User login
To the Editor:
Onychomycosis is a common nail disease that frequently is caused by dermatophytes and is diagnosed by direct microscopy. Conventional diagnostic methods are often time consuming and can produce false-positive or false-negative results. We report a case of onychomycosis diagnosed by confocal microscopy and confirmed with routine potassium hydroxide (KOH) examination and fungal culture. Confocal microscopy is a reliable, practical, and noninvasive technique in the diagnosis of onychomycosis.
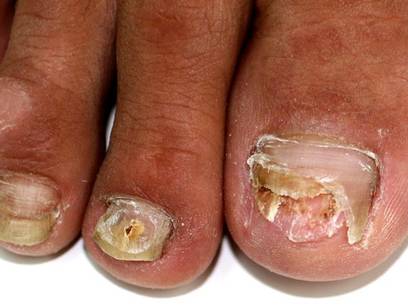
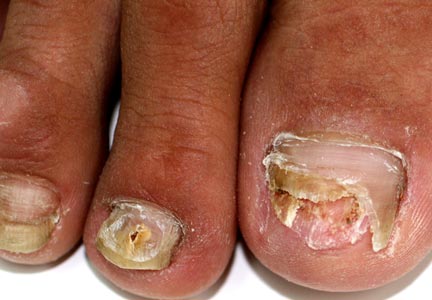
A 46-year-old woman presented with yellow-brown discoloration and dystrophy of the toenails (Figure 1) that had become worse over a 5-year period. She was otherwise healthy and had no other dermatologic problems. Examination revealed yellow-brown discoloration, subungual hyperkeratosis, and onycholysis of the toenails. Clinically, a diagnosis of onychomycosis was made. Potassium hydroxide examination of a scraping from the subungual region showed fungal elements. Trichophyton rubrum on Sabouraud dextrose agar was determined.
|
We performed both in vivo and in vitro confocal laser scanning microscopic examination of the nail of the right great toe (Figure 2). For the diagnosis of onychomycosis in our case, we used a multilaser reflectance confocal microscope (RCM) with a wavelength of 786 nm. In vivo confocal microscopy of the nail revealed branching hyphae just below the surface of the nail plate. Hyphae were seen as refractile, bright, linear structures along the laminates of the nail.
Onychomycosis is a common condition affecting 5.5% of the population worldwide and representing 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.1,2 There are many methods available to confirm the clinical diagnosis of onychomycosis by detecting the causative organisms. Direct microscopic examination of the scraping with a KOH culture, histopathologic assessment with periodic acid–Schiff staining, immunofluorescence analysis with calcofluor white staining, enzyme analysis, and polymerase chain reaction can be used for diagnosis of fungal infections. The most frequently used diagnostic method for onychomycosis is KOH examination of the scraping; however, fungal culture and histopathologic examination also can be used in cases having diagnostic difficulties.1,3,4 There are many studies comparing the efficacies of these methods in the literature.5-9
The causative fungal agent should be determined with at least 1 laboratory method due to the high cost, long duration, and serious potential adverse effects of systemic antifungal treatment. Direct microscopic examination with KOH in the diagnosis of onychomycosis is simple, fast, and inexpensive. However, inadequate material, using crystallized KOH for hydrolysis, insufficient or too much hydrolysis of scrapings, inappropriate staining, and not scanning all areas in the microscopy produce false-negative results. Similarly, secondary contamination of hair, cotton, yarn, or air bubbles mimicking fungal structures can cause false-positive results.9,10
Fungal culture is another diagnostic method that is accepted as the gold standard for diagnosis of onychomycosis.9 However, fungal cultures were positive in only 43% to 50% of all cases of onychomycosis that were diagnosed with other methods,11,12 which may be due to the loss of viability and ability of the fungi to grow in culture media during the transport. A major advantage of fungal culture is that the fungal agent can be classified as dermatophyte, nondermatophyte, mold, or yeast. However, culture does determine if the growing fungi is contamination or the real pathogen. Moreover, it is necessary to wait 3 to 4 weeks for culture results. For nondermatophyte fungi this time may be much longer.12
In vivo RCM is a noninvasive imaging method that allows optical en face sectioning of the living tissue with high resolution. Currently, RCM has a wide range of applications, such as the evaluation of both benign and malignant skin lesions in clinical dermatology.13
In vivo RCM was used first by Hongcharu et al.14 The diagnoses of onychomycosis and fungal hyphae were shown both in vivo and in vitro.14 The sensitivity and specificity of confocal examination in the diagnosis of onychomycosis is not known yet. Large clinical trials are needed to assess the sensitivity and specificity of this method in diagnosing fungal infections.
