User login
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
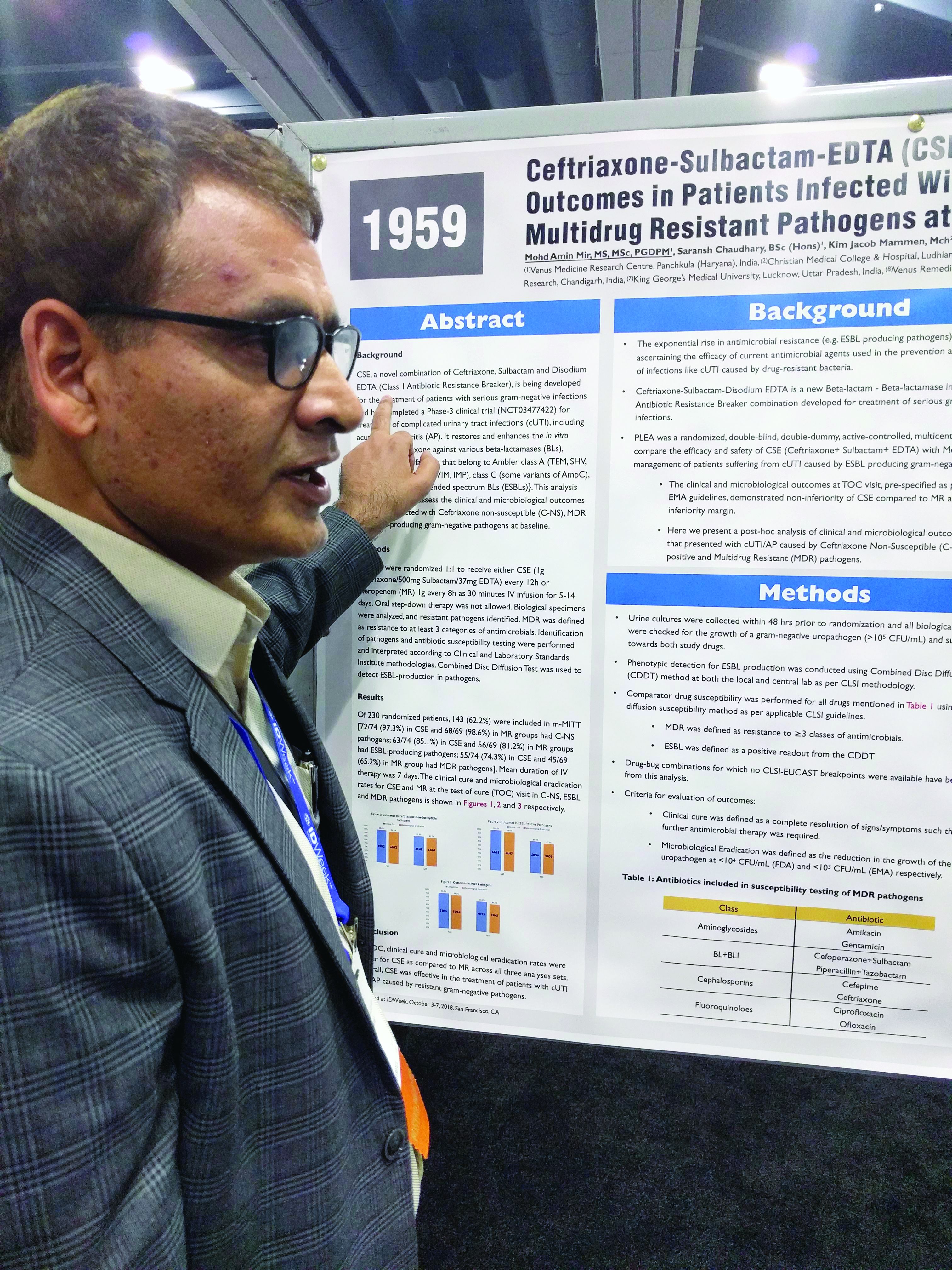
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
SAN FRANCISCO – A combination of ceftriaxone, a beta-lactamase inhibitor, and disodium ethylenediaminetetraacetic acid (EDTA) is superior to meropenem in the treatment of complicated urinary tract infections caused by extended-spectrum beta-lactamase (ESBL) gram-negative bacteria, according to a new study.
