User login
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
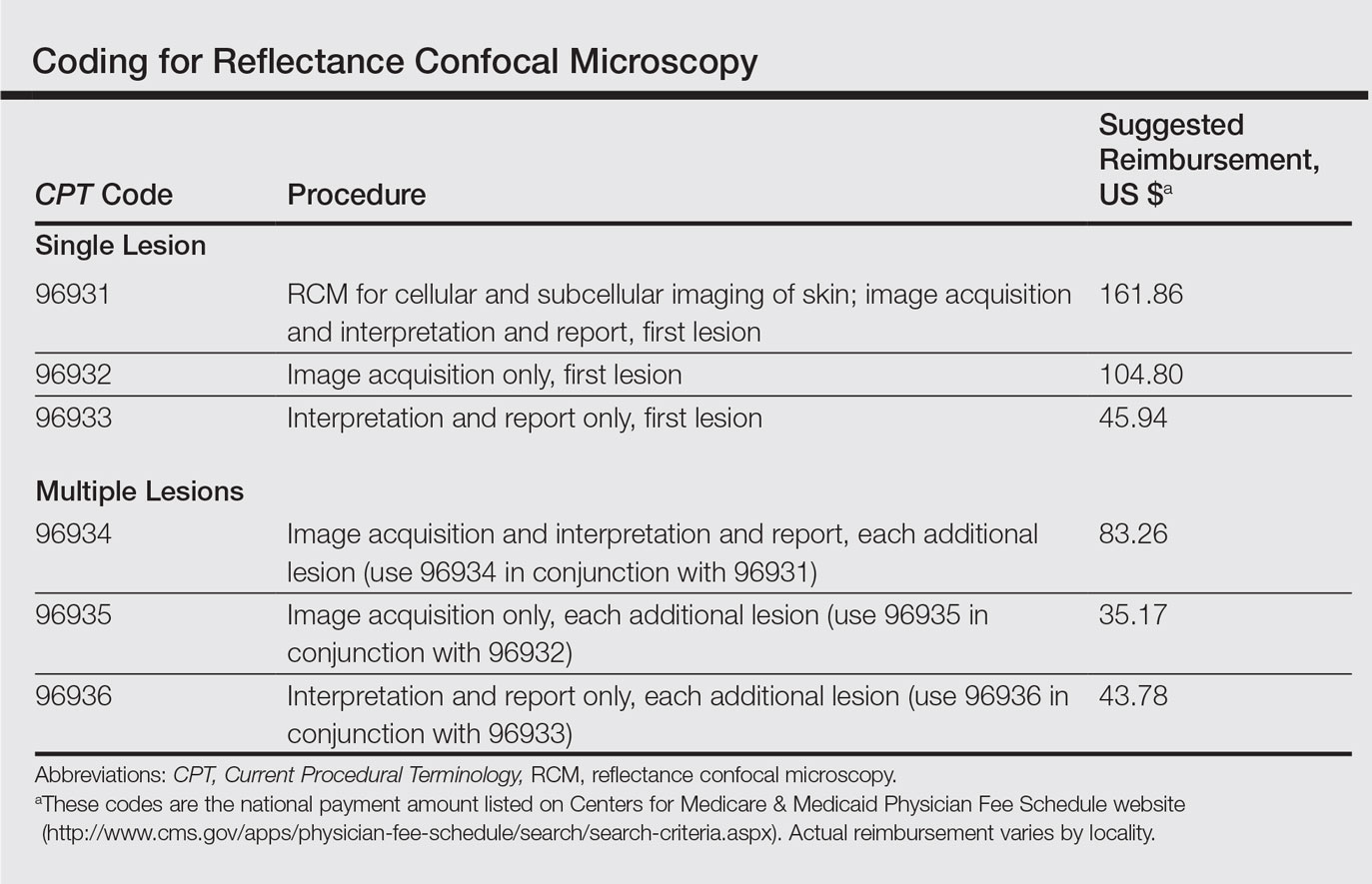
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
Reflectance confocal microscopy (RCM) imaging received Category I Current Procedural Terminology (CPT) codes by the Centers for Medicare & Medicaid Services in January 2016 and can now be submitted to insurance companies with reimbursement comparable to a skin biopsy or a global skin pathology service.1 This fairly new technology is a US Food and Drug Administration–cleared noninvasive imaging modality that provides high-resolution in vivo cellular images of the skin. It has been shown to be efficacious in differentiating benign and malignant skin lesions, increasing diagnostic accuracy, and reducing the number of unnecessary skin biopsies that are performed. In addition to skin cancer diagnosis, RCM imaging also can help guide management of malignant lesions by detecting lateral margins prior to surgery as well as monitoring the lesion over time for treatment efficacy or recurrence. The potential impact of RCM imaging is tremendous, and reimbursement may lead to increased use in clinical practice to the benefit of our patients. Herein, we present a brief review of RCM imaging and reimbursement as well as the benefits and limitations of this new technology for dermatologists.
