User login
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
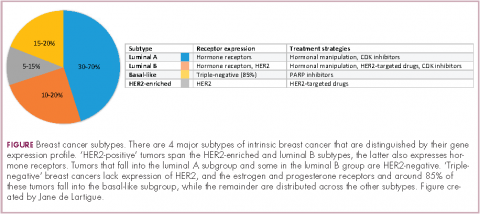
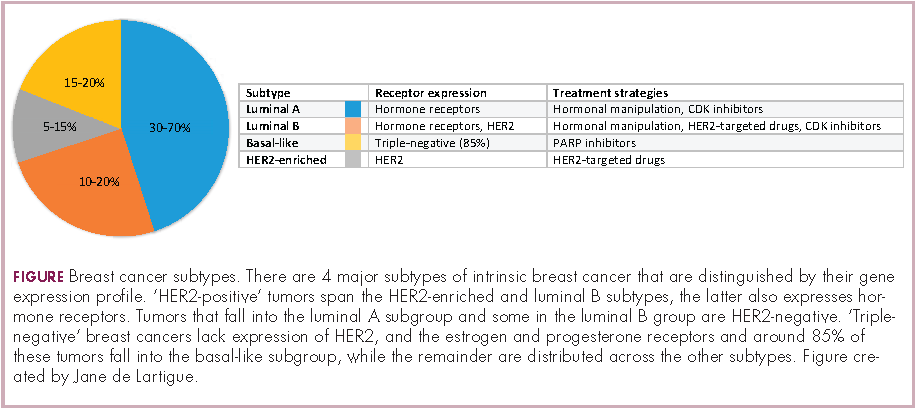
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
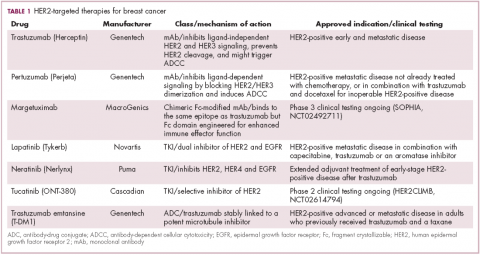
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
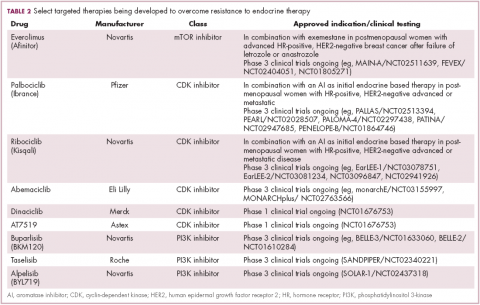
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38
As our understanding of the biology of breast cancer has improved, treatment has become increasingly personalized. Targeted therapies continue to significantly improve patient outcomes in multiple subtypes, with several recent drug approvals. Here, we discuss some of these latest developments.
A disease of many faces
Clinically speaking, breast cancers can be divided into at least 5 subtypes on the basis of the genes they express (Figure 1). The luminal subtypes make up the largest proportion and are characterized by the expression of hormone receptor (HR) genes. Luminal A tumors are negative for human epidermal growth factor receptor 2 (HER2; HER2-negative), whereas luminal B tumors often co-express the HER2 genes.1
The remainder of HER2-positive patients fall into the HER2-enriched category, in which HER2 expression is the defining characteristic. Basal-like tumors, meanwhile, represent the most heterogeneous subtype, overlapping to a large extent with tumors dubbed “triple-negative” because of their lack of either HER2 or ESR1 and PGR gene expression. The fifth subtype is known as normal breast-like and remains poorly characterized.
In recent years, there have been significant advancements in the genomic characterization of breast cancer that have begun to provide a more comprehensive understanding of the driver molecular mechanisms, which has helped to explain some of the limitations of current targeted approaches and to reveal new possible treatments, with a shift toward increasingly personalized strategies.2
HER2: what’s neu?
