User login
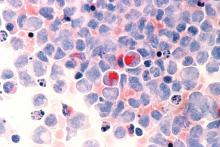
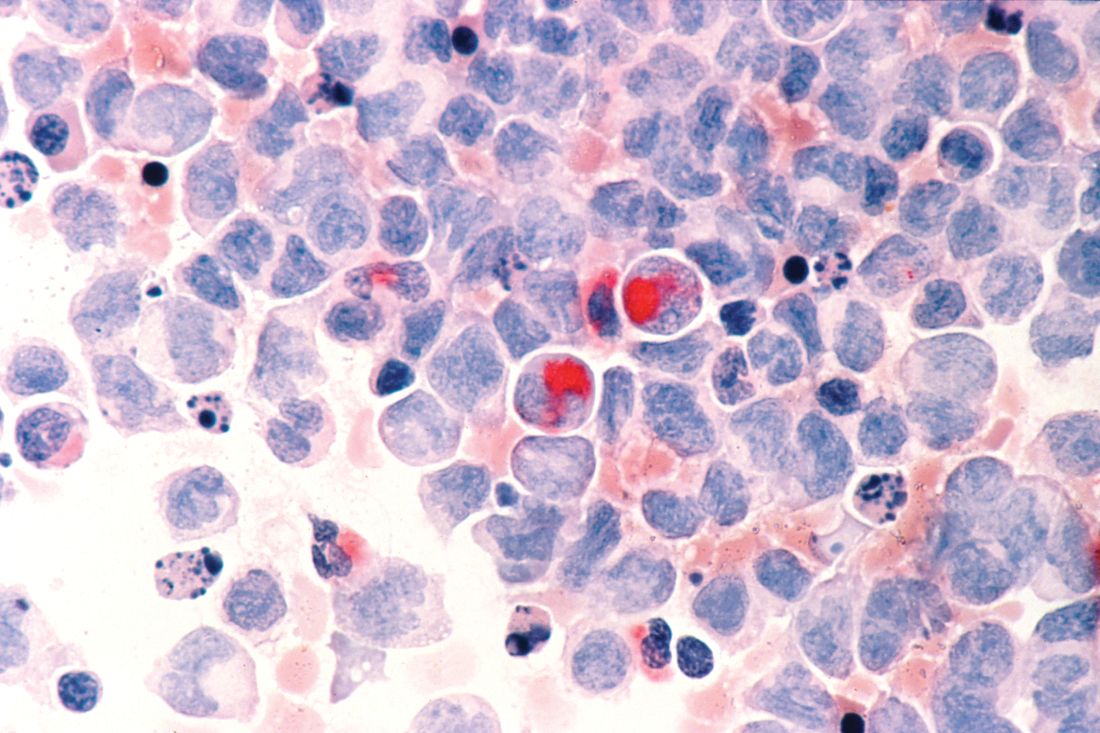
New York – For unfit elderly patients with acute myeloid leukemia (AML), venetoclax may be one of the most promising potential options that is emerging, according to an expert in the field.
The oral B-cell lymphoma 2 (BCL-2) inhibitor is the treatment that some in the AML community are “most excited about” for this population, Eunice S. Wang, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., said at the National Comprehensive Cancer Network (NCCN) Annual Congress: Hematologic Malignancies.
Venetoclax is currently approved by the Food and Drug Administration for previously treated chronic lymphocytic leukemia (CLL), alone or in combination with rituximab. It has been granted four Breakthrough Therapy designations from the FDA in AML. In July 2018, AbbVie submitted a Supplemental New Drug Application to the FDA for its use in combination with a hypomethylating agent (HMA) or in combination with low-dose cytarabine (LDAC) for the treatment of newly diagnosed AML patients who are ineligible for intensive chemotherapy.
“This agent doesn’t work on its own but had worked in the refractory setting and can be a great option upfront,” Dr. Wang said.
About half the patients were alive at 1 year following treatment with venetoclax plus low-dose chemotherapy, whether that was LDAC or an HMA, she said, commenting on recently reported results.
In a phase 1b trial, venetoclax was evaluated in combination with either azacitidine or decitabine. In recently reported preliminary results that included 57 patients aged 65 years or older who were ineligible for standard induction therapy, the combination was well tolerated and had promising activity (Lancet Oncol. 2018 Feb;19[2]:216-28).
Overall, 35 patients (61%) had complete remission (CR) or complete remission with incomplete marrow recovery (CRi).
In another report on this same trial, which included 33 patients from a single participating center who received venetoclax and azacytidine, the overall response rate was 91%, including 19 (58%) with CR and 9 (27%) with CRi (Blood. 2017 Dec;130 [Suppl 1]:181).
A separate phase 1/2 trial examined venetoclax plus LDAC in treatment-naive elderly patients unfit for intensive chemotherapy. In the 1-year outcomes that have been reported, the observed CR/CRi rate was 62%, median overall survival was an “encouraging” 11.4 months, and the observed 12-month overall survival was 46% in 61 patients treated at a venetoclax dose of 600 mg (Blood. 2017 Dec;130 [Suppl 1]:890).
In those 61 patients, treatment-related grade 3/4 adverse events included thrombocytopenia in 59%, neutropenia in 46%, febrile neutropenia in 36%, anemia in 28%, decreased white blood cell count in 26%, and one case of tumor lysis syndrome, according to the report.
Based on those findings in a cohort of patients with poor risk features, venetoclax 600 mg plus LDAC was carried forward to be evaluated in an ongoing phase 3 study, investigators noted at the time.
Dr. Wang reported financial relationships with AbbVie, Amgen, ImmunoGen, Incyte, Novartis, and Otsuka.
New York – For unfit elderly patients with acute myeloid leukemia (AML), venetoclax may be one of the most promising potential options that is emerging, according to an expert in the field.