Reflectance Confocal Microscopy
In vivo RCM allows us to visualize the epidermis in real time on a cellular level down to the papillary dermis at a high resolution (×30) comparable to histologic examination. With optical sections 3- to 5-µm thick and a lateral resolution of 0.5 to 1.0 µm, RCM produces a stack of 500×500-µm2 images up to a depth of approximately 200 µm.2,3 At any chosen depth, these smaller images are stitched together with sophisticated software into a block, or mosaic, increasing the field of view to up to 8×8 mm2. Imaging is performed in en face planes oriented parallel to the skin surface, similar to dermoscopy.
Current CPT Guidelines and Reimbursement
The CPT codes for RCM imaging provide reimbursement on a per-lesion basis and are similar to those used for skin biopsy and pathology (Table).1 Codes 96931 through 96933 are used for imaging of a single lesion on a patient. The first code—96931—is used when image acquisition, interpretation, and report creation are carried out by a single clinician. The next 2 codes are used when one clinician acquires the image—96932—comparable to the technical component of a pathology code, while another reads it and creates the report—96933—similar to a dermatopathologist billing for the professional component of a pathology report. For patients presenting with multiple lesions, the next 3 codes—96934, 96935, and 96936—are used in conjunction with the applicable first code for each additional lesion with similar global, technical, and professional components. Because these codes are not in the radiology or pathology sections of CPT, a single code cannot be used with modifier -TC (technical component) and modifier -26, as they are in those sections.
The wide-probe VivaScope 1500 (Caliber I.D., Inc) currently is the only confocal device that can be reported with a CPT code and routinely reimbursed. The handheld VivaScope 3000 (Caliber I.D., Inc) can only view a small stack and does not have the ability to acquire a full mosaic image; it is not covered by these codes.
Images can be viewed as a stack captured at the same horizontal position but at sequential depths or as a mosaic, which has a larger field of view but is limited to a single plane. To appropriately assess a lesion, clinicians must obtain a mosaic that needs to be assessed at multiple layers for a diagnosis to be made because it is a cross-section view.
Diagnosis
Studies have demonstrated the usefulness of RCM imaging in the diagnosis of a wide range of skin diseases, including melanoma and nonmelanoma skin cancers, infectious diseases, and inflammatory and autoimmune conditions, as well as wound healing and skin aging. Reflectance confocal microscopy imaging is not limited to the skin; it can be used to evaluate the hair, nails, oral mucosa, and other organs.
According to several studies, RCM imaging notably increases the diagnostic accuracy and detection rate of skin cancers over clinical and dermoscopic examination alone and therefore can act as an aid in differentiating lesions that are benign versus those that are suspicious and should be biopsied.