Onychomycosis is a contagious infectious disease characterized by hyphae proliferation in the nail plate. Definitive diagnosis is necessary before treatment because onychomycosis can be mistaken for many infectious or noninfectious skin diseases with nail involvement. Conventional methods are time consuming, laborious, and less reliable. Instead of high-cost procedures, in vivo confocal microscopic examination can be a rapid and reliable diagnostic method for onychomycosis in the near future.
1. Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659-672.
2. Kaur R, Kashyap B, Bhalla P. Onychomycosis—epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108-116.
3. Richardson MD. Diagnosis and pathogenesis of dermatophyte infections. Br J Clin Pract Suppl. 1990;71:98-102.
4. Jensen RH, Arendrup MC. Molecular diagnosis of dermato-phyte infections. Curr Opin Infect Dis. 2012;25:126-134.
5. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
6. Gianni C, Morelli V, Cerri A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283-288.
7. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. 1998;61:217-219.
8. Wilsmann-Theis D, Sareika F, Bieber T, et al. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2011;25:235-237.
9. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
10. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
11. Daniel CR 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
12. Borkowski P, Williams M, Holewinski J, et al. Onychomycosis: an analysis of 50 cases and a comparison of diagnostic techniques. J Am Podiatr Med Assoc. 2001;91:351-355.
13. Rajadhyaksha M, Gonzalez S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293-303.
14. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42(2, pt 1):214-216.
To the Editor:
Onychomycosis is a common nail disease that frequently is caused by dermatophytes and is diagnosed by direct microscopy. Conventional diagnostic methods are often time consuming and can produce false-positive or false-negative results. We report a case of onychomycosis diagnosed by confocal microscopy and confirmed with routine potassium hydroxide (KOH) examination and fungal culture. Confocal microscopy is a reliable, practical, and noninvasive technique in the diagnosis of onychomycosis.
A 46-year-old woman presented with yellow-brown discoloration and dystrophy of the toenails (Figure 1) that had become worse over a 5-year period. She was otherwise healthy and had no other dermatologic problems. Examination revealed yellow-brown discoloration, subungual hyperkeratosis, and onycholysis of the toenails. Clinically, a diagnosis of onychomycosis was made. Potassium hydroxide examination of a scraping from the subungual region showed fungal elements. Trichophyton rubrum on Sabouraud dextrose agar was determined.
|
We performed both in vivo and in vitro confocal laser scanning microscopic examination of the nail of the right great toe (Figure 2). For the diagnosis of onychomycosis in our case, we used a multilaser reflectance confocal microscope (RCM) with a wavelength of 786 nm. In vivo confocal microscopy of the nail revealed branching hyphae just below the surface of the nail plate. Hyphae were seen as refractile, bright, linear structures along the laminates of the nail.
Onychomycosis is a common condition affecting 5.5% of the population worldwide and representing 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.1,2 There are many methods available to confirm the clinical diagnosis of onychomycosis by detecting the causative organisms. Direct microscopic examination of the scraping with a KOH culture, histopathologic assessment with periodic acid–Schiff staining, immunofluorescence analysis with calcofluor white staining, enzyme analysis, and polymerase chain reaction can be used for diagnosis of fungal infections. The most frequently used diagnostic method for onychomycosis is KOH examination of the scraping; however, fungal culture and histopathologic examination also can be used in cases having diagnostic difficulties.1,3,4 There are many studies comparing the efficacies of these methods in the literature.5-9
The causative fungal agent should be determined with at least 1 laboratory method due to the high cost, long duration, and serious potential adverse effects of systemic antifungal treatment. Direct microscopic examination with KOH in the diagnosis of onychomycosis is simple, fast, and inexpensive. However, inadequate material, using crystallized KOH for hydrolysis, insufficient or too much hydrolysis of scrapings, inappropriate staining, and not scanning all areas in the microscopy produce false-negative results. Similarly, secondary contamination of hair, cotton, yarn, or air bubbles mimicking fungal structures can cause false-positive results.9,10
Fungal culture is another diagnostic method that is accepted as the gold standard for diagnosis of onychomycosis.9 However, fungal cultures were positive in only 43% to 50% of all cases of onychomycosis that were diagnosed with other methods,11,12 which may be due to the loss of viability and ability of the fungi to grow in culture media during the transport. A major advantage of fungal culture is that the fungal agent can be classified as dermatophyte, nondermatophyte, mold, or yeast. However, culture does determine if the growing fungi is contamination or the real pathogen. Moreover, it is necessary to wait 3 to 4 weeks for culture results. For nondermatophyte fungi this time may be much longer.12
In vivo RCM is a noninvasive imaging method that allows optical en face sectioning of the living tissue with high resolution. Currently, RCM has a wide range of applications, such as the evaluation of both benign and malignant skin lesions in clinical dermatology.13
In vivo RCM was used first by Hongcharu et al.14 The diagnoses of onychomycosis and fungal hyphae were shown both in vivo and in vitro.14 The sensitivity and specificity of confocal examination in the diagnosis of onychomycosis is not known yet. Large clinical trials are needed to assess the sensitivity and specificity of this method in diagnosing fungal infections.
