User login
An Unusual Skin Infection With Achromobacter xylosoxidans
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
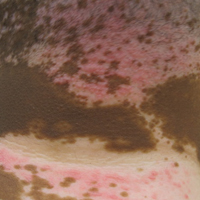
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.
The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
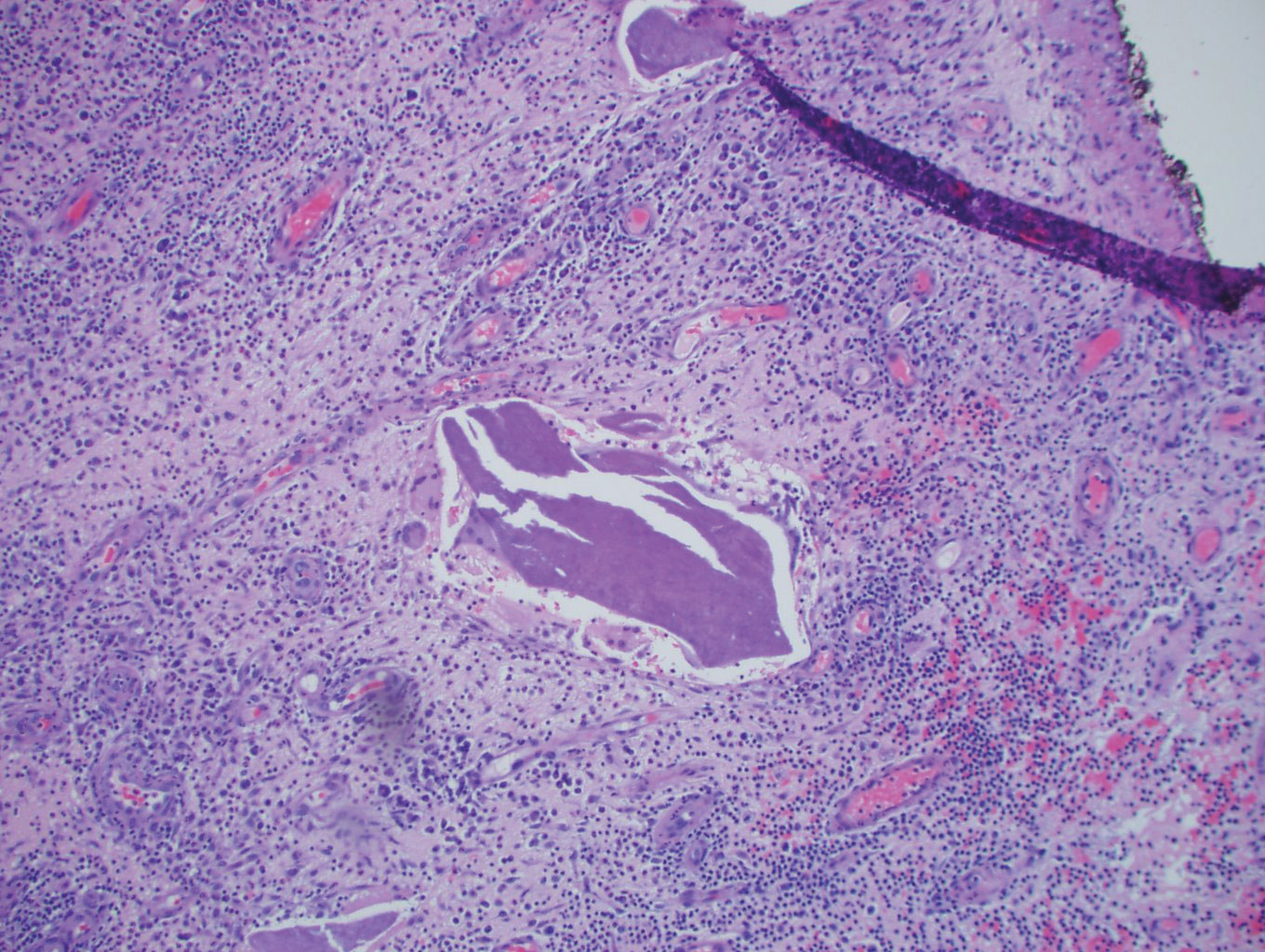
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).

The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.

The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).


The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
Case Report
A 50-year-old woman presented with a sore, tender, red lump on the right superior buttock of 5 months’ duration. Five months prior to presentation the patient used this area to attach the infusion set for an insulin pump, which was left in place for 7 days as opposed to the 2 or 3 days recommended by the device manufacturer. A firm, slightly tender lump formed, similar to prior scars that had developed from use of the insulin pump. However, the lump began to grow and get softer. It was intermittently warm and red. Although the area was sore and tender, she never had any major pain. She also denied any fever, malaise, or other systemic symptoms.
The patient indicated a medical history of type 1 diabetes mellitus diagnosed at 9 years of age; hypertension; asthma; gastroesophageal reflux disease; allergic rhinitis; migraine headaches; depression; hidradenitis suppurativa that resolved after surgical excision; and recurrent vaginal yeast infections, especially when taking antibiotics. She had a surgical history of hidradenitis suppurativa excision at the inguinal folds, bilateral carpal tunnel release, tubal ligation, abdominoplasty, and cholecystectomy. The patient’s current medications included insulin aspart, mometasone furoate, inhaled fluticasone, pantoprazole, cetirizine, spironolactone, duloxetine, sumatriptan, fluconazole, topiramate, and enalapril.
Physical examination revealed normal vital signs and the patient was afebrile. She had no swollen or tender lymph nodes. There was a 5.5×7.0-cm, soft, tender, erythematous subcutaneous mass with no visible punctum or overlying epidermal change on the right superior buttock (Figure 1). Based on the history and physical examination, the differential diagnosis included subcutaneous fat necrosis, epidermal inclusion cyst, and an abscess.

The patient was scheduled for excision of the mass the day after presenting to the clinic. During excision, 10 mL of thick purulent liquid was drained. A sample of the liquid was sent for Gram stain, aerobic and anaerobic culture, and antibiotic sensitivities. Necrotic-appearing adipose and fibrotic tissues were dissected and extirpated through an elliptical incision and submitted for pathologic evaluation.
Histopathology showed a subcutaneous defect with palisaded granulomatous inflammation and sclerosis (Figure 2). There was no detection of microorganisms with Grocott-Gomori methenamine-silver, tissue Gram, or acid-fast stains. There was a focus of acellular material embedded within the inflammation (Figure 3). The Gram stain of the purulent material showed few white blood cells and rare gram-negative bacilli. Culture grew moderate Achromobacter xylosoxidans resistant to cefepime, cefotaxime, and gentamicin. The culture was susceptible to ceftazidime, imipenem, levofloxacin, piperacillin, and trimethoprim-sulfamethoxazole (TMP-SMX).


