User login
Glitter Effects of Nail Art on Optical Coherence Tomography
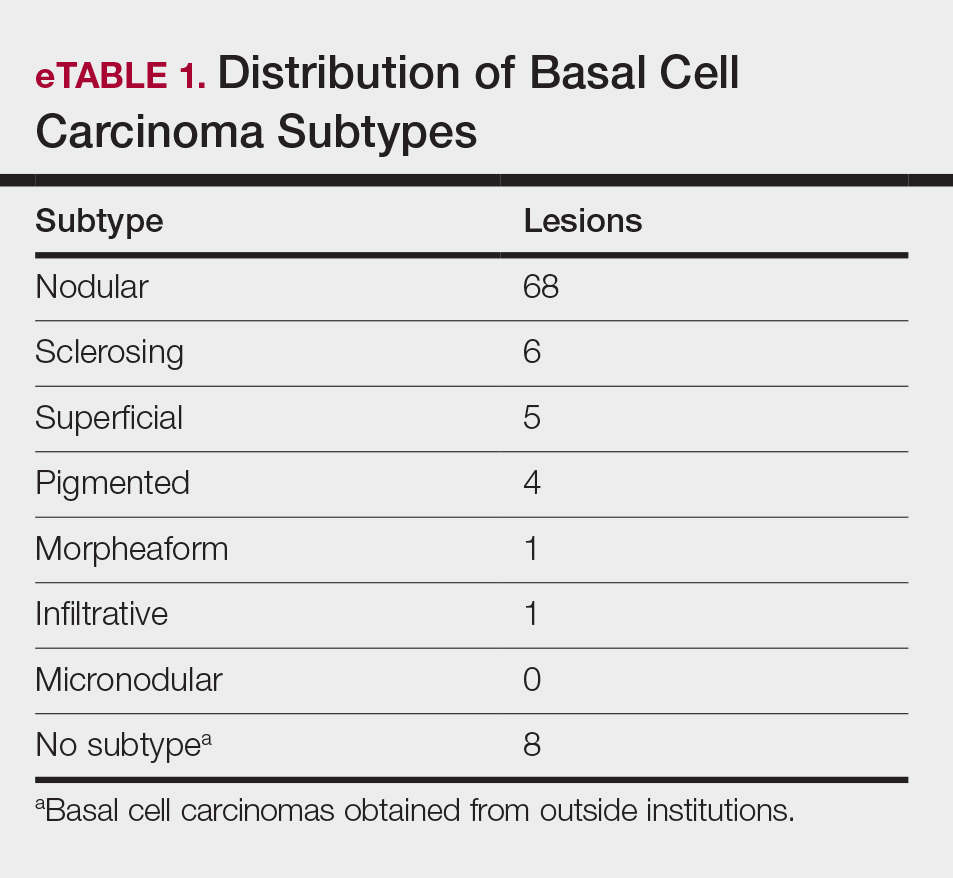
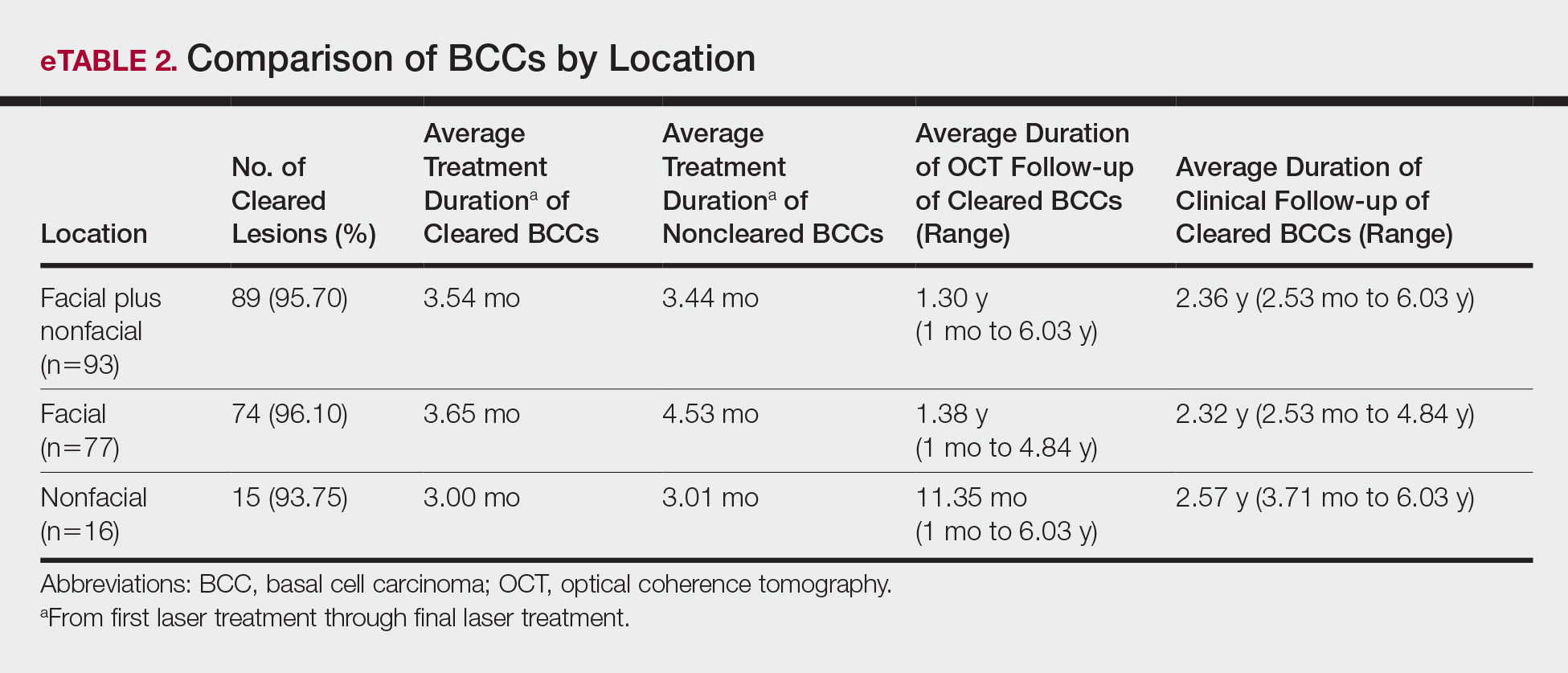