Onychomycosis is a contagious infectious disease characterized by hyphae proliferation in the nail plate. Definitive diagnosis is necessary before treatment because onychomycosis can be mistaken for many infectious or noninfectious skin diseases with nail involvement. Conventional methods are time consuming, laborious, and less reliable. Instead of high-cost procedures, in vivo confocal microscopic examination can be a rapid and reliable diagnostic method for onychomycosis in the near future.
To the Editor:
Onychomycosis is a common nail disease that frequently is caused by dermatophytes and is diagnosed by direct microscopy. Conventional diagnostic methods are often time consuming and can produce false-positive or false-negative results. We report a case of onychomycosis diagnosed by confocal microscopy and confirmed with routine potassium hydroxide (KOH) examination and fungal culture. Confocal microscopy is a reliable, practical, and noninvasive technique in the diagnosis of onychomycosis.
A 46-year-old woman presented with yellow-brown discoloration and dystrophy of the toenails (Figure 1) that had become worse over a 5-year period. She was otherwise healthy and had no other dermatologic problems. Examination revealed yellow-brown discoloration, subungual hyperkeratosis, and onycholysis of the toenails. Clinically, a diagnosis of onychomycosis was made. Potassium hydroxide examination of a scraping from the subungual region showed fungal elements. Trichophyton rubrum on Sabouraud dextrose agar was determined.
|
We performed both in vivo and in vitro confocal laser scanning microscopic examination of the nail of the right great toe (Figure 2). For the diagnosis of onychomycosis in our case, we used a multilaser reflectance confocal microscope (RCM) with a wavelength of 786 nm. In vivo confocal microscopy of the nail revealed branching hyphae just below the surface of the nail plate. Hyphae were seen as refractile, bright, linear structures along the laminates of the nail.
Onychomycosis is a common condition affecting 5.5% of the population worldwide and representing 20% to 40% of all onychopathies and approximately 30% of cutaneous mycotic infections.1,2 There are many methods available to confirm the clinical diagnosis of onychomycosis by detecting the causative organisms. Direct microscopic examination of the scraping with a KOH culture, histopathologic assessment with periodic acid–Schiff staining, immunofluorescence analysis with calcofluor white staining, enzyme analysis, and polymerase chain reaction can be used for diagnosis of fungal infections. The most frequently used diagnostic method for onychomycosis is KOH examination of the scraping; however, fungal culture and histopathologic examination also can be used in cases having diagnostic difficulties.1,3,4 There are many studies comparing the efficacies of these methods in the literature.5-9
The causative fungal agent should be determined with at least 1 laboratory method due to the high cost, long duration, and serious potential adverse effects of systemic antifungal treatment. Direct microscopic examination with KOH in the diagnosis of onychomycosis is simple, fast, and inexpensive. However, inadequate material, using crystallized KOH for hydrolysis, insufficient or too much hydrolysis of scrapings, inappropriate staining, and not scanning all areas in the microscopy produce false-negative results. Similarly, secondary contamination of hair, cotton, yarn, or air bubbles mimicking fungal structures can cause false-positive results.9,10
Fungal culture is another diagnostic method that is accepted as the gold standard for diagnosis of onychomycosis.9 However, fungal cultures were positive in only 43% to 50% of all cases of onychomycosis that were diagnosed with other methods,11,12 which may be due to the loss of viability and ability of the fungi to grow in culture media during the transport. A major advantage of fungal culture is that the fungal agent can be classified as dermatophyte, nondermatophyte, mold, or yeast. However, culture does determine if the growing fungi is contamination or the real pathogen. Moreover, it is necessary to wait 3 to 4 weeks for culture results. For nondermatophyte fungi this time may be much longer.12
In vivo RCM is a noninvasive imaging method that allows optical en face sectioning of the living tissue with high resolution. Currently, RCM has a wide range of applications, such as the evaluation of both benign and malignant skin lesions in clinical dermatology.13
In vivo RCM was used first by Hongcharu et al.14 The diagnoses of onychomycosis and fungal hyphae were shown both in vivo and in vitro.14 The sensitivity and specificity of confocal examination in the diagnosis of onychomycosis is not known yet. Large clinical trials are needed to assess the sensitivity and specificity of this method in diagnosing fungal infections.