The patient was prescribed oral TMP-SMX (160 mg of TMP and 800 mg of SMX) twice daily for 10 days. The patient tolerated the procedure and the subsequent antibiotics well. The patient had normal levels of IgA, IgG, and IgM, as well as a negative screening test for human immunodeficiency virus. She healed well from the surgical procedure and has had no recurrence of symptoms.
Comment
Achromobacter xylosoxidans is a nonfermentative, non–spore-forming, motile, gram-negative, aerobic, catalase-positive and oxidase-positive flagellate bacterium. It is an emerging pathogen that was first isolated in 1971 from patients with chronic otitis media.1 Since its recognition, it has been documented to cause a variety of infections, including pneumonia, meningitis, osteomyelitis, endocarditis, and bacteremia, as well as abdominal, urinary tract, ocular, and skin and soft tissue infections.2,3 Those affected usually are immunocompromised, have hematologic disorders, or have indwelling catheters.4 Strains of A xylosoxidans have shown resistance to multiple antibiotics including penicillins, cephalosporins, carbapenems, aminoglycosides, macrolides, fluoroquinolones, and TMP-SMX. Achromobacter xylosoxidans has been documented to form biofilms on plastics, including on contact lenses, urinary and intravenous catheters, and reusable tissue dispensers treated with disinfectant solution.4-6 One study demonstrated that A xylosoxidans is even capable of biodegradation of plastic, using the plastic as its sole source of carbon.7
Our case illustrates an indolent infection with A xylosoxidans forming a granulomatous abscess at the site of an insulin pump that was left in place for 7 days in an immunocompetent patient. Although infections with A xylosoxidans in patients with urinary or intravenous catheters have been reported,4 our case is unique, as the insulin pump was the source of such an infection. It is possible that the subcutaneous focus of acellular material described on the pathology report represented a partially biodegraded piece of the insulin pump catheter that broke off and was serving as a nidus of infection for A xylosoxidans. Although multidrug resistance is common, the culture grown from our patient was susceptible to TMP-SMX, among other antibiotics. Our patient was treated successfully with surgical excision, drainage, and a 10-day course of TMP-SMX.
Conclusion
Health care providers should recognize A xylosoxidans as an emerging pathogen that is capable of forming biofilms on “disinfected” surfaces and medical products, especially plastics. Achromobacter xylosoxidans may be resistant to multiple antibiotics and can cause infections with various presentations.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
- Yabuuchi E, Oyama A. Achromobacter xylosoxidans n. sp. from human ear discharge. Jpn J Microbiol. 1971;15:477-481.
- Rodrigues CG, Rays J, Kanegae MY. Native-valve endocarditis caused by Achromobacter xylosoxidans: a case report and review of literature. Autops Case Rep. 2017;7:50-55.
- Tena D, Martínez NM, Losa C, et al. Skin and soft tissue infection caused by Achromobacter xylosoxidans: report of 14 cases. Scand J Infect Dis. 2014;46:130-135.
- Pérez Barragán E, Sandino Pérez J, Corbella L, et al. Achromobacter xylosoxidans bacteremia: clinical and microbiological features in a 10-year case series. Rev Esp Quimioter. 2018;31:268-273.
- Konstantinović N, Ćirković I, Đukić S, et al. Biofilm formation of Achromobacter xylosoxidans on contact lens. Acta Microbiol Immunol Hung. 2017;64:293-300.
- Günther F, Merle U, Frank U, et al. Pseudobacteremia outbreak of biofilm-forming Achromobacter xylosoxidans—environmental transmission. BMC Infect Dis. 2016;16:584.
- Kowalczyk A, Chyc M, Ryszka P, et al. Achromobacter xylosoxidans as a new microorganism strain colonizing high-density polyethylene as a key step to its biodegradation. Environ Sci Pollut Res Int. 2016;23:11349-11356.
Practice Points
- Achromobacter xylosoxidans is an emerging pathogen primarily in the immunocompromised patient.
- Achromobacter xylosoxidans can form biofilms on plastics treated with disinfectant solution, including medical products.
- Strains of A xylosoxidans have shown multiantibiotic resistance.
Lichen Planus Pemphigoides Treated With Ustekinumab
Case Report
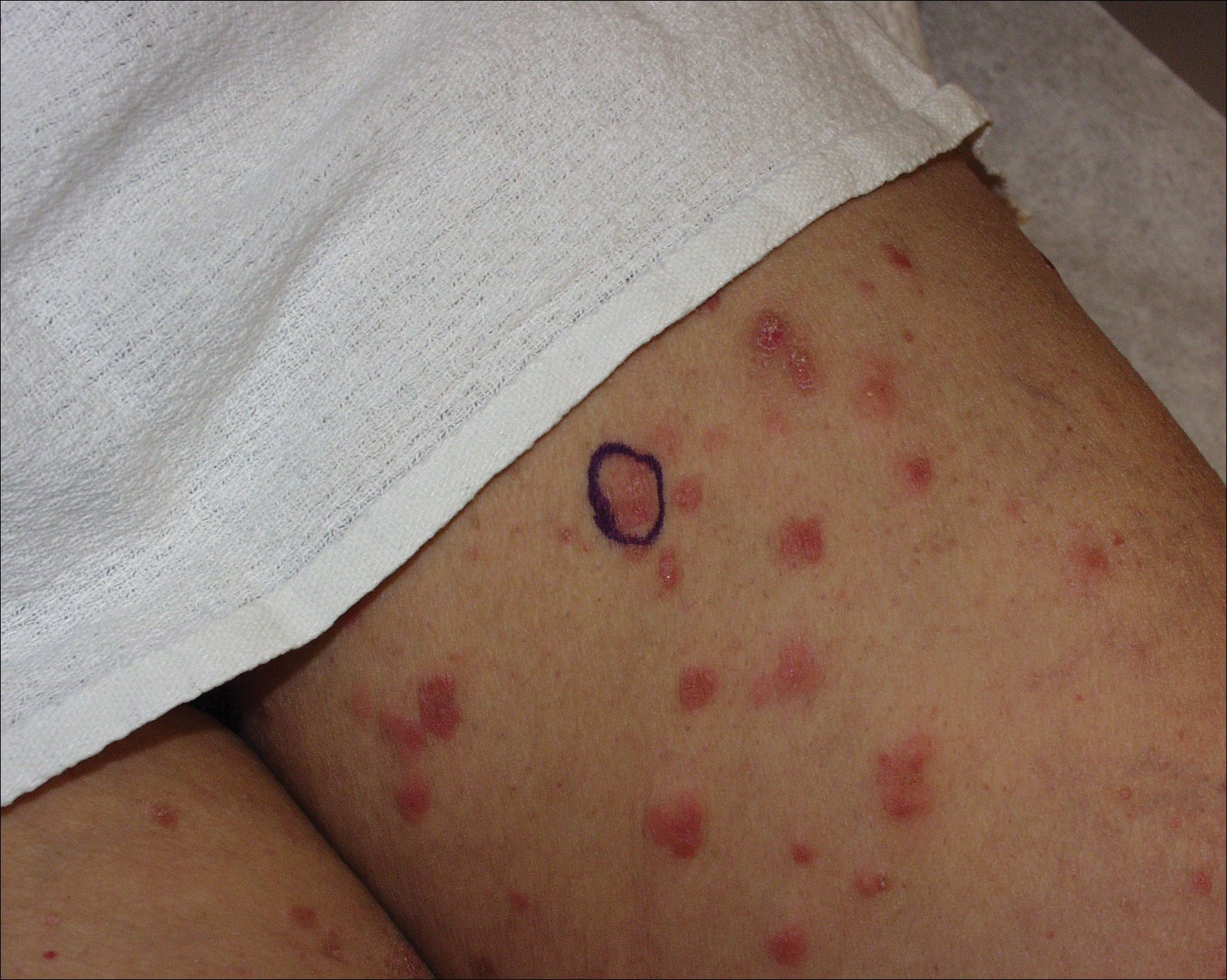
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.
Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.
The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
Case Report
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.

Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.

The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
Case Report
A 71-year-old woman presented with pink to violaceous, flat-topped, polygonal papules consistent with lichen planus (LP) on the volar wrists, extensor elbows, and bilateral lower legs of 3 years’ duration. She also had erythematous, violaceous, infiltrated plaques with microvesiculation on the bilateral thighs of several months’ duration (Figure 1). She reported pruritus, burning, and discomfort. Her medical history included type 2 diabetes mellitus, hypertension, and asthma with no history of skin rashes. A complete physical examination was performed. Age-appropriate screening for malignancy was negative. Hepatitis B and C antibody serologies were negative. Her medications at the time included risedronate and atenolol, which she had been taking for several years.

Punch biopsies from perilesional skin were submitted for hematoxylin and eosin staining and direct immunofluorescence (DIF). Histopathology showed a subepidermal blistering disease with tissue eosinophilia consistent with lichen planus pemphigoides (LPP)(Figure 2); direct immunofluorescence was positive for IgG, C3, and type IV collagen at the dermoepidermal junction. Serum BP180 was positive at 51 U/mL (reference range, <14 U/mL) and BP230 was negative. She was then started on tetracycline (500 mg twice daily), nicotinamide (500 mg twice daily), prednisone (5 mg daily), and dapsone (100 mg daily).
After 3 months without improvement, tetracycline and nicotinamide were discontinued, prednisone was increased to 10 mg daily, and dapsone was continued. A repeat biopsy was taken from a new area of involvement on the left lower leg, which revealed a psoriasiform dermatitis with interface changes. The DIF was positive for IgG and C3 along the basement membrane. A serum indirect immunofluorescence for BP180 also was positive.

