User login
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
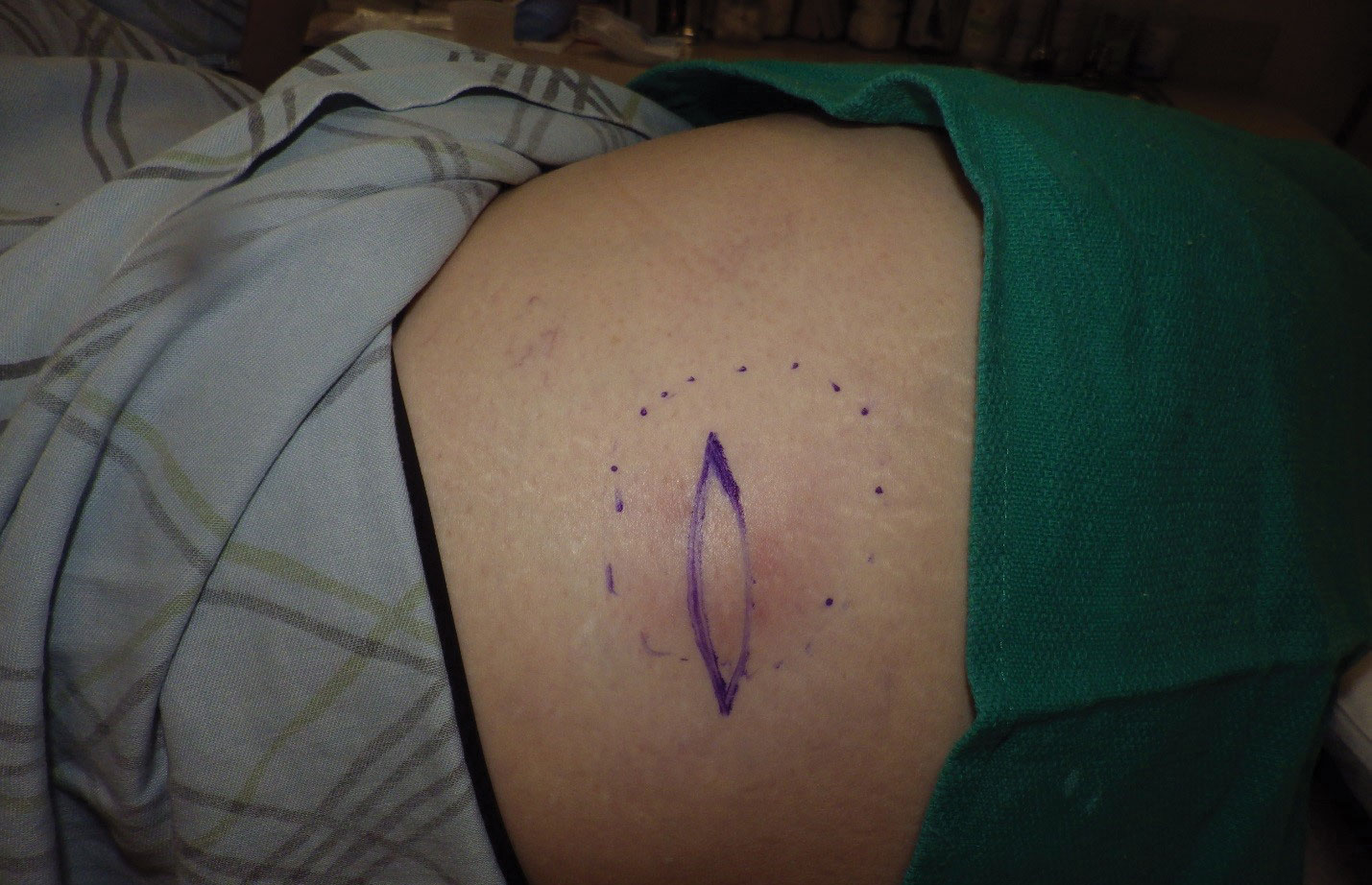
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.
The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
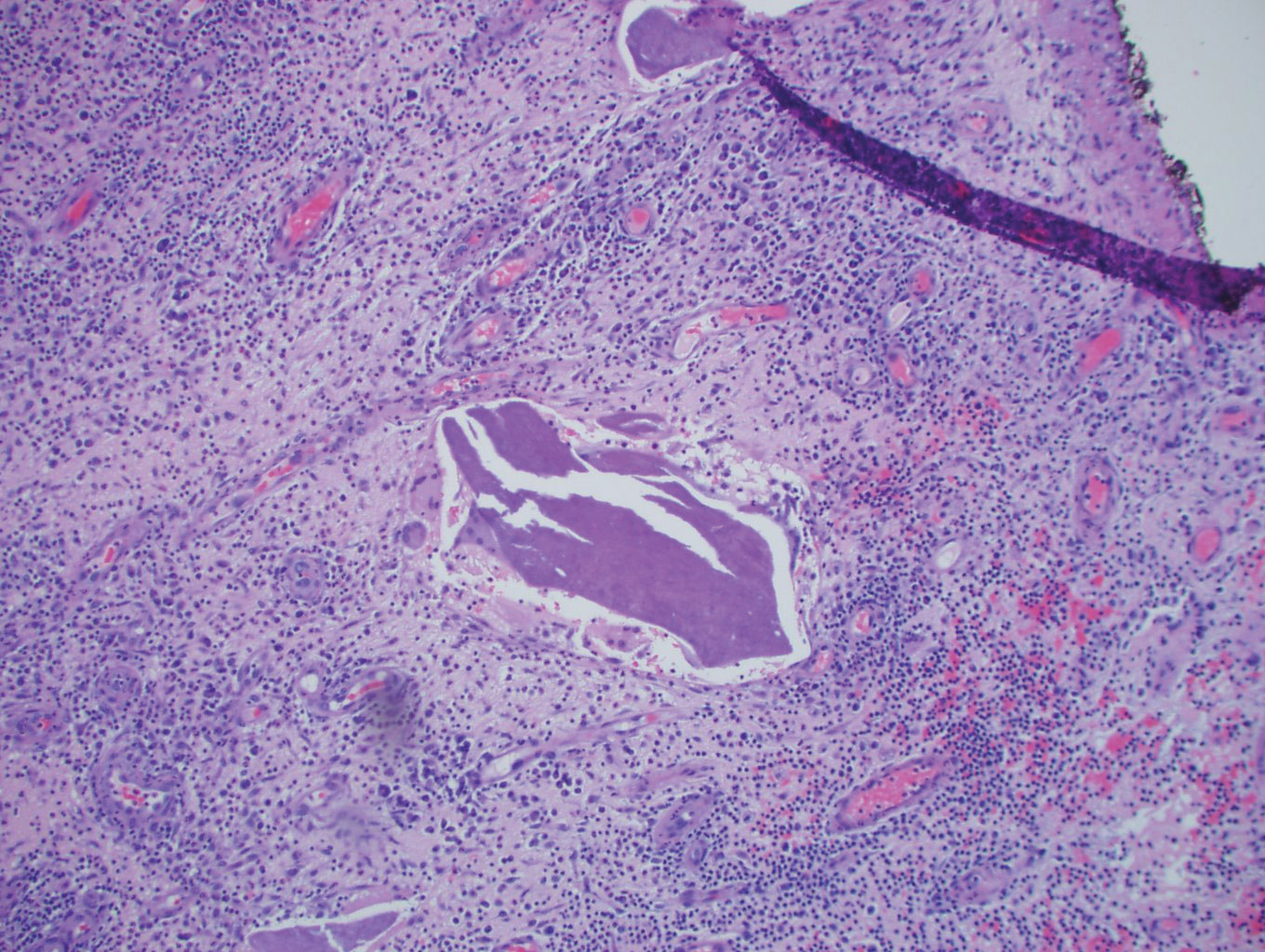
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).

The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.

The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).


The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.

The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).


The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
Practice Points
- Achromobacter xylosoxidans is an emerging pathogen primarily in the immunocompromised patient.
- Achromobacter xylosoxidans can form biofilms on plastics treated with disinfectant solution, including medical products.
- Strains of A xylosoxidans have shown multiantibiotic resistance.