The oral B-cell lymphoma 2 (BCL-2) inhibitor is the treatment that some in the AML community are “most excited about” for this population, Eunice S. Wang, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., said at the National Comprehensive Cancer Network (NCCN) Annual Congress: Hematologic Malignancies.
Venetoclax is currently approved by the Food and Drug Administration for previously treated chronic lymphocytic leukemia (CLL), alone or in combination with rituximab. It has been granted four Breakthrough Therapy designations from the FDA in AML. In July 2018, AbbVie submitted a Supplemental New Drug Application to the FDA for its use in combination with a hypomethylating agent (HMA) or in combination with low-dose cytarabine (LDAC) for the treatment of newly diagnosed AML patients who are ineligible for intensive chemotherapy.
“This agent doesn’t work on its own but had worked in the refractory setting and can be a great option upfront,” Dr. Wang said.
About half the patients were alive at 1 year following treatment with venetoclax plus low-dose chemotherapy, whether that was LDAC or an HMA, she said, commenting on recently reported results.
In a phase 1b trial, venetoclax was evaluated in combination with either azacitidine or decitabine. In recently reported preliminary results that included 57 patients aged 65 years or older who were ineligible for standard induction therapy, the combination was well tolerated and had promising activity (Lancet Oncol. 2018 Feb;19[2]:216-28).
Overall, 35 patients (61%) had complete remission (CR) or complete remission with incomplete marrow recovery (CRi).
In another report on this same trial, which included 33 patients from a single participating center who received venetoclax and azacytidine, the overall response rate was 91%, including 19 (58%) with CR and 9 (27%) with CRi (Blood. 2017 Dec;130 [Suppl 1]:181).
A separate phase 1/2 trial examined venetoclax plus LDAC in treatment-naive elderly patients unfit for intensive chemotherapy. In the 1-year outcomes that have been reported, the observed CR/CRi rate was 62%, median overall survival was an “encouraging” 11.4 months, and the observed 12-month overall survival was 46% in 61 patients treated at a venetoclax dose of 600 mg (Blood. 2017 Dec;130 [Suppl 1]:890).
In those 61 patients, treatment-related grade 3/4 adverse events included thrombocytopenia in 59%, neutropenia in 46%, febrile neutropenia in 36%, anemia in 28%, decreased white blood cell count in 26%, and one case of tumor lysis syndrome, according to the report.
Based on those findings in a cohort of patients with poor risk features, venetoclax 600 mg plus LDAC was carried forward to be evaluated in an ongoing phase 3 study, investigators noted at the time.
Dr. Wang reported financial relationships with AbbVie, Amgen, ImmunoGen, Incyte, Novartis, and Otsuka.
New York – For unfit elderly patients with acute myeloid leukemia (AML), venetoclax may be one of the most promising potential options that is emerging, according to an expert in the field.
The oral B-cell lymphoma 2 (BCL-2) inhibitor is the treatment that some in the AML community are “most excited about” for this population, Eunice S. Wang, MD, of Roswell Park Comprehensive Cancer Center in Buffalo, N.Y., said at the National Comprehensive Cancer Network (NCCN) Annual Congress: Hematologic Malignancies.
Venetoclax is currently approved by the Food and Drug Administration for previously treated chronic lymphocytic leukemia (CLL), alone or in combination with rituximab. It has been granted four Breakthrough Therapy designations from the FDA in AML. In July 2018, AbbVie submitted a Supplemental New Drug Application to the FDA for its use in combination with a hypomethylating agent (HMA) or in combination with low-dose cytarabine (LDAC) for the treatment of newly diagnosed AML patients who are ineligible for intensive chemotherapy.
“This agent doesn’t work on its own but had worked in the refractory setting and can be a great option upfront,” Dr. Wang said.
About half the patients were alive at 1 year following treatment with venetoclax plus low-dose chemotherapy, whether that was LDAC or an HMA, she said, commenting on recently reported results.
In a phase 1b trial, venetoclax was evaluated in combination with either azacitidine or decitabine. In recently reported preliminary results that included 57 patients aged 65 years or older who were ineligible for standard induction therapy, the combination was well tolerated and had promising activity (Lancet Oncol. 2018 Feb;19[2]:216-28).
Overall, 35 patients (61%) had complete remission (CR) or complete remission with incomplete marrow recovery (CRi).
In another report on this same trial, which included 33 patients from a single participating center who received venetoclax and azacytidine, the overall response rate was 91%, including 19 (58%) with CR and 9 (27%) with CRi (Blood. 2017 Dec;130 [Suppl 1]:181).
A separate phase 1/2 trial examined venetoclax plus LDAC in treatment-naive elderly patients unfit for intensive chemotherapy. In the 1-year outcomes that have been reported, the observed CR/CRi rate was 62%, median overall survival was an “encouraging” 11.4 months, and the observed 12-month overall survival was 46% in 61 patients treated at a venetoclax dose of 600 mg (Blood. 2017 Dec;130 [Suppl 1]:890).
In those 61 patients, treatment-related grade 3/4 adverse events included thrombocytopenia in 59%, neutropenia in 46%, febrile neutropenia in 36%, anemia in 28%, decreased white blood cell count in 26%, and one case of tumor lysis syndrome, according to the report.
Based on those findings in a cohort of patients with poor risk features, venetoclax 600 mg plus LDAC was carried forward to be evaluated in an ongoing phase 3 study, investigators noted at the time.
Dr. Wang reported financial relationships with AbbVie, Amgen, ImmunoGen, Incyte, Novartis, and Otsuka.
EXPERT ANALYSIS FROM THE NCCN ANNUAL CONGRESS: HEMATOLOGIC MALIGNANCIES