The patient developed mild hemolytic anemia on dapsone; the medication was eventually discontinued. Subsequent treatments included adequate trials of azathioprine, mycophenolate mofetil, and hydroxychloroquine. Azathioprine (150 mg daily) and hydroxychloroquine (400 mg daily) treatment failed. She initially improved on mycophenolate mofetil (500 mg in the morning and 1000 mg in the evening) with flattening of the papules on the arms and legs and decreased erythema. However, mycophenolate mofetil eventually lost its efficacy and was discontinued.
Because several medications failed (ie, tetracycline, nicotinamide, prednisone, dapsone, azathioprine, mycophenolate mofetil, hydroxychloroquine), she was started on ustekinumab (45 mg) initial loading dose by subcutaneous injection (patient’s weight, 63 kg). At 4 weeks, the patient was given the second subcutaneous injection of ustekinumab (45 mg). She experienced marked improvement with no new lesions. The prior lesions also had decreased in size and were only slightly pink. The prednisone dose was tapered to 5 mg daily.
She had near-complete resolution of the skin lesions 12 weeks after the second dose of ustekinumab. Since then, she has had some recrudescence of the papulosquamous lesions but no vesicles or bullae. With the exception of occasional scattered pink papules on the forearms, her condition greatly improved on ustekinumab. She is no longer taking any of the other medications with the exception of prednisone (down to 1 mg daily) with a plan to gradually taper completely off of it.
Comment
Clinical Presentation
Lichen planus pemphigoides is a rare autoimmune subepidermal blistering disease with few cases reported in the literature. It is considered a clinical variation of bullous pemphigoid (BP) or a coexistence of LP and BP.1,2 It is characterized by bullous lesions developing on LP papules as well as on clinically uninvolved areas of the skin. It has been reported that LPP is provoked by several medications including cinnarizine, captopril, ramipril, simvastatin, psoralen plus UVA, and antituberculous medications (eg, isoniazid, rifampin, ethambutol, pyrazinamide).1 Risedronate or atenolol have not been reported to cause LPP, LP, or BP; however, according to Litt,3 a lichenoid drug eruption has been associated with atenolol. Furthermore, some cases of LPP demonstrate overlapping characteristics with paraneoplastic pemphigus and have been associated with internal malignancy. Hamada et al4 described a case of LPP coupled with colon adenocarcinoma and numerous keratoacanthomas. The earliest depiction of the coexistence of a case of mainstream LP complicated by an extensive bullous eruption was by Kaposi5 in 1892. He coined the term lichen ruber pemphigoides.5
Compared to BP, LPP is believed to affect a younger age group and have a less serious clinical course. The mean age of onset of LPP is in the third to fourth decades of life, while BP typically presents in the sixth decade. When comparing the location of bullae in LPP versus BP, the lesions of LPP tend to occur on the limbs, while BP tends to occur on the trunk.6
Clinically, LPP is distinguished by the existence of bullous lesions developing atop of the lesions of LP as well as on normal skin, with the latter being more commonplace. A classic example of LPP is characterized by an initial episode of traditional LP lesions often having severe pruritus, with or without patches of erythema, with the sudden eruption of tense bullae. These bullae commonly appear on the extremities and can appear over the normal skin, erythematous patches, or preexisting papules.7 In the atypical clinical presentations of this dubious skin condition, the bullae may only be seen on the lesions of LP.8 There also could be a lichenoid erythrodermic manifestation of a bullous eruption.9
Oral lesions of LPP have been described but had not been studied immunopathologically until Allen et al10 portrayed a 59-year-old man with cutaneous and oral lesions of LPP. They performed biopsies on the oral lesions and examined them by routine light microscopy and immunofluorescent techniques. The fine keratotic striae on the anterior buccal mucosal lesions were clinically consistent with oral LP. Perilesional tissue in conjunction with ulceration of the posterior buccal mucosa demonstrated histologic and immunopathologic alterations consistent with BP.10
Histopathology
Histopathologically, the lesions of LP show a bandlike lymphohistiocytic infiltrate, colloid bodies in the dermis, irregular acanthosis with saw-toothed rete ridges, orthokeratosis, wedge-shaped hypergranulosis, and liquefaction degeneration of the basal layer. Direct immunofluorescence shows mainly IgM and C3 deposited on colloid bodies, fibrin, and fibrinogen.11 The histopathology of the bullous lesion of LPP depicts a subepidermal bulla with variable diffuse or sparse lymphohistiocytic infiltrate and frequent eosinophils with or without neutrophils in the upper dermis. The existence of C3 alone or with IgG along the dermoepidermal junction gives confirmation on DIF.7
Autoantibodies
The expression of IgG autoantibodies directed against the basement membrane zone distinguishes LPP from bullous LP.2 IgG autoantibodies to either one or both the 230-kDa and 180-kDa BP (type XVII collagen) antigens has been demonstrated with LPP.4,12-14 Hamada et al4 described a histologic pattern more consistent with paraneoplastic pemphigus. It has been suggested that injury to the basal cells in LP or damage due to other courses of therapy such as psoralen plus UVA unveil suppressed antigenic determinants or produce new antigens, leading to antibody development and production of BP.12,15
Zillikens et al2 performed a study to identify the target antigen of LPP autoantibodies. They used sera from patients with LPP (n=4) and stained the epidermal side of salt-split human skin in a configuration identical to BP sera. In BP, the autoimmune response is directed against BP180, a hemidesmosomal transmembrane collagenous glycoprotein. They demonstrated that sera from BP patients largely reacted with a set of 4 epitopes (MCW-0 through MCW-3) grouped within a 45 amino acid stretch of the major noncollagenous extracellular domain (NC16A) of BP180. By immunoblotting and enzyme-linked immunosorbent assay, LPP sera also were compellingly reactive with recombinant BP180 NC16A. Lichen planus pemphigoides epitopes were additionally mapped using a series of overlapping recombinant segments of the NC16A domain. The authors demonstrated that all LPP sera reacted with amino acids 46 through 59 of domain NC16A, a protein portion that was previously shown to be unreactive with BP sera. In addition, they showed that 2 LPP sera reacted with the immunodominant antigenic region related to BP. Furthermore, they identified a unique epitope within the BP180 NC16A domain—MCW-4—which was distinctively recognized by sera from patients with LPP.2
Pathogenesis
The pathogenesis of both LP and BP has been linked to multiple cytokines that induce apoptosis in basal keratinocytes. Implicated cytokines include IFN-γ, tumor necrosis factor α (TNF-α), IL-1, IL-6, and IL-8, as well as other apoptosis-related molecules, such as Fas/Apo-1 and Bcl-2 in LP.16-18 Soluble E-selectin, vascular endothelial growth factor, IL-1β, IL-8, IL-5, transforming growth factor β1, and TNF-α were found to be elevated in either blister fluid or sera of BP patients.15-17
Management
Lichen planus pemphigoides usually responds well to traditional therapies, with systemic steroids being the most efficacious treatment of extensive disease.12,13 Other options include tetracycline and nicotinamide, isotretinoin, dapsone, and immunosuppressive drugs such as systemic cortico-steroids.12 Demirçay et al12 described a patient with skin lesions that rapidly cleared after the administration of oral methylprednisolone (48 mg/d) and oral dapsone (100 mg/d). The methylprednisolone and dapsone were withdrawn after 12 and 16 weeks, respectively. There was no recurrence during the 1-year follow-up period.12 et al19 described a patient who was treated with pulsed intravenous corticosteroids and continued to develop new papular and vesicular skin lesions. However, when oral acitretin was added to the patient’s regimen, the skin lesions cleared.19 There are several case reports of the successful use of hydroxychloroquine in LP.20,21
Cutaneous, nail, and oral LP also can be treated with TNF-α inhibitors (eg, adalimumab, etanercept) with resolution of lesions.22-25 However, we have not been able to find any reports of treating LPP with biologic medications in a search of PubMed articles indexed for MEDLINE using the terms lichen planus pemphigoides and biologic treatments/therapies. Given the fact that TNF-α and other inflammatory cytokines are involved in the pathogenesis of BP and LP, it is feasible that they also may be involved in the pathogenesis of LPP.
In our patient with cutaneous LPP, we chose to use ustekinumab instead of a primary TNF-α inhibitor because ustekinumab indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22, which also could have played a role in the patient’s disease. Our goal was to use ustekinumab as a potential corticosteroid-sparing agent. Ustekinumab greatly improved her skin condition and allowed us to discontinue other medications.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Zillikens D, Caux F, Mascaro JM, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Litt J. Litt’s Drug Eruptions and Reactions Manual. 18th Ed. London, England: Informa Healthcare; 2011.
- Hamada T, Fujimoto W, Okazaki F, et al. Lichen planus pemphigoides and multiple keratoacanthomas associated with colon adenocarcinoma. Br J Dermatol. 2004;151:252-254.
- Kaposi M. Lichen ruber pemphigoides. Arch Derm Syph. 1892;343-346.
- Swale VJ, Black MM, Bhogal BS. Lichen planus pemphigoides: two case reports. Clin Exp Dermatol. 1998;23:132-135.
- Okochi H, Nashiro K, Tsuchida T, et al. Lichen planus pemphigoides: case reports and results of immunofluorescence and immunoelectron microscopic study. J Am Acad Dermatol. 1990;22:626-631.
- Mendiratta V, Asati DP, Koranne RV. Lichen planus pemphigoides in an Indian female. Indian J Dermatol. 2005;50:224-226.
- Joly P, Tanasescu S, Wolkenstein P, et al. Lichenoid erythrodermic bullous pemphigoid of the African patient. J Am Acad Dermatol. 1998;39:691-697.
- Allen , , R. Lichen planus pemphigoides: report of a case with oral lesions. Oral Surg Oral Med Oral Pathol. 1987;63:184-188.
- Rapini RP. Practical Dermatopathology. Philadelphia, PA: Mosby Elsevier; 2005.
- Demirçay Z, Baykal C, Demirkesen C. Lichen planus pemphigoides: report of two cases. Int J Dermatol. 2001;40:757-759.
- Sakuma-Oyama Y, Powell AM, Albert S, et al. Lichen planus pemphigoides evolving into pemphigoid nodularis. Clin Exp Dermatol. 2004;28:613-616.
- Hsu S, Ghohestani RF, Uitto J. Lichen planus pemphigoides with IgG autoantibodies to the 180kd bullous pemphigoid antigen (type XVII collagen). J Am Acad Dermatol. 2000;42:136-141.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Ameglio F, D’Auria L, Cordiali-Fei P, et al. Bullous pemphigoid and pemphigus vulgaris: correlated behaviour of serum VEGF, sE-selectin and TNF-alpha levels. J Biol Regul Homeost Agents. 1997;11:148-153.
- Ameglio F, D’auria L, Bonifati C, et al. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: relationships with disease intensity. Br J Dermatol. 1998;138:611-614.
- D’Auria L, Mussi A, Bonifati C, et al. Increased serum IL-6, TNF-alpha and IL-10 levels in patients with bullous pemphigoid: relationships with disease activity. J Eur Acad Dermatol Venereol. 1999;12:11-15.
- , ,, . Treatment of lichen planus pemphigoides with acitretin and pulsed corticosteroids. Hautarzt. 2003;54:268-273.
- Eisen D. Hydroxychloroquine sulfate (Plaquenil) improves oral lichen planus: an open trial. J Am Acad Dermatol. 1993;28:609-612.
- James WD, Berger T, Elston D. Andrews’ Diseases of the Skin. 11th ed. Philadelphia, PA: Mosby Elsevier; 2011.
- Holló P, Szakonyi J, Kiss D, et al. Successful treatment of lichen planus with adalimumab. Acta Derm Venereol. 2012;92:385-386.
- Yarom N. Etanercept for the management of oral lichen planus. Am J Clin Dermatol. 2007;8:121.
- Chao TJ. Adalimumab in the management of cutaneous and oral lichen planus. Cutis. 2009;84:325-328.
- Irla N, Schneiter T, Haneke E, et al. Nail lichen planus: successful treatment with etanercept. Case Rep Dermatol. 2010;2:173-176.
- Lichen planus pemphigoides (LPP) is a rare autoimmune subepidermal blistering disease with few cases reported in the literature.
- Because tumor necrosis factor 11α (TNF-11α) and other inflammatory cytokines are involved in the pathogenesis of bullous pemphigoid and lichen planus, it is feasible that they also may be involved in the pathogenesis of LPP.