Onychomycosis is a contagious infectious disease characterized by hyphae proliferation in the nail plate. Definitive diagnosis is necessary before treatment because onychomycosis can be mistaken for many infectious or noninfectious skin diseases with nail involvement. Conventional methods are time consuming, laborious, and less reliable. Instead of high-cost procedures, in vivo confocal microscopic examination can be a rapid and reliable diagnostic method for onychomycosis in the near future.
1. Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659-672.
2. Kaur R, Kashyap B, Bhalla P. Onychomycosis—epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108-116.
3. Richardson MD. Diagnosis and pathogenesis of dermatophyte infections. Br J Clin Pract Suppl. 1990;71:98-102.
4. Jensen RH, Arendrup MC. Molecular diagnosis of dermato-phyte infections. Curr Opin Infect Dis. 2012;25:126-134.
5. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
6. Gianni C, Morelli V, Cerri A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283-288.
7. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. 1998;61:217-219.
8. Wilsmann-Theis D, Sareika F, Bieber T, et al. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2011;25:235-237.
9. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
10. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
11. Daniel CR 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
12. Borkowski P, Williams M, Holewinski J, et al. Onychomycosis: an analysis of 50 cases and a comparison of diagnostic techniques. J Am Podiatr Med Assoc. 2001;91:351-355.
13. Rajadhyaksha M, Gonzalez S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293-303.
14. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42(2, pt 1):214-216.
1. Singal A, Khanna D. Onychomycosis: diagnosis and management. Indian J Dermatol Venereol Leprol. 2011;77:659-672.
2. Kaur R, Kashyap B, Bhalla P. Onychomycosis—epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108-116.
3. Richardson MD. Diagnosis and pathogenesis of dermatophyte infections. Br J Clin Pract Suppl. 1990;71:98-102.
4. Jensen RH, Arendrup MC. Molecular diagnosis of dermato-phyte infections. Curr Opin Infect Dis. 2012;25:126-134.
5. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
6. Gianni C, Morelli V, Cerri A, et al. Usefulness of histological examination for the diagnosis of onychomycosis. Dermatology. 2001;202:283-288.
7. Machler BC, Kirsner RS, Elgart GW. Routine histologic examination for the diagnosis of onychomycosis: an evaluation of sensitivity and specificity. Cutis. 1998;61:217-219.
8. Wilsmann-Theis D, Sareika F, Bieber T, et al. New reasons for histopathological nail-clipping examination in the diagnosis of onychomycosis. J Eur Acad Dermatol Venereol. 2011;25:235-237.
9. Reisberger EM, Abels C, Landthaler M, et al. Histopathological diagnosis of onychomycosis by periodic acid-Schiff-stained nail clippings. Br J Dermatol. 2003;148:749-754.
10. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
11. Daniel CR 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
12. Borkowski P, Williams M, Holewinski J, et al. Onychomycosis: an analysis of 50 cases and a comparison of diagnostic techniques. J Am Podiatr Med Assoc. 2001;91:351-355.
13. Rajadhyaksha M, Gonzalez S, Zavislan JM, et al. In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology. J Invest Dermatol. 1999;113:293-303.
14. Hongcharu W, Dwyer P, Gonzalez S, et al. Confirmation of onychomycosis by in vivo confocal microscopy. J Am Acad Dermatol. 2000;42(2, pt 1):214-216.