Reflectance confocal microscopy has been shown to have a mean sensitivity of 94% (range, 92%–96%) and specificity of 83% (range, 81%–84%) for all types of skin cancer when used with dermoscopy.4 In particular, for melanocytic lesions that are ambiguous on dermoscopy, RCM used in addition to dermoscopy increases the mean sensitivity and specificity for melanoma diagnosis to 93% (range, 89%–96%) and 76% (range, 68%–83%), respectively.5 Although these reported sensitivities are comparable to dermoscopy, the specificity is superior, especially for detecting hypomelanotic and amelanotic melanomas, which often lack specific features on dermoscopy.6-8
The combination of RCM with dermoscopy has reduced the number of unnecessary excisions of benign nevi by more than 50% when compared to dermoscopy alone.9 One study showed that the number needed to treat (ie, excise) a melanoma decreased from 14.6 with dermoscopy alone to 6.8 when guided by dermoscopy and RCM imaging.9 In a similar study, the number needed to treat dropped from 19.41 with dermoscopy alone to 6.25 with dermoscopy and RCM.10
These studies were not looking to evaluate RCM as a replacement test but rather as an add-on test to dermoscopy. Reflectance confocal microscopy imaging takes longer than dermoscopy for each lesion; therefore, RCM should only be used as an adjunctive tool to dermoscopy and not as an initial screening test. Consequentially, a dermatologist skilled in dermoscopy is essential in deciding which lesions would be appropriate for subsequent RCM imaging.
In Vivo Margin Mapping as an Adjunct to Surgery
Oftentimes, tumor margins are poorly defined and can be difficult to map clinically and dermoscopically. Studies have demonstrated the use of RCM in delineation of surgical margins prior to surgery or excisional biopsies.11,12 Alternatively, when complete removal at biopsy would be impractical (eg, for extremely large lesions or lesions located in cosmetically sensitive areas such as the face), RCM can be used to pick the best site for an appropriate biopsy, which decreases the chance of sampling error due to skip lesions and increases histologic accuracy.
Nonsurgical Treatment Monitoring
One advantage of RCM over conventional histology is that RCM imaging leaves the tissue intact, allowing dynamic changes to be studied over time, which is useful for monitoring nonmelanoma skin cancers and lentigo maligna being treated with noninvasive therapeutic modalities.13 If not as a definitive treatment, RCM can act as an adjunct for surgery by monitoring reduction in lesion size prior to Mohs micrographic surgery, thereby decreasing the resulting surgical defect.14
Limitations
Imaging Depth
Although RCM is a revolutionary device in the field of dermatology, it has several limitations. With a maximal imaging depth of 350 µm, the imaging resolution decreases substantially with depth, limiting accurate interpretation to 200 µm. Reflectance confocal microscopy can only image the superficial portion of a lesion; therefore, deep tumor margins cannot be assessed. Hypertrophic or hyperkeratotic lesions, including lesions on the palms and soles, also are unable to be imaged with RCM. This limitation in depth penetration makes treatment monitoring impossible for invasive lesions that extend into the dermal layer.
Difficult-to-Reach Areas
Another limitation is the difficulty imaging areas such as the ocular canthi, nasal alae, or helices of the ear due to the wide probe size on the VivaScope 1500. The advent of the smaller handheld VivaScope 3000 device allows for improved imaging of concave services and difficult lesions at the risk of less accurate imaging, low field of view, and no reimbursement at present.
False-Positive Results
Although RCM has been shown to be helpful in reducing unnecessary biopsies, there still is the issue of false-positives on imaging. False-positives most commonly occur in nevi with severe atypia or when Langerhans cells are present that cannot always be differentiated from melanocytic cells.3,15,16 One prospective study found 7 false-positive results from 63 sites using RCM for the diagnosis of lentigo malignas.16 False-negatives can occur in the presence of inflammatory infiltrates and scar tissue that can hide cellular morphology or in sampling errors due to skip lesions.3,16
Time Efficiency
The time required for acquisition of RCM mosaics and stacks followed by reading and interpretation can be substantial depending on the size and complexity of the lesion, which is a major limitation for use of RCM in busy dermatology practices; therefore, RCM should be reserved for lesions selected to undergo biopsy that are clinically equivocal for malignancy prior to RCM examination.17 It would not be cost-effective or time effective to evaluate lesions that either clinically or dermoscopically have a high probability of malignancy; however, patients and physicians may opt for increased specificity at the expense of time, particularly when a lesion is located on a cosmetically sensitive area, as patients can avoid initial histologic biopsy and gain the cosmetic benefit of going straight to surgery versus obtaining an initial diagnostic biopsy.
Cost
Lastly, the high cost involved in purchasing an RCM device and the training involved to use and interpret RCM images currently limits RCM to large academic centers. Reimbursement may make more widespread use feasible. In any event, RCM imaging should be part of the curriculum for both dermatology and pathology trainees.