- Ustekinumab may be used to treat LPP as a potential corticosteroid-sparing agent because it indirectly blocks TNF-α, as well as other proinflammatory cytokines such as IFN-γ, IL-17, and IL-22.
Telmisartan-Induced Lichen Planus Eruption Manifested on Vitiliginous Skin
To the Editor:
A 39-year-old man with a history of hypertension and vitiligo presented with a rapid-onset, generalized, pruritic rash covering the body of 4 weeks’ duration. He reported that the rash progressively worsened after developing mild sunburn. The patient stated that the rash was extremely pruritic with a burning sensation and was tender to touch. He was treated with betamethasone valerate cream 0.1% by an outside physician and an over-the-counter anti-itch lotion with no notable improvement. His only medication was telmisartan-hydrochlorothiazide (HCTZ) for hypertension. He denied any drug allergies.
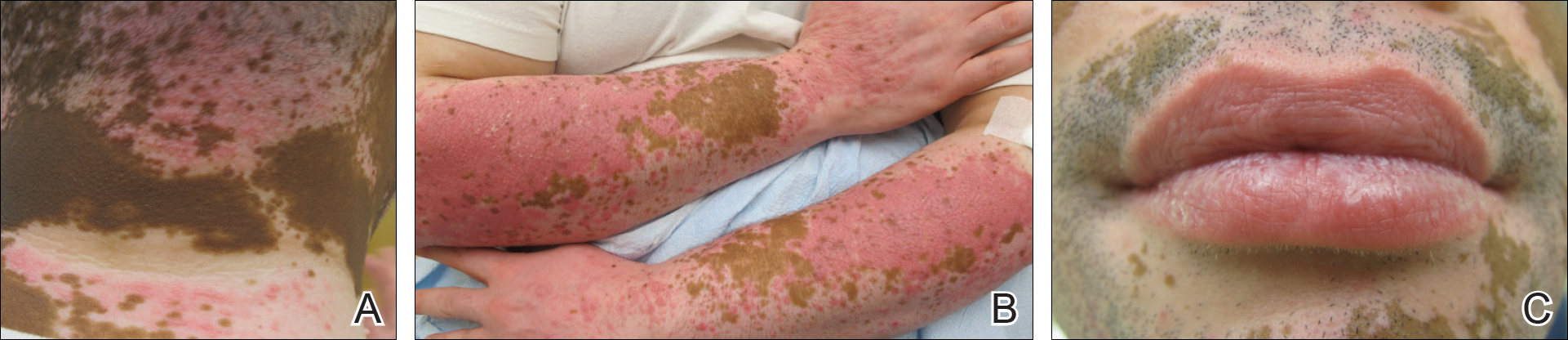
Physical examination revealed multiple discrete and coalescent planar erythematous papules and plaques involving only the depigmented vitiliginous skin of the forehead, eyelids, and nape of the neck (Figure 1A), and confluent on the lateral aspect of the bilateral forearms (Figure 1B), dorsal aspect of the right hand, and bilateral dorsi of the feet. Wickham striae were noted on the lips (Figure 1C). A clinical diagnosis of lichen planus (LP) was made. The patient initially was prescribed halobetasol propionate ointment 0.05% twice daily. He reported notable relief of pruritus with reduction of overall symptoms and new lesion formation.
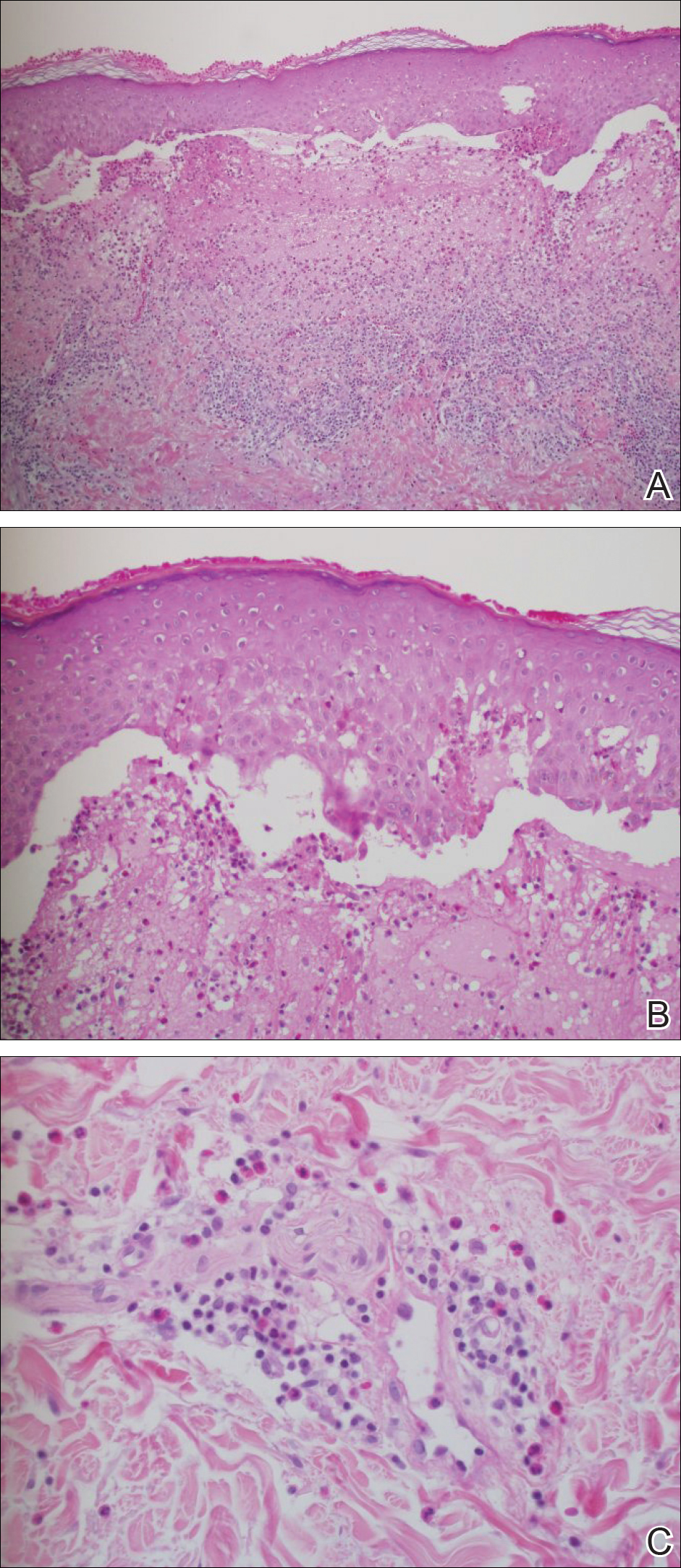
A 4-mm punch biopsy was performed on the left forearm. Histopathology revealed LP. Microscopic examination of the hematoxylin and eosin–stained specimen revealed a bandlike lymphohistiocytic infiltrate that extended across the papillary dermis, focally obscuring the dermoepidermal junction where there were vacuolar changes and colloid bodies. The epidermis showed sawtooth rete ridges, wedge-shaped foci of hypergranulosis, and compact hyperkeratosis (Figure 2).
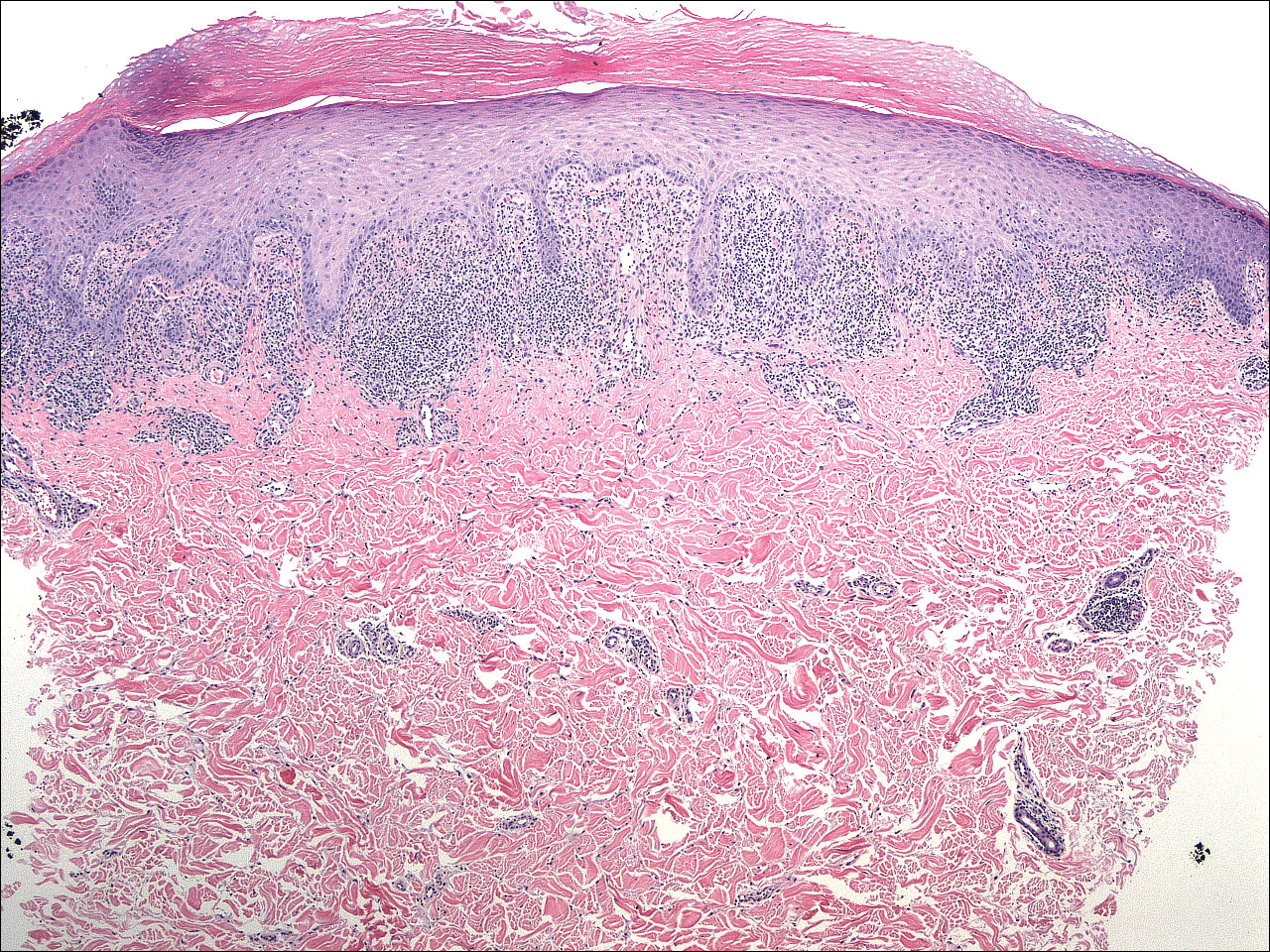
On further questioning during follow-up, the patient revealed that his hypertensive medication was changed from HCTZ, which he had been taking for the last 8 years, to the combination antihypertensive medication telmisartan-HCTZ before the onset of the skin eruption. Due to the temporal relationship between the new medication and onset of the eruption, the clinical impression was highly suspicious for drug-induced eruptive LP with Köbner phenomenon caused by the recent sunburn. Systemic workup for underlying causes of LP was negative. Laboratory tests revealed normal complete blood cell counts. The hepatitis panel included hepatitis A antibodies; hepatitis B surface, e antigen, and core antibodies; hepatitis B surface antigen and e antibodies; hepatitis C antibodies; and antinuclear antibodies, which were all negative.
The patient continued to develop new pruritic papules clinically consistent with LP. He was instructed to return to his primary care physician to change the telmisartan-HCTZ to a different class of antihypertensive medication. His medication was changed to atenolol. The patient also was instructed to continue the halobetasol propionate ointment 0.05% twice daily to the affected areas.
The patient returned for a follow-up visit 1 month later and reported notable improvement in pruritus and near-complete resolution of the LP after discontinuation of telmisartan-HCTZ. He also noted some degree of perifollicular repigmentation of the vitiliginous skin that had been unresponsive to prior therapy (Figure 3).
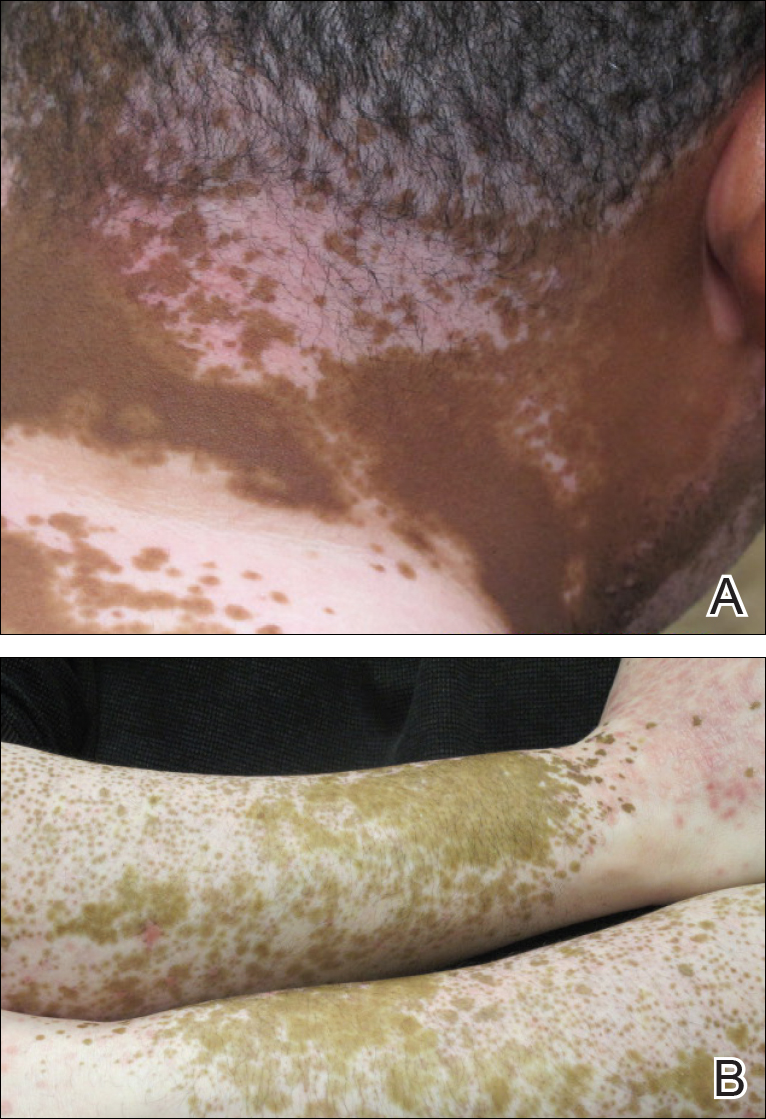
Lichen planus is a pruritic and inflammatory papulosquamous skin condition that presents as scaly, flat-topped, violaceous, polygonal-shaped papules commonly involving the flexor surface of the arms and legs, oral mucosa, scalp, nails, and genitalia. Clinically, LP can present in various forms including actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, and vesicular/bullous types. Koebnerization is common, especially in the linear form of LP. There are no specific laboratory findings or serologic markers seen in LP.
The exact cause of LP remains unknown. Clinical observations and anecdotal evidence have directed the cell-mediated immune response to insulting agents such as medications or contact allergy to metals triggering an abnormal cellular immune response. Various viral agents have been reported including hepatitis C virus, human herpesvirus, herpes simplex virus, and varicella-zoster virus.1-5 Other factors such as seasonal change and the environment may contribute to the development of LP and an increase in the incidence of LP eruption has been observed from January to July throughout the United States.6 Lichen planus also has been associated with other altered immune-related disease such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosis, and myasthenia gravis.7 Increased levels of emotional stress, particularly related to family members, often is related to the onset or aggravation of symptoms.8,9
Many drug-related LP-like and lichenoid eruptions have been reported with antihypertensive drugs, antimalarial drugs, diuretics, antidepressants, nonsteroidal anti-inflammatory drugs, antimicrobial drugs, and metals. In particular, medications such as captopril, enalapril, labetalol, propranolol, chlorothiazide, HCTZ, methyldopa, chloroquine, hydroxychloroquine, quinacrine, gold salts, penicillamine, and quinidine commonly are reported to induce lichenoid drug eruption.10
Several inflammatory papulosquamous skin conditions should be considered in the differential diagnosis before confirming the diagnosis of LP. It is important to rule out lupus erythematosus, especially if the oral mucosa and scalp are involved. In addition, erosive paraneoplastic pemphigus involving primarily the oral mucosa can resemble oral LP. Nail diseases such as psoriasis, onychomycosis, and alopecia areata should be considered as the differential diagnosis of nail disease. Genital involvement also can be seen in psoriasis and lichen sclerosus.
Treatment of LP is mainly symptomatic because of the benign nature of the disease and the high spontaneous remission rate with varying amount of time. If drugs, dental/metal implants, or underlying viral infections are the identifiable triggering factors of LP, the offending agents should be discontinued or removed. Additionally, topical or systemic treatments can be given depending on the severity of the disease, focusing mainly on symptomatic relief as well as the balance of risks and benefits associated with treatment.
Treatment options include topical and intralesional corticosteroids. Systemic medications such as oral corticosteroids and/or acitretin commonly are used in acute, severe, and disseminated cases, though treatment duration varies depending on the clinical response. Other systemic agents used to treat LP include griseofulvin, metronidazole, sulfasalazine, cyclosporine, and mycophenolate mofetil.
Phototherapy is considered an alternative therapy, especially for recalcitrant LP. UVA1 and narrowband UVB (wavelength, 311 nm) have been reported to effectively treat long-standing and therapy-resistant LP.11 In addition, a small study used the excimer laser (wavelength, 308 nm), which is well tolerated by patients, to treat focal recalcitrant oral lesions with excellent results.12 Photochemotherapy has been used with notable improvement, but the potential of carcinogenicity, especially in patients with Fitzpatrick skin types I and II, has limited its use.13
Our patient developed an unusual extensive LP eruption involving only vitiliginous skin shortly after initiation of the combined antihypertensive medication telmisartan-HCTZ, an angiotensin receptor blocker with a thiazide diuretic. Telmisartan and other angiotensin receptor blockers have not been reported to trigger LP; HCTZ is listed as one of the common drugs causing photosensitivity and LP.14,15 Although it is possible that our patient exhibited a delayed lichenoid drug eruption from the HCTZ, it is noteworthy that he did not experience a single episode of LP during his 8-year history of taking HCTZ. Instead, he developed the LP eruption shortly after the addition of telmisartan to his HCTZ antihypertensive regimen. The temporal relationship led us to direct the patient to the prescribing physician to discontinue telmisartan-HCTZ. After changing his antihypertensive medication to atenolol, the patient presented with improvement within the first month and near-complete resolution 2 months after the discontinuation of telmisartan-HCTZ.
Our patient’s LP lesions only manifested on the skin affected by vitiligo, sparing the normal-pigmented skin. Studies have demonstrated an increased ratio of CD8+ T cells to CD4+ T cells as well as increased intercellular adhesion molecule 1 at the dermal level.10,16 Both vitiligo and LP share some common histopathologic features including highly populated CD8+ T cells and intercellular adhesion molecule 1. In our case, LP was triggered on the vitiliginous skin by telmisartan. Vitiligo in combination with trauma induced by sunburn may represent the trigger that altered the cellular immune response and created the telmisartan-induced LP. As a result, the LP eruption was confined to the vitiliginous skin lesions.
Perifollicular repigmentation was observed in our patient after the LP lesions resolved; the patient’s vitiligo was unresponsive to prior treatment. The inflammatory process occurring in LP may exert and interfere in the underlying autoimmune cytotoxic effect toward the melanocytes and the melanin synthesis. It may be of interest to find out if the inflammatory response of LP has a positive influence on the effect of melanogenesis pathways or on the underlying autoimmune-related inflammatory process in vitiligo. Further studies are needed to investigate the role of immunotherapy targeting specific inflammatory pathways and the impact on the repigmentation in vitiligo.
Acknowledgment—Special thanks to Paul Chu, MD (Port Chester, New York).
- Pilli M, Zerbini A, Vescovi P, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446-1452.
- De Vries HJ, van Marle J, Teunissen MB, et al. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154:361-364.
- De Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213-219.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. 2001;45:614-615.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Sadr-Ashkevari S. Familial actinic lichen planus: case reports in two brothers. Arch Int Med. 2001;4:204-206.
- Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-441.
- Mahood JM. Familial lichen planus. Arch Dermatol. 1983;119:292-294.
- Shimizu M, Higaki Y, Higaki M, et al. The role of granzyme B-expressing CD8-positive T cells in apoptosis of keratinocytes in lichen planus. Arch Dermatol Res. 1997;289:527-532.
- Bécherel PA, Bussel A, Chosidow O, et al. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805.
- Trehan M, Taylar CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004;140:415-420.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23:15-19.
- Fellner MJ. Lichen planus. Int J Dermatol. 1980;19:71-75.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345-372.
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
To the Editor:
A 39-year-old man with a history of hypertension and vitiligo presented with a rapid-onset, generalized, pruritic rash covering the body of 4 weeks’ duration. He reported that the rash progressively worsened after developing mild sunburn. The patient stated that the rash was extremely pruritic with a burning sensation and was tender to touch. He was treated with betamethasone valerate cream 0.1% by an outside physician and an over-the-counter anti-itch lotion with no notable improvement. His only medication was telmisartan-hydrochlorothiazide (HCTZ) for hypertension. He denied any drug allergies.
Physical examination revealed multiple discrete and coalescent planar erythematous papules and plaques involving only the depigmented vitiliginous skin of the forehead, eyelids, and nape of the neck (Figure 1A), and confluent on the lateral aspect of the bilateral forearms (Figure 1B), dorsal aspect of the right hand, and bilateral dorsi of the feet. Wickham striae were noted on the lips (Figure 1C). A clinical diagnosis of lichen planus (LP) was made. The patient initially was prescribed halobetasol propionate ointment 0.05% twice daily. He reported notable relief of pruritus with reduction of overall symptoms and new lesion formation.

