ti] OR effectiveness[ti] OR evaluation[ti] OR feasibility[ti]) AND (trial[ti] OR studies[ti] OR study[ti] OR program[ti] OR design[ti])} OR clinical trial[pt] OR randomized controlled trial[pt] OR epidemiologic studies[mh] OR evaluation studies[mh] OR comparative study[mh] OR feasibility studies[mh] OR intervention studies[mh] OR program evaluation[mh] OR epidemiologic research design[mh] OR systematic5 | 2,688,847 |
| 10 | #8 AND #9 | 748 |
| 11 | #5 OR #10 | 806 |
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.
- ,,,.The medical emergency team.Anaesth Intensive Care.1995;23(2):183–186.
- ,,,.The 100 000 Lives Campaign: setting a goal and a deadline for improving health care quality.JAMA.2006;295(3):324–7.
- ,,,,.Impact of a critical care outreach team on critical care readmissions and mortality.Acta Anaesthesiol Scand2004;48:1096–1100.
- ,.The patient‐at‐risk team.Anaesthesia.2000;55(2):198.
- ,,, et al.Findings of the First Consensus Conference on Medical Emergency Teams.Crit Care Med.2006;34:2463–2478.
- .Recognition of patients who require emergency assistance: a descriptive study.Heart Lung.2000;29(4):262–268.
- ,,, et al.Antecedents to hospital deaths.Intern Med J.2001;31:343–348.
- ,,, et al.Duration of life‐threatening antecedents prior to intensive care admission.Intensive Care Med.2002;28:1629–1634.
- ,,,,.The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team.Resuscitation.2002;54:125–131.
- ,,,,,.A comparison of antecedents to cardiac arrests, deaths and emergency intensive care admissions in Australia and New Zealand, and the United Kingdom—the ACADEMIA study.Resuscitation.2004;62:275–282.
- ,,,.Validation of a modified Early Warning Score in medical admissions.QJM.2001;94:521–526.
- ,,,,.Inpatient transfers to the intensive care unit: delays are associated with increased mortality and morbidity.J Gen Intern Med.2003;18(2):77–83.
- ,,,,.Clinical antecedents to in‐hospital cardiopulmonary arrest.Chest.1990;98:1388–1392.
- ,.Developing strategies to prevent inhospital cardiac arrest: analyzing responses of physicians and nurses in the hours before the event.Crit Care Med.1994;22(2):244–247.
- ,,.Introduction to the rapid response systems series.Jt Comm J Qual Patient Saf.2006;32:359–360.
- ,,, et al.Rates of in‐hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team.Med J Aust.2000;173:236–240.
- ,,,,.The patient‐at‐risk team: identifying and managing seriously ill ward patients.Anaesthesia.1999;54:853–860.
- .The medical emergency team: no evidence to justify not implementing change.Med J Aust.2000;173:228–229.
- ,.The medical emergency team, evidence‐based medicine and ethics.Med J Aust.2003;179:313–315.
- ,,.Rapid response teams—walk, don't run.JAMA.2006;296:1645–1647.
- ,,, et al.Investigating the effectiveness of critical care outreach services: a systematic review.Intensive Care Med.2006;32:1713–1721.
- ,,,,,.Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study.BMJ.2002;324:387–390.
- ,,, et al.A prospective before‐and‐after trial of a medical emergency team.Med J Aust.2003;179:283–287.
- .Out of our reach? Assessing the impact of introducing a critical care outreach service.Anaesthesia.2003;58:882–885.
- Cochrane Collaboration Effective Practice and Organisation of Care group. Available at: http://www.epoc.uottawa.ca/inttime.pdf.Accessed August 4,2006.
- ,,.Experimental and Quasi‐Experimental Designs for Generalized Causal Inference.Boston, MA:Houghton Mifflin;2002.
- ,,, et al.Guidelines for the uniform reporting of data for Medical Emergency Teams.Resuscitation.2006;68(1):11–25.
- ,.The intracluster correlation coefficient in cluster randomisation.BMJ.1998;316:1455.