An estimated 18%-20% of breast tumors are HER2 positive, displaying amplification of the HER2/neu gene or overexpression of its protein product.3 Historically, HER2 positivity correlated with a highly aggressive and metastatic form of disease, conferring poor prognosis.4,5 The HER2-targeted monoclonal antibody (mAb), trastuzumab serves as a prime example of the power of personalized medicine. Evidence suggests that trastuzumab has altered the natural history of HER2-positive breast cancer, such that trastuzumab-treated patients with HER2-positive breast cancer now have a better prognosis than do patients with HER2-negative disease.6,7
Several additional HER2-targeted drugs have joined trastuzumab on the market, including other mAbs, small molecule tyrosine kinase inhibitors (TKIs), and an antibody–drug conjugate that combines the specificity of a mAb with the anti-tumor potency of a cytotoxic drug. These drugs have further improved patient outcomes in both early and advanced disease settings (Table 1).
The most recent regulatory approval was for neratinib, a potent TKI inhibiting all members of the HER protein family. On the basis of the phase 3 ExteNET study, neratinib was granted approval by the US Food and Drug Administration (FDA) for extended adjuvant treatment of patients with HER2-positive, early-stage breast cancer previously treated with trastuzumab. In a 5-year analysis of the study, invasive disease-free survival (DFS) was 90.4% with neratinib, compared with 87.9% with placebo (hazard ratio [HR], 0.74; P = .017).8,9
The tide of advancements in HER2-targeted therapy looks set to continue in the coming years as potentially practice-changing data emerges from ongoing clinical trials and, as the patent on trastuzumab has expired, a number of biosimilars, such as MYL-1401O have the potential to help patients who may not have access to trastuzumab.10
One of the biggest remaining challenges is identifying drugs that can effectively treat patients with brain metastases because the blood–brain barrier presents an impediment to the delivery of effective concentrations of anticancer drugs. Initially, it was hoped that the small molecule inhibitors lapatinib and neratinib could cross the blood–brain barrier and may be more effective in patients with brain metastases, but that hypothesis has not borne out in randomized clinical trials.11
Tucatinib (ONT-380) has shown significant promise in this respect. In a phase 1 trial, ONT-380 had significant efficacy in patients with and without central nervous system metastases; the overall response rate (ORR) in the CNS was 36%. ONT-380 is also notable for its specificity for HER2, without significant inhibition of HER1 and EGFR, which could translate into a better toxicity profile.12
Doubling down on resistant tumors
Since the success of HER2-targeted therapy is limited by the development of resistance, there has been significant interest in assessing the potential of dual HER2 blockade, exploiting the unique mechanisms of action of different drugs in combination therapy, and ensuring more complete inhibition of the HER2 pathway. Although numerous different combinations have been tested, a double antibody combination has proved most effective.
In fact, dual HER2 targeting with trastuzumab and pertuzumab in combination with chemotherapy has replaced a trastuzumab-chemotherapy regimen as the new standard of care in the metastatic setting. A 6-month improvement in progression-free survival (PFS) sealed FDA approval for the combination and in a recently published final analysis of the trial overall survival (OS) was also improved to a level unprecedented in the first-line setting.13,14The double antibody combination has also been successful in the neoadjuvant setting. Approval followed the results of the phase 2 NeoSphere trial, in which the combination was associated with a significant improvement in pathologic complete response (pCR) rate, a measure that acts as a surrogate for improved survival in the neoadjuvant setting. In a 5-year analysis of the NeoSphere trial, improved pCR did indeed translate into improved PFS and DFS.15,16
The results of the phase 3 APHINITY trial evaluating this combination in the adjuvant setting have been hotly anticipated. In a presentation at the 2017 American Society of Clinical Oncology (ASCO) meeting in June, the study authors reported that in 4,085 patients with operable HER2-positive disease, it significantly reduced the risk of disease recurrence or death compared with trastuzumab and chemotherapy alone.17
There is an ongoing effort to determine if it is possible to de-escalate treatment by removing the chemotherapy component. At least in the neoadjuvant setting, pCR rates in the chemotherapy-free arms of several studies suggest that a proportion of patients might benefit from this strategy15,18,19 and the challenge now is to identify them. To that end, the phase 2 PAMELA trial identified the HER2-enriched subtype as a strong predictor of response to neoadjuvant dual blockade (lapatinib and trastuzumab) without chemotherapy. The pCR rate was 40.6% for the combination in patients with the HER2-enriched subtype of breast cancer and only 10% in patients with non–HER2-enriched tumors.20
Targeting resistance to endocrine therapy
Another coup for personalized medicine in breast cancer is the treatment of hormone receptor–positive cases with endocrine therapy, which has become the cornerstone of treatment in the metastatic and adjuvant settings. Those drugs are designed to block the growth-stimulating effects of the estrogen and progesterone hormones on tumor cells. They include the selective estrogen receptor (ER) modulator tamoxifen, aromatase inhibitors (AIs) such as letrozole, anastrozole, and exemestane, which work by blocking the activity of the aromatase enzyme that converts androgens into estrogens, and the selective estrogen-receptor down-regulator fulvestrant.