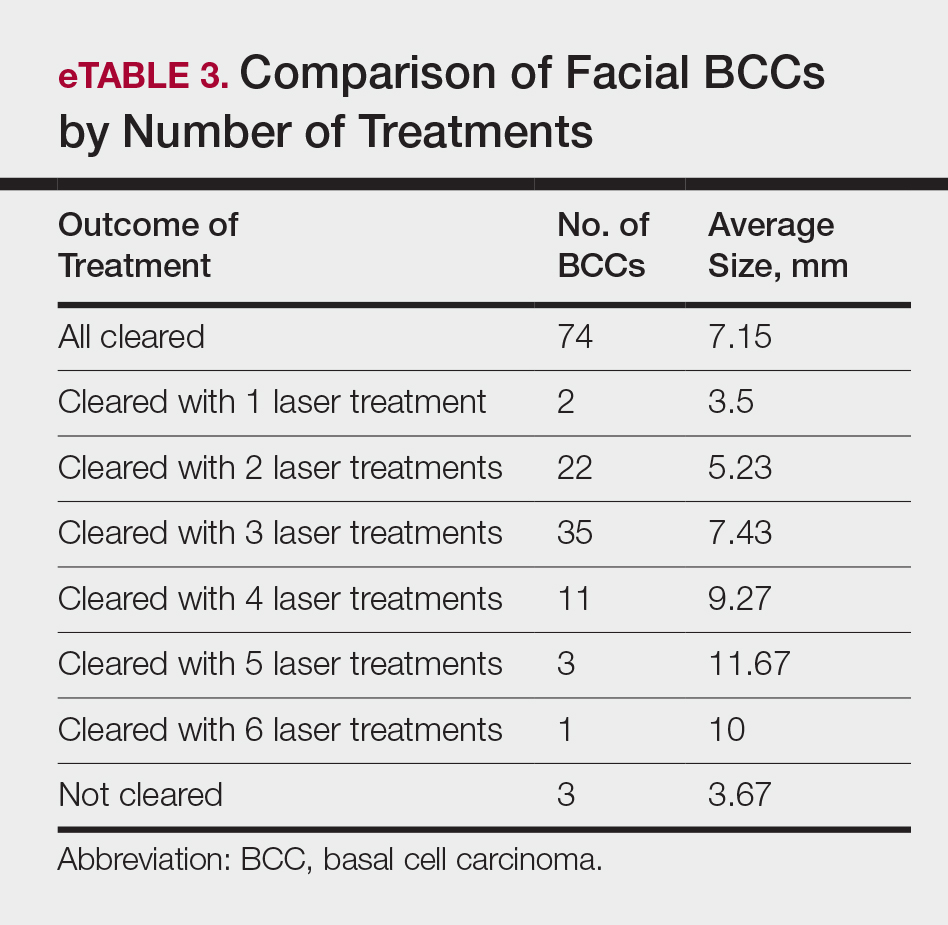
Practice Gap
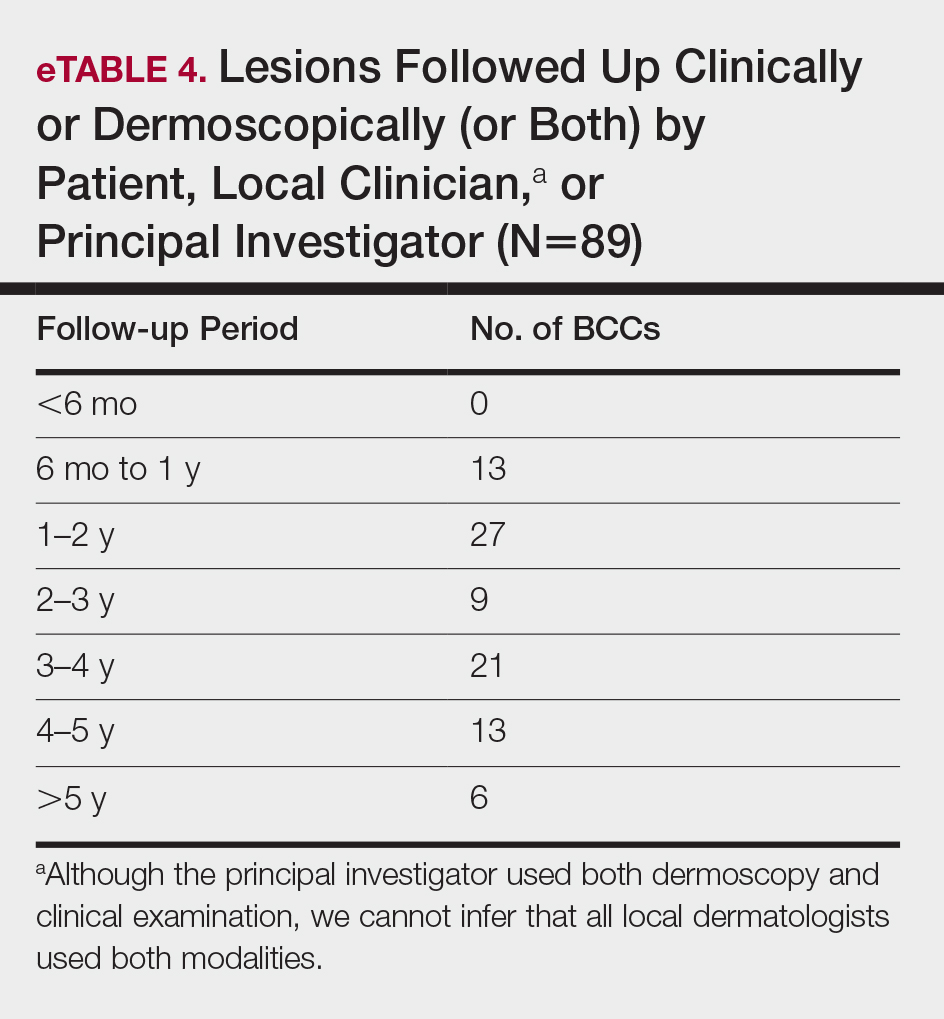