- ,.Issues in the meta‐analysis of cluster randomized trials.Stat Med.2002;21:2971–2980.
- ,,,.Measuring inconsistency in meta‐analyses.BMJ.2003;327:557–560.
- ,,.Establishing a rapid response team (RRT) in an academic hospital: one year's experience.J Hosp Med.2006;1:296–305.
- ,,, et al.A multidisciplinary community hospital program for early and rapid resuscitation of shock in nontrauma patients.Chest.2005;127:1729–1743.
- ,,, et al.Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates.Crit Care Med.2004;32:916–921.
- ,,,,,.Use of medical emergency team responses to reduce hospital cardiopulmonary arrests.Qual Saf Health Care.2004;13:251–254.
- ,,, et al.Introducing critical care outreach: a ward‐randomised trial of phased introduction in a general hospital.Intensive Care Med.2004;30:1398–1404.
- ,,, et al.Introduction of the medical emergency team (MET) system: a cluster‐randomised controlled trial.Lancet.2005;365:2091–2097.
- ,,,,.Reduction of paediatric in‐patient cardiac arrest and death with a medical emergency team: preliminary results.Arch Dis Child.2005;90:1148–1152.
- ,,, et al.The effect of transition from a traditional code team to a rapid response team in a children's center: a before and after intervention trial [abstract].Crit Care Med.2005;33(12 suppl):A17.
- ,,,,.Improved hospital mortality by institution of a rapid response team in a university hospital.Chest.2005;128(suppl S):182S.
- ,,,.Evaluation of a medical emergency team one year after implementation.Resuscitation.2004;61(3):257–263.
- ,,.CONSORT statement: extension to cluster randomised trials.BMJ.2004;328:702–708.
- ,.Evidence‐Based Quality Improvement: The State Of The Science.Health Aff.2005;24(1):138–150.
- ,,,,.Hospital nurse staffing and patient mortality, nurse burnout, and job dissatisfaction.JAMA.2002;288:1987–1993.
- ,,, et al.Evaluation of triage decisions for intensive care admission.Crit Care Med.1999;27:1073–1079.
- ,,,,.Rationing of intensive care unit services. An everyday occurrence.JAMA.1986;255:1143–1146.
- ,,,.How do physicians adapt when the coronary care unit is full? A prospective multicenter study.JAMA.1987;257:1181–1185.
- ,,,,.The association between hospital overcrowding and mortality among patients admitted via Western Australian emergency departments.Med J Aust.2006;184:208–212.
- ,,,,,.Physician staffing patterns and clinical outcomes in critically ill patients: a systematic review.JAMA.2002;288:2151–2'62.
- ,,,,,.Implementation of a voluntary hospitalist service at a community teaching hospital: improved clinical efficiency and patient outcomes.Ann Intern Med.2002;137:859–865.
- .Critical care outreach team's effect on patient outcome: other conclusions are possible.BMJ.2004;328:347; author reply
- The “MERIT” Trial of medical emergency teams in Australia: an analysis of findings and implications for the 100,000 Lives Campaign. Institute for Healthcare Improvement,2006. Available at: http://www.ihi.org/NR/rdonlyres/F3401FEF‐2179‐4403‐8F67–B9255C57E207/0/LancetAnalysis81505.pdf. Accessed August 17, 2006.
- ,,, et al.Toward evidence‐based quality improvement. evidence (and its limitations) of the effectiveness of guideline dissemination and implementation strategies 1966‐1998.J Gen Intern Med.2006;21(suppl 2):S14–S20.
- ,,, et al.lessons learned about implementing research evidence into clinical practice. Experiences from VA QUERI.J Gen Intern Med.2006;21(suppl 2):S21–S24.
- ,,,,,.Improving the quality of reports of meta‐analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta‐analyses.Lancet.1999;354:1896–1900.
- ,,,,,.Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital.J Crit Care.2003;18(2):87–94.
- ,,, et al.Long term effect of a medical emergency team on cardiac arrests in a teaching hospital.Crit Care.2005;9:R808–R815.