As with HER2-targeted therapy, patients treated with endocrine therapy often develop resistance. Activation of alternate signaling cascades, such as the P13K–Akt–mTOR (phosphatidylinositol-3-kinase–Akt–mammalian target of rapamycin) pathway, or downstream targets of ER signaling, including the cyclin-dependent kinases, CDK4 and CDK6, have emerged as important mechanisms of resistance.21,22
Drugs directed against these secondary targets, aimed to enhance the efficacy of endocrine therapies, have shown significant promise (Table 2). The mTOR inhibitor everolimus received FDA approval in 2012 in combination with exemestane for the treatment of advanced HR-positive, HER2-negative breast cancer.23 More recently, everolimus has also proven effective in combination with either fulvestrant or letrozole, according to the phase 2 PrECOG 0102 and BOLERO-4 studies, both doubling PFS compared with endocrine therapy alone.24,25
Buparlisib is an oral reversible pan-PI3K inhibitor, and the results of the first phase 3 trial of this drug in metastatic breast cancer (MBC) were recently reported. Among 1,147 postmenopausal women with HR-positive, HER2-negative MBC that progressed on or after AI therapy, the combination of buparlisib and fulvestrant prolonged PFS compared with fulvestrant alone (median PFS, 6.9 vs 5 months; HR,0.78; P < .001). However, Novartis, which was developing buparlisib, reported that the combination will not be pursued further due to increased toxicity.26
Two other PI3K inhibitors are currently in phase 3 clinical trials; taselisib and alpelisib, both selective PI3K-alpha inhibitors. The results of a phase 1 dose-escalation study of taselisib were recently published and the ORR among patients with PIK3CA-mutant solid tumors was 36%, including responses in 4 patients with breast cancer.27 Meanwhile, alpelisib has also demonstrated early promise in combination with both letrozole and fulvestrant in patients with ER-positive MBC refractory to endocrine therapy. In combination with letrozole, the clinical benefit rate was 35% overall (44% in patients with PIK3CA mutations, compared with 20% in patients with wild-type PIK3CA status). The combination of alpesilib and fulvestrant produced an ORR of 27%, and both combinations were well tolerated.28,29
Another exciting therapeutic avenue is CDK4 and CDK6 inhibitors. These proteins are critical regulators of cell cycle progression, ensuring transition from G1 to S phase occurs at the appropriate time. The CDK pathway is also a downstream target of ER activation and, unsurprisingly, aberrant expression of the proteins involved in this pathway is commonly observed in breast tumors.