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
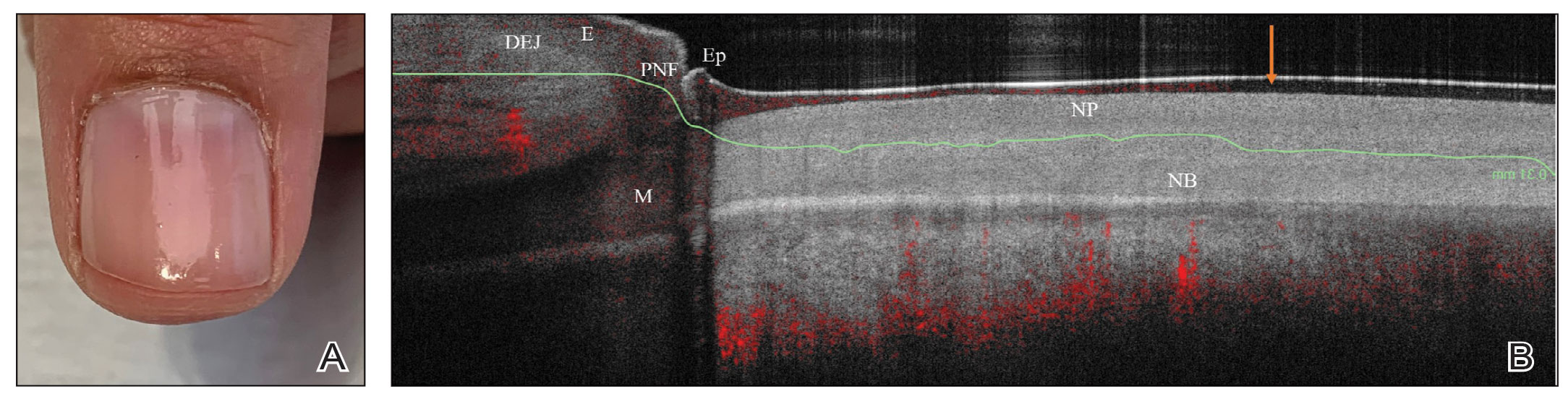
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).
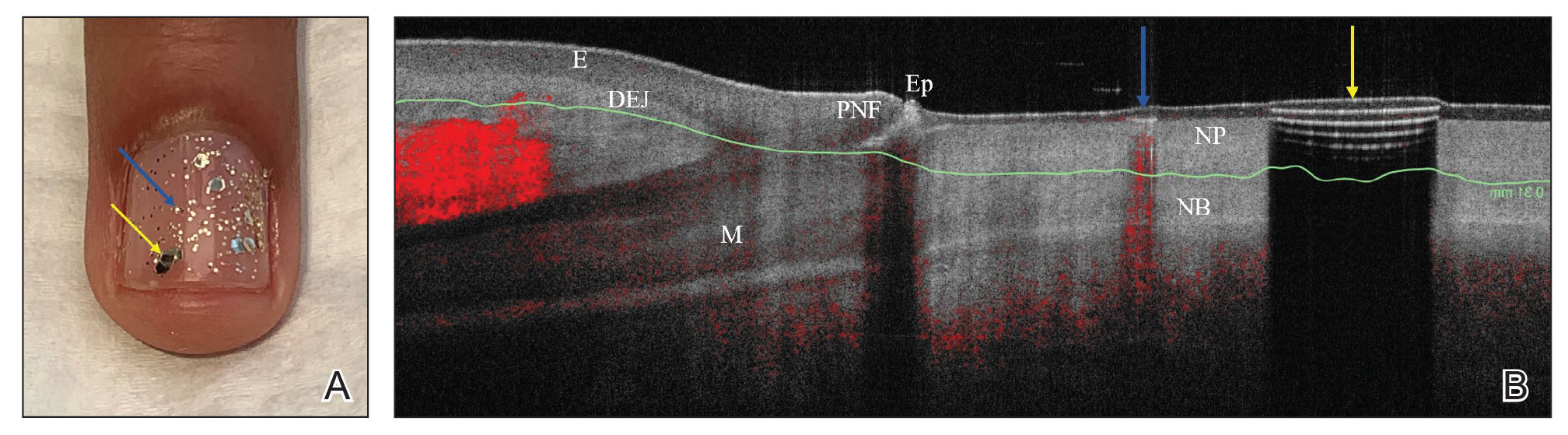
We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.
Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
Practice Gap
Nail art can skew the results of optical coherence tomography (OCT), a noninvasive imaging technology that is used to visualize nail morphology in diseases such as psoriatic arthritis and onychomycosis, with a penetration depth of 2 mm and high-resolution images.1 Few studies have evaluated the effects of nail art on OCT. Saleah and colleagues1 found that clear, semitransparent, and red nail polishes do not interfere with visualization of the nail plate, whereas nontransparent gel polish and art stones obscure the image. They did not comment on the effect of glitter nail art in their study, though they did test 1 nail that contained glitter.1 Monpeurt et al2 compared matte and glossy nail polishes. They found that matte polish was readily identifiable from the nail plate, whereas glossy polish presented a greater number of artifacts.2
The Solution
We looked at 3 glitter nail polishes—gold, pink, and silver—that were scanned by OCT to assess the effect of the polish on the resulting image. We determined that glitter particles completely obscured the nail bed and nail plate, regardless of color (Figure 1). Glossy clear polish imparted a distinct film on the top of the nail plate that did not obscure the nail plate or the nail bed (Figure 2).

We conclude that glitter nail polish contains numerous reflective solid particles that interfere with OCT imaging of the nail plate and nail bed. As a result, we recommend removal of nail art to properly assess nail pathology. Because removal may need to be conducted by a nail technician, the treating clinician should inform the patient ahead of time to come to the appointment with bare (ie, unpolished) nails.

Practice Implications
Bringing awareness to the necessity of removing nail art prior to OCT imaging is crucial because many patients partake in its application, and removal may require the involvement of a professional nail technician. If a patient can be made aware that they should remove all nail art in advance, they will be better prepared for an OCT imaging session. Such a protocol increases efficiency, decreases diagnostic delay, and reduces cost associated with multiple office visits.
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
- Saleah S, Kim P, Seong D, et al. A preliminary study of post-progressive nail-art effects on in vivo nail plate using optical coherence tomography-based intensity profiling assessment. Sci Rep. 2021;11:666. doi:10.1038/s41598-020-79497-3
- Monpeurt C, Cinotti E, Hebert M, et al. Thickness and morphology assessment of nail polishes applied on nails by high-definition optical coherence tomography. Skin Res Technol. 2018;24:156-157. doi:10.1111/srt.12406
Mobile App Usage Among Dermatology Residents in America
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