A 4-mm punch biopsy was performed on the left forearm. Histopathology revealed LP. Microscopic examination of the hematoxylin and eosin–stained specimen revealed a bandlike lymphohistiocytic infiltrate that extended across the papillary dermis, focally obscuring the dermoepidermal junction where there were vacuolar changes and colloid bodies. The epidermis showed sawtooth rete ridges, wedge-shaped foci of hypergranulosis, and compact hyperkeratosis (Figure 2).

On further questioning during follow-up, the patient revealed that his hypertensive medication was changed from HCTZ, which he had been taking for the last 8 years, to the combination antihypertensive medication telmisartan-HCTZ before the onset of the skin eruption. Due to the temporal relationship between the new medication and onset of the eruption, the clinical impression was highly suspicious for drug-induced eruptive LP with Köbner phenomenon caused by the recent sunburn. Systemic workup for underlying causes of LP was negative. Laboratory tests revealed normal complete blood cell counts. The hepatitis panel included hepatitis A antibodies; hepatitis B surface, e antigen, and core antibodies; hepatitis B surface antigen and e antibodies; hepatitis C antibodies; and antinuclear antibodies, which were all negative.
The patient continued to develop new pruritic papules clinically consistent with LP. He was instructed to return to his primary care physician to change the telmisartan-HCTZ to a different class of antihypertensive medication. His medication was changed to atenolol. The patient also was instructed to continue the halobetasol propionate ointment 0.05% twice daily to the affected areas.
The patient returned for a follow-up visit 1 month later and reported notable improvement in pruritus and near-complete resolution of the LP after discontinuation of telmisartan-HCTZ. He also noted some degree of perifollicular repigmentation of the vitiliginous skin that had been unresponsive to prior therapy (Figure 3).