Future Directions
In vivo RCM is a noninvasive imaging modality that allows for real-time evaluation of the skin. Used in conjunction with dermoscopy, RCM can substantially improve diagnostic accuracy and reduce the number of unnecessary biopsies. Now that RCM has finally gained foundational CPT codes and insurance reimbursement, there may be a growing demand for clinicians to incorporate this technology into their clinical practice.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Que SK, Fraga-Braghiroli N, Grant-Kels JM, et al. Through the looking glass: basics and principles of reflectance confocal microscopy [published online June 4, 2015]. J Am Acad Dermatol. 2015;73:276-284.
- Rajadhyaksha M, Marghoob A, Rossi A, et al. Reflectance confocal microscopy of skin in vivo: from bench to bedside [published online October 27, 2016]. Lasers Surg Med. 2017;49:7-19.
- Xiong YD, Ma S, Li X, et al. A meta-analysis of reflectance confocal microscopy for the diagnosis of malignant skin tumours. J Eur Acad Dermatol Venereol. 2016;30:1295-1302.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Busam KJ, Hester K, Charles C, et al. Detection of clinically amelanotic malignant melanoma and assessment of its margins by in vivo confocal scanning laser microscopy. Arch Dermatol. 2001;137:923-929.
- Losi A, Longo C, Cesinaro AM, et al. Hyporeflective pagetoid cells: a new clue for amelanotic melanoma diagnosis by reflectance confocal microscopy. Br J Dermatol. 2014;171:48-54.
- Guitera P, Menzies SQ, Argenziano G, et al. Dermoscopy and in vivo confocal microscopy are complementary techniques for the diagnosis of difficult amelanotic and light-coloured skin lesions [published online October 12, 2016]. Br J Dermatol. 2016;175:1311-1319.
- Pellacani G, Pepe P, Casari A, et al. Reflectance confocal microscopy as a second-level examination in skin oncology improves diagnostic accuracy and saves unnecessary excisions: a longitudinal prospective study. Br J Dermatol. 2014;171:1044-1051.
- Pellacani G, Witkowski A, Cesinaro AM, et al. Cost-benefit of reflectance confocal microscopy in the diagnostic performance of melanoma. J Eur Acad Dermatol Venereol. 2016;30:413-419.
- Champin J, Perrot JL, Cinotti E, et al. In vivo reflectance confocal microscopy to optimize the spaghetti technique for defining surgical margins of lentigo maligna. Dermatol Surg. 2014;40:247-256.
- Hibler BP, Cordova M, Wong RJ, et al. Intraoperative real-time reflectance confocal microscopy for guiding surgical margins of lentigo maligna melanoma. Dermatol Surg. 2015;41:980-983.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:202a10.
- Torres A, Niemeyer A, Berkes B, et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg. 2004;30(12, pt 1):1462-1469.
- Hashemi P, Pulitzer MP, Scope A, et al. Langerhans cells and melanocytes share similar morphologic features under in vivo reflectance confocal microscopy: a challenge for melanoma diagnosis. J Am Acad Dermatol. 2012;66:452-462.
- Menge TD, Hibler BP, Cordova MA, et al. Concordance of handheld reflectance confocal microscopy (RCM) with histopathology in the diagnosis of lentigo maligna (LM): a prospective study. J Am Acad Dermatol. 2016;74:1114-1120.
- Borsari S, Pampena R, Lallas A, et al. Clinical indications for use of reflectance confocal microscopy for skin cancer diagnosis. JAMA Dermatol. 2016;152:1093-1098.
Practice Points
- Reflectance confocal microscopy (RCM) recently received Category I Current Procedural Terminology codes for reimbursement comparable to a skin biopsy.
- When used in combination with dermoscopy, RCM has been shown to increase diagnostic accuracy of skin cancer.
- Reflectance confocal microscopy also is useful in surgical treatment planning and monitoring nonsurgical treatments over time.
- Limitations of RCM imaging include low imaging depth, difficulty in imaging certain areas of the skin, learning curve for interpreting these images, and the cost of equipment.