The post-hoc analysis also found that the three-drug combination – known as CSE – is noninferior to meropenem in multidrug-resistant (MDR) and ceftriaxone-nonsusceptible (C-NS) pathogens.
CSE is aimed at the growing problem of antibiotic resistance, particularly the mechanisms used by bacteria to counter beta-lactamase inhibitors. EDTA chelates zinc and calcium, and many of the resistance mechanisms rely on one or the other of these ions to function. In in vitro models, the combination of sulbactam and EDTA restores activity of ceftriaxone against various beta-lactamases.
Mohd Amin Mir, MD, head of clinical research at the Venus Medicine Research Center, Panchkula, India, and presenter of the study, said that, in the case of efflux pumps, “when there is EDTA present, it chelates the calcium, and that means there is no energy for the efflux pump to throw out the drug.”
The penems, which include meropenem, are a class of synthetic antibiotics with an unsaturated beta-lactam ring. Like other antibiotics, they are under assault from antibiotic resistance, especially beta-lactamase enzymes. “Penems are very precious drugs. The objective of developing [EDTA combinations] is to save the penems,” Dr. Mir said at an annual scientific meeting on infectious diseases.
The PLEA trial randomized 143 patients with complicated urinary tract infections or acute pyelonephritis to CSE (1 g ceftriaxone/500 mg sulbactam/37 mg EDTA) every 12 hours or 1 g meropenem (MR) as a 30-minute intravenous infusion over 30 minutes. Patients received treatment for 5-14 days.
The original study demonstrated that CSE is noninferior to meropenem at a 10% noninferiority margin. The researchers conducted a post-hoc analysis of patients who presented with complicated UTIs or acute pyelonephritis cases that were C-NS, ESBL-positive, or multidrug-resistant (MDR) pathogens. The researchers defined MDR as resistance to three or more categories of antimicrobial agents.
Of patients who received CSE, 97.3% had pathogens that were nonsusceptible to ceftriaxone, as did 98.6% of those who received MR; 85.1% of CSE and 81.2% of MR patients had an ESBL-producing pathogen; and 74.3% of infections in the CSE group were MDR, as were 65.2% of the MR group.
In all three resistant phenotypes, CSE at least trended to more favorable outcomes. In the MDR group, 96.4% of CSE patients achieved a clinical cure, compared with 88.9% in the MR group, and 94.5% achieved microbial eradication, compared with 86.75% in the MR group.
In the ESBL subgroup, 100% of patients in the CSE group achieved a clinical cure, compared with 89.3%, while 98.4% had complete eradication in the CSE group, compared with 87.5%. In the C-NS subgroup, 95.8% in the CSE group achieved a clinical cure, compared with 91.2%, and 94.4% achieved eradication, compared with 89.7% in the MR group.
In the ESBL subgroup, the lower bound of the 95% confidence interval of the between-group difference was greater than 0, indicating superiority of CSE over MR for both clinical cure and eradication. In the MDR and C-NS subgroups, CSE achieved noninferiority at a –10% margin.
CSE is currently commercially available in India, and the manufacturer is now seeking approval in Europe and the United States.
SOURCE: Mir MA et al. IDWeek 2018, Abstract 1959.
REPORTING FROM IDWEEK 2018
Key clinical point:
Major finding: The combination was noninferior in the context of different resistant subtypes.
Study details: Posthoc analysis of a randomized, controlled trial (n = 143).
Disclosures: The study was funded by Venus Medicine Research Center, which employs Dr. Mir.
Source: Mir MA et al. IDWeek 2018, Abstract 1959.