Lichen planus is a pruritic and inflammatory papulosquamous skin condition that presents as scaly, flat-topped, violaceous, polygonal-shaped papules commonly involving the flexor surface of the arms and legs, oral mucosa, scalp, nails, and genitalia. Clinically, LP can present in various forms including actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, and vesicular/bullous types. Koebnerization is common, especially in the linear form of LP. There are no specific laboratory findings or serologic markers seen in LP.
The exact cause of LP remains unknown. Clinical observations and anecdotal evidence have directed the cell-mediated immune response to insulting agents such as medications or contact allergy to metals triggering an abnormal cellular immune response. Various viral agents have been reported including hepatitis C virus, human herpesvirus, herpes simplex virus, and varicella-zoster virus.1-5 Other factors such as seasonal change and the environment may contribute to the development of LP and an increase in the incidence of LP eruption has been observed from January to July throughout the United States.6 Lichen planus also has been associated with other altered immune-related disease such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosis, and myasthenia gravis.7 Increased levels of emotional stress, particularly related to family members, often is related to the onset or aggravation of symptoms.8,9
Many drug-related LP-like and lichenoid eruptions have been reported with antihypertensive drugs, antimalarial drugs, diuretics, antidepressants, nonsteroidal anti-inflammatory drugs, antimicrobial drugs, and metals. In particular, medications such as captopril, enalapril, labetalol, propranolol, chlorothiazide, HCTZ, methyldopa, chloroquine, hydroxychloroquine, quinacrine, gold salts, penicillamine, and quinidine commonly are reported to induce lichenoid drug eruption.10
Several inflammatory papulosquamous skin conditions should be considered in the differential diagnosis before confirming the diagnosis of LP. It is important to rule out lupus erythematosus, especially if the oral mucosa and scalp are involved. In addition, erosive paraneoplastic pemphigus involving primarily the oral mucosa can resemble oral LP. Nail diseases such as psoriasis, onychomycosis, and alopecia areata should be considered as the differential diagnosis of nail disease. Genital involvement also can be seen in psoriasis and lichen sclerosus.
Treatment of LP is mainly symptomatic because of the benign nature of the disease and the high spontaneous remission rate with varying amount of time. If drugs, dental/metal implants, or underlying viral infections are the identifiable triggering factors of LP, the offending agents should be discontinued or removed. Additionally, topical or systemic treatments can be given depending on the severity of the disease, focusing mainly on symptomatic relief as well as the balance of risks and benefits associated with treatment.
Treatment options include topical and intralesional corticosteroids. Systemic medications such as oral corticosteroids and/or acitretin commonly are used in acute, severe, and disseminated cases, though treatment duration varies depending on the clinical response. Other systemic agents used to treat LP include griseofulvin, metronidazole, sulfasalazine, cyclosporine, and mycophenolate mofetil.
Phototherapy is considered an alternative therapy, especially for recalcitrant LP. UVA1 and narrowband UVB (wavelength, 311 nm) have been reported to effectively treat long-standing and therapy-resistant LP.11 In addition, a small study used the excimer laser (wavelength, 308 nm), which is well tolerated by patients, to treat focal recalcitrant oral lesions with excellent results.12 Photochemotherapy has been used with notable improvement, but the potential of carcinogenicity, especially in patients with Fitzpatrick skin types I and II, has limited its use.13
Our patient developed an unusual extensive LP eruption involving only vitiliginous skin shortly after initiation of the combined antihypertensive medication telmisartan-HCTZ, an angiotensin receptor blocker with a thiazide diuretic. Telmisartan and other angiotensin receptor blockers have not been reported to trigger LP; HCTZ is listed as one of the common drugs causing photosensitivity and LP.14,15 Although it is possible that our patient exhibited a delayed lichenoid drug eruption from the HCTZ, it is noteworthy that he did not experience a single episode of LP during his 8-year history of taking HCTZ. Instead, he developed the LP eruption shortly after the addition of telmisartan to his HCTZ antihypertensive regimen. The temporal relationship led us to direct the patient to the prescribing physician to discontinue telmisartan-HCTZ. After changing his antihypertensive medication to atenolol, the patient presented with improvement within the first month and near-complete resolution 2 months after the discontinuation of telmisartan-HCTZ.
Our patient’s LP lesions only manifested on the skin affected by vitiligo, sparing the normal-pigmented skin. Studies have demonstrated an increased ratio of CD8+ T cells to CD4+ T cells as well as increased intercellular adhesion molecule 1 at the dermal level.10,16 Both vitiligo and LP share some common histopathologic features including highly populated CD8+ T cells and intercellular adhesion molecule 1. In our case, LP was triggered on the vitiliginous skin by telmisartan. Vitiligo in combination with trauma induced by sunburn may represent the trigger that altered the cellular immune response and created the telmisartan-induced LP. As a result, the LP eruption was confined to the vitiliginous skin lesions.
Perifollicular repigmentation was observed in our patient after the LP lesions resolved; the patient’s vitiligo was unresponsive to prior treatment. The inflammatory process occurring in LP may exert and interfere in the underlying autoimmune cytotoxic effect toward the melanocytes and the melanin synthesis. It may be of interest to find out if the inflammatory response of LP has a positive influence on the effect of melanogenesis pathways or on the underlying autoimmune-related inflammatory process in vitiligo. Further studies are needed to investigate the role of immunotherapy targeting specific inflammatory pathways and the impact on the repigmentation in vitiligo.
Acknowledgment—Special thanks to Paul Chu, MD (Port Chester, New York).
To the Editor:
A 39-year-old man with a history of hypertension and vitiligo presented with a rapid-onset, generalized, pruritic rash covering the body of 4 weeks’ duration. He reported that the rash progressively worsened after developing mild sunburn. The patient stated that the rash was extremely pruritic with a burning sensation and was tender to touch. He was treated with betamethasone valerate cream 0.1% by an outside physician and an over-the-counter anti-itch lotion with no notable improvement. His only medication was telmisartan-hydrochlorothiazide (HCTZ) for hypertension. He denied any drug allergies.
Physical examination revealed multiple discrete and coalescent planar erythematous papules and plaques involving only the depigmented vitiliginous skin of the forehead, eyelids, and nape of the neck (Figure 1A), and confluent on the lateral aspect of the bilateral forearms (Figure 1B), dorsal aspect of the right hand, and bilateral dorsi of the feet. Wickham striae were noted on the lips (Figure 1C). A clinical diagnosis of lichen planus (LP) was made. The patient initially was prescribed halobetasol propionate ointment 0.05% twice daily. He reported notable relief of pruritus with reduction of overall symptoms and new lesion formation.