Palbociclib became the first FDA-approved member of this drug class, receiving accelerated approval in patients with HR-positive, HER2-negative metastatic breast cancer, in combination with letrozole in 2015. This became full regulatory approval in combination with any AI earlier this year, following the phase 3 PALOMA-3 study, in which the combination of palbociclib and fulvestrant (accelerated approval was based upon a trial testing palbociclib and letrozole) improved PFS by 5 months (HR, 0.46; P < .0001).30
In addition, a second CDK4/6 inhibitor hit the market this year. Ribociclib demonstrated a significant PFS benefit in combination with letrozole; median PFS was 25.3 months, compared with 16 months for letrozole alone, translating to a 44% reduction in the risk of disease progression or death.31
Abemaciclib, which has greater selectivity for CDK4 than its predecessors, also appears to be heading towards approval. It was granted priority review by the FDA based on data from the MONARCH-2 trial, showing a significant improvement in PFS for the combination of abemaciclib and fulvestrant (median PFS, 16.4 vs 9.3 months for fulvestrant alone; HR, 0.553; P < .001).32
Teasing out ‘HER2-positive’ subtypes
Until recently, “HER2-positive” and “HR-positive” tumors have been treated as separate subtypes, despite the fact that about half of HER2-positive tumors fall into the luminal A subtype and are also HR-positive. Patients were typically treated with HER2-targeted therapy regardless of their endocrine status because of the aggressive nature of HER2-positive disease.
Increasingly, researchers are reconsidering this view, especially as several studies have shown differential response rates to HER2-targeted therapy in HR-positive compared with HR-negative patients and accumulating evidence suggests that there is significant crosstalk between the HER2 and HR pathways, which may be responsible for the development of resistance with both treatment paradigms.
Findings from several studies have shown a benefit to combining HER2-targeted and hormonal therapies in patients with luminal (HR-positive), HER2-positive disease. In the metastatic setting, the results of the phase 2 PERTAIN study, presented at the 2017 ASCO annual meeting suggest that dual HER2 blockade could prove even more effective. The addition of pertuzumab to a combination of trastuzumab and an AI improved PFS by more than 3 months (median PFS, 19.89 vs 15.8 months; HR, 0.65; P = .007).33
The clinical application of these combinations may be limited by the additional cost – several studies have suggested that they are not cost effective – and toxicity, but have served to drive the development of new clinical trial designs as the importance of considering luminal and nonluminal HER2-positive tumors has become increasingly apparent.
PARP inhibitors make a dent in BRCA1/2-mutated cancers
The most renowned breast cancer genes, BRCA1 and BRCA2 are present in about 5%-10% of all breast cancers. They play a central role in the homologous recombination pathway that fixes double-strand breaks in the DNA. Genome sequencing studies have revealed that the presence of the BRCA1/2 genes and other DNA repair defects is highest among patients with the basal-like subtype of breast cancer, in particular those who have triple-negative disease.34,35
This type of breast cancer has proved stubbornly resistant to efforts to improve patient outcomes with targeted therapies. BRCA1/2 mutations and other DNA repair defects that confer a so-called BRCAness phenotype, render tumor cells dependent on other pathways for DNA repair and there has been considerable interest in therapeutically exploiting this through the development of inhibitors of the poly(ADP-ribose) polymerase (PARP) enzyme, which is involved in the repair of single-strand breaks in the DNA. The double damage to DNA repair mechanisms through PARP inhibition in patients with BRCA1/2-mutant tumors proves overwhelming to cancerous cells.
Despite more than a decade of investigation in breast cancer, PARP inhibitors have yet to yield any FDA-approved treatment options. That may be set to change imminently, following the success of olaparib (Table 3). In the first randomized phase 3 trial of a PARP inhibitor in breast cancer (OlympiAD), olaparib was compared with standard chemotherapy in patients with BRCA1/2-mutated MBC who had received up to 2 previous lines of chemotherapy. Olaparib reduced the risk of disease progression by 42% compared with standard chemotherapy and was well tolerated.36
The novel PARP inhibitor talazoparib, which is the most potent to date, is also demonstrating significant efficacy in clinical trials. The results of the phase 2 ABRAZO trial were presented at the ASCO annual meeting. Two cohorts were treated; the first included 49 patients who had responded to their last platinum-containing regimen for metastatic disease and progressed more than 8 weeks after last platinum dose and the other included 35 patients previously treated with 3 or more nonplatinum regimens for metastatic disease. ORR was 28% across the 2 cohorts; 23% and 33% in BRCA1- and BRCA2-mutant carriers, respectively; and 26% in patients with triple-negative breast cancer.37 PARP inhibition is not faring so well in early-stage triple-negative disease; a phase 3 trial of veliparib in combination with chemotherapy did not meet its primary endpoint.38