User login
Dosage windows could be difficult to understand pharmacologically, but for a partial agonist the presumed mechanism could be more evident. Clinicians should be aware that more is not always better, meaning that with partial agonist drugs a higher dosage might not lead to greater patient response. With brexpiprazole, a dopamine D2 partial agonist FDA-approved for schizophrenia and an adjunct for major depressive disorder (MDD),1 moderation is best because of
Recommended dosage
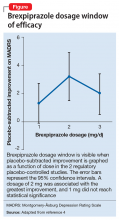
Two placebo-controlled studies2,3 examined brexpiprazole dosages of 1, 2, and 3 mg/d. The recommended dosage of 2 mg/d for MDD was determined by changes in Montgomery-Åsburg Depression Rating Scale scores (Figure).4 Lower dosages of 1 mg/d did not reach statistical significance, and 3 mg/d were less effective than the intermediate dosage of 2 mg/d. This result suggests a window of efficacy for brexpiprazole for MDD. This therapeutic window likely applies to most patients; however, patient-specific variables could alter the optimum dosage.
Dosage window
Brexpiprazole has high affinity for dopamine D2, D3, serotonin 5-HT1A, 5-HT2A, norepinephrine α1B, and α2 Creceptors. At relatively low drug concentrations, brexpiprazole achieves high receptor occupancy. At receptors for which brexpiprazole is a partial agonist (5-HT1A, D2, D3) the drug blocks the receptor and stimulates it at a fraction of the endogenous neurotransmitter. With a very high affinity agent, the endogenous neurotransmitter could be completely excluded from interacting with these receptors if brexpiprazole occupancy is high. At lower dosages, the drug occupies only a fraction of the receptors, allowing the endogenous neurotransmitters to continue interacting with their receptors, thereby magnifying the signal of that receptor above baseline.
1. FDA approves Rexulti (brexpiprazole) as adjunctive treatment for adults with major depressive disorder and as a treatment for adults with schizophrenia [news release]. Valby, Denmark; Tokyo, Japan: H. Lundbeck A/S (Lundbeck); Otsuka Pharmaceutical Co., Ltd; July 11, 2015. http://investor.lundbeck.com/ releasedetail.cfm?Release ID=921621. Accessed October 3, 2015.
2. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9): 1232-1240.
3. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebocontrolled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231.
4. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
Dosage windows could be difficult to understand pharmacologically, but for a partial agonist the presumed mechanism could be more evident. Clinicians should be aware that more is not always better, meaning that with partial agonist drugs a higher dosage might not lead to greater patient response. With brexpiprazole, a dopamine D2 partial agonist FDA-approved for schizophrenia and an adjunct for major depressive disorder (MDD),1 moderation is best because of
Recommended dosage
Two placebo-controlled studies2,3 examined brexpiprazole dosages of 1, 2, and 3 mg/d. The recommended dosage of 2 mg/d for MDD was determined by changes in Montgomery-Åsburg Depression Rating Scale scores (Figure).4 Lower dosages of 1 mg/d did not reach statistical significance, and 3 mg/d were less effective than the intermediate dosage of 2 mg/d. This result suggests a window of efficacy for brexpiprazole for MDD. This therapeutic window likely applies to most patients; however, patient-specific variables could alter the optimum dosage.
Dosage window
Brexpiprazole has high affinity for dopamine D2, D3, serotonin 5-HT1A, 5-HT2A, norepinephrine α1B, and α2 Creceptors. At relatively low drug concentrations, brexpiprazole achieves high receptor occupancy. At receptors for which brexpiprazole is a partial agonist (5-HT1A, D2, D3) the drug blocks the receptor and stimulates it at a fraction of the endogenous neurotransmitter. With a very high affinity agent, the endogenous neurotransmitter could be completely excluded from interacting with these receptors if brexpiprazole occupancy is high. At lower dosages, the drug occupies only a fraction of the receptors, allowing the endogenous neurotransmitters to continue interacting with their receptors, thereby magnifying the signal of that receptor above baseline.
Dosage windows could be difficult to understand pharmacologically, but for a partial agonist the presumed mechanism could be more evident. Clinicians should be aware that more is not always better, meaning that with partial agonist drugs a higher dosage might not lead to greater patient response. With brexpiprazole, a dopamine D2 partial agonist FDA-approved for schizophrenia and an adjunct for major depressive disorder (MDD),1 moderation is best because of
Recommended dosage
Two placebo-controlled studies2,3 examined brexpiprazole dosages of 1, 2, and 3 mg/d. The recommended dosage of 2 mg/d for MDD was determined by changes in Montgomery-Åsburg Depression Rating Scale scores (Figure).4 Lower dosages of 1 mg/d did not reach statistical significance, and 3 mg/d were less effective than the intermediate dosage of 2 mg/d. This result suggests a window of efficacy for brexpiprazole for MDD. This therapeutic window likely applies to most patients; however, patient-specific variables could alter the optimum dosage.
Dosage window
Brexpiprazole has high affinity for dopamine D2, D3, serotonin 5-HT1A, 5-HT2A, norepinephrine α1B, and α2 Creceptors. At relatively low drug concentrations, brexpiprazole achieves high receptor occupancy. At receptors for which brexpiprazole is a partial agonist (5-HT1A, D2, D3) the drug blocks the receptor and stimulates it at a fraction of the endogenous neurotransmitter. With a very high affinity agent, the endogenous neurotransmitter could be completely excluded from interacting with these receptors if brexpiprazole occupancy is high. At lower dosages, the drug occupies only a fraction of the receptors, allowing the endogenous neurotransmitters to continue interacting with their receptors, thereby magnifying the signal of that receptor above baseline.
1. FDA approves Rexulti (brexpiprazole) as adjunctive treatment for adults with major depressive disorder and as a treatment for adults with schizophrenia [news release]. Valby, Denmark; Tokyo, Japan: H. Lundbeck A/S (Lundbeck); Otsuka Pharmaceutical Co., Ltd; July 11, 2015. http://investor.lundbeck.com/ releasedetail.cfm?Release ID=921621. Accessed October 3, 2015.
2. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9): 1232-1240.
3. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebocontrolled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231.
4. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.
1. FDA approves Rexulti (brexpiprazole) as adjunctive treatment for adults with major depressive disorder and as a treatment for adults with schizophrenia [news release]. Valby, Denmark; Tokyo, Japan: H. Lundbeck A/S (Lundbeck); Otsuka Pharmaceutical Co., Ltd; July 11, 2015. http://investor.lundbeck.com/ releasedetail.cfm?Release ID=921621. Accessed October 3, 2015.
2. Thase ME, Youakim JM, Skuban A, et al. Adjunctive brexpiprazole 1 and 3 mg for patients with major depressive disorder following inadequate response to antidepressants: a phase 3, randomized, double-blind study. J Clin Psychiatry. 2015;76(9): 1232-1240.
3. Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a phase 3, randomized, placebocontrolled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224-1231.
4. Rexulti [package insert]. Rockville, MD: Otsuka; 2015.