A 4-mm punch biopsy was performed on the left forearm. Histopathology revealed LP. Microscopic examination of the hematoxylin and eosin–stained specimen revealed a bandlike lymphohistiocytic infiltrate that extended across the papillary dermis, focally obscuring the dermoepidermal junction where there were vacuolar changes and colloid bodies. The epidermis showed sawtooth rete ridges, wedge-shaped foci of hypergranulosis, and compact hyperkeratosis (Figure 2).

On further questioning during follow-up, the patient revealed that his hypertensive medication was changed from HCTZ, which he had been taking for the last 8 years, to the combination antihypertensive medication telmisartan-HCTZ before the onset of the skin eruption. Due to the temporal relationship between the new medication and onset of the eruption, the clinical impression was highly suspicious for drug-induced eruptive LP with Köbner phenomenon caused by the recent sunburn. Systemic workup for underlying causes of LP was negative. Laboratory tests revealed normal complete blood cell counts. The hepatitis panel included hepatitis A antibodies; hepatitis B surface, e antigen, and core antibodies; hepatitis B surface antigen and e antibodies; hepatitis C antibodies; and antinuclear antibodies, which were all negative.
The patient continued to develop new pruritic papules clinically consistent with LP. He was instructed to return to his primary care physician to change the telmisartan-HCTZ to a different class of antihypertensive medication. His medication was changed to atenolol. The patient also was instructed to continue the halobetasol propionate ointment 0.05% twice daily to the affected areas.
The patient returned for a follow-up visit 1 month later and reported notable improvement in pruritus and near-complete resolution of the LP after discontinuation of telmisartan-HCTZ. He also noted some degree of perifollicular repigmentation of the vitiliginous skin that had been unresponsive to prior therapy (Figure 3).

Lichen planus is a pruritic and inflammatory papulosquamous skin condition that presents as scaly, flat-topped, violaceous, polygonal-shaped papules commonly involving the flexor surface of the arms and legs, oral mucosa, scalp, nails, and genitalia. Clinically, LP can present in various forms including actinic, annular, atrophic, erosive, follicular, hypertrophic, linear, pigmented, and vesicular/bullous types. Koebnerization is common, especially in the linear form of LP. There are no specific laboratory findings or serologic markers seen in LP.
The exact cause of LP remains unknown. Clinical observations and anecdotal evidence have directed the cell-mediated immune response to insulting agents such as medications or contact allergy to metals triggering an abnormal cellular immune response. Various viral agents have been reported including hepatitis C virus, human herpesvirus, herpes simplex virus, and varicella-zoster virus.1-5 Other factors such as seasonal change and the environment may contribute to the development of LP and an increase in the incidence of LP eruption has been observed from January to July throughout the United States.6 Lichen planus also has been associated with other altered immune-related disease such as ulcerative colitis, alopecia areata, vitiligo, dermatomyositis, morphea, lichen sclerosis, and myasthenia gravis.7 Increased levels of emotional stress, particularly related to family members, often is related to the onset or aggravation of symptoms.8,9
Many drug-related LP-like and lichenoid eruptions have been reported with antihypertensive drugs, antimalarial drugs, diuretics, antidepressants, nonsteroidal anti-inflammatory drugs, antimicrobial drugs, and metals. In particular, medications such as captopril, enalapril, labetalol, propranolol, chlorothiazide, HCTZ, methyldopa, chloroquine, hydroxychloroquine, quinacrine, gold salts, penicillamine, and quinidine commonly are reported to induce lichenoid drug eruption.10
Several inflammatory papulosquamous skin conditions should be considered in the differential diagnosis before confirming the diagnosis of LP. It is important to rule out lupus erythematosus, especially if the oral mucosa and scalp are involved. In addition, erosive paraneoplastic pemphigus involving primarily the oral mucosa can resemble oral LP. Nail diseases such as psoriasis, onychomycosis, and alopecia areata should be considered as the differential diagnosis of nail disease. Genital involvement also can be seen in psoriasis and lichen sclerosus.
Treatment of LP is mainly symptomatic because of the benign nature of the disease and the high spontaneous remission rate with varying amount of time. If drugs, dental/metal implants, or underlying viral infections are the identifiable triggering factors of LP, the offending agents should be discontinued or removed. Additionally, topical or systemic treatments can be given depending on the severity of the disease, focusing mainly on symptomatic relief as well as the balance of risks and benefits associated with treatment.
Treatment options include topical and intralesional corticosteroids. Systemic medications such as oral corticosteroids and/or acitretin commonly are used in acute, severe, and disseminated cases, though treatment duration varies depending on the clinical response. Other systemic agents used to treat LP include griseofulvin, metronidazole, sulfasalazine, cyclosporine, and mycophenolate mofetil.
Phototherapy is considered an alternative therapy, especially for recalcitrant LP. UVA1 and narrowband UVB (wavelength, 311 nm) have been reported to effectively treat long-standing and therapy-resistant LP.11 In addition, a small study used the excimer laser (wavelength, 308 nm), which is well tolerated by patients, to treat focal recalcitrant oral lesions with excellent results.12 Photochemotherapy has been used with notable improvement, but the potential of carcinogenicity, especially in patients with Fitzpatrick skin types I and II, has limited its use.13
Our patient developed an unusual extensive LP eruption involving only vitiliginous skin shortly after initiation of the combined antihypertensive medication telmisartan-HCTZ, an angiotensin receptor blocker with a thiazide diuretic. Telmisartan and other angiotensin receptor blockers have not been reported to trigger LP; HCTZ is listed as one of the common drugs causing photosensitivity and LP.14,15 Although it is possible that our patient exhibited a delayed lichenoid drug eruption from the HCTZ, it is noteworthy that he did not experience a single episode of LP during his 8-year history of taking HCTZ. Instead, he developed the LP eruption shortly after the addition of telmisartan to his HCTZ antihypertensive regimen. The temporal relationship led us to direct the patient to the prescribing physician to discontinue telmisartan-HCTZ. After changing his antihypertensive medication to atenolol, the patient presented with improvement within the first month and near-complete resolution 2 months after the discontinuation of telmisartan-HCTZ.
Our patient’s LP lesions only manifested on the skin affected by vitiligo, sparing the normal-pigmented skin. Studies have demonstrated an increased ratio of CD8+ T cells to CD4+ T cells as well as increased intercellular adhesion molecule 1 at the dermal level.10,16 Both vitiligo and LP share some common histopathologic features including highly populated CD8+ T cells and intercellular adhesion molecule 1. In our case, LP was triggered on the vitiliginous skin by telmisartan. Vitiligo in combination with trauma induced by sunburn may represent the trigger that altered the cellular immune response and created the telmisartan-induced LP. As a result, the LP eruption was confined to the vitiliginous skin lesions.
Perifollicular repigmentation was observed in our patient after the LP lesions resolved; the patient’s vitiligo was unresponsive to prior treatment. The inflammatory process occurring in LP may exert and interfere in the underlying autoimmune cytotoxic effect toward the melanocytes and the melanin synthesis. It may be of interest to find out if the inflammatory response of LP has a positive influence on the effect of melanogenesis pathways or on the underlying autoimmune-related inflammatory process in vitiligo. Further studies are needed to investigate the role of immunotherapy targeting specific inflammatory pathways and the impact on the repigmentation in vitiligo.
Acknowledgment—Special thanks to Paul Chu, MD (Port Chester, New York).
- Pilli M, Zerbini A, Vescovi P, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446-1452.
- De Vries HJ, van Marle J, Teunissen MB, et al. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154:361-364.
- De Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213-219.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. 2001;45:614-615.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Sadr-Ashkevari S. Familial actinic lichen planus: case reports in two brothers. Arch Int Med. 2001;4:204-206.
- Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-441.
- Mahood JM. Familial lichen planus. Arch Dermatol. 1983;119:292-294.
- Shimizu M, Higaki Y, Higaki M, et al. The role of granzyme B-expressing CD8-positive T cells in apoptosis of keratinocytes in lichen planus. Arch Dermatol Res. 1997;289:527-532.
- Bécherel PA, Bussel A, Chosidow O, et al. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805.
- Trehan M, Taylar CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004;140:415-420.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23:15-19.
- Fellner MJ. Lichen planus. Int J Dermatol. 1980;19:71-75.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345-372.
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
- Pilli M, Zerbini A, Vescovi P, et al. Oral lichen planus pathogenesis: a role for the HCV-specific cellular immune response. Hepatology. 2002;36:1446-1452.
- De Vries HJ, van Marle J, Teunissen MB, et al. Lichen planus is associated with human herpesvirus type 7 replication and infiltration of plasmacytoid dendritic cells. Br J Dermatol. 2006;154:361-364.
- De Vries HJ, Teunissen MB, Zorgdrager F, et al. Lichen planus remission is associated with a decrease of human herpes virus type 7 protein expression in plasmacytoid dendritic cells. Arch Dermatol Res. 2007;299:213-219.
- Requena L, Kutzner H, Escalonilla P, et al. Cutaneous reactions at sites of herpes zoster scars: an expanded spectrum. Br J Dermatol. 1998;138:161-168.
- Al-Khenaizan S. Lichen planus occurring after hepatitis B vaccination: a new case. J Am Acad Dermatol. 2001;45:614-615.
- Boyd AS, Neldner KH. Lichen planus. J Am Acad Dermatol. 1991;25:593-619.
- Sadr-Ashkevari S. Familial actinic lichen planus: case reports in two brothers. Arch Int Med. 2001;4:204-206.
- Manolache L, Seceleanu-Petrescu D, Benea V. Lichen planus patients and stressful events. J Eur Acad Dermatol Venereol. 2008;22:437-441.
- Mahood JM. Familial lichen planus. Arch Dermatol. 1983;119:292-294.
- Shimizu M, Higaki Y, Higaki M, et al. The role of granzyme B-expressing CD8-positive T cells in apoptosis of keratinocytes in lichen planus. Arch Dermatol Res. 1997;289:527-532.
- Bécherel PA, Bussel A, Chosidow O, et al. Extracorporeal photochemotherapy for chronic erosive lichen planus. Lancet. 1998;351:805.
- Trehan M, Taylar CR. Low-dose excimer 308-nm laser for the treatment of oral lichen planus. Arch Dermatol. 2004;140:415-420.
- Wackernagel A, Legat FJ, Hofer A, et al. Psoralen plus UVA vs. UVB-311 nm for the treatment of lichen planus. Photodermatol Photoimmunol Photomed. 2007;23:15-19.
- Fellner MJ. Lichen planus. Int J Dermatol. 1980;19:71-75.
- Moore DE. Drug-induced cutaneous photosensitivity: incidence, mechanism, prevention and management. Drug Saf. 2002;25:345-372.
- Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
Practice Points
- Lichen planus (LP) is a T-cell–mediated autoimmune disease that affects the skin and often the mucosa, nails, and scalp.
- The etiology of LP is unknown. It can be induced by a variety of medications and may spread through the isomorphic phenomenon.
- Immune factors play a role in the development of LP, drug-induced LP, and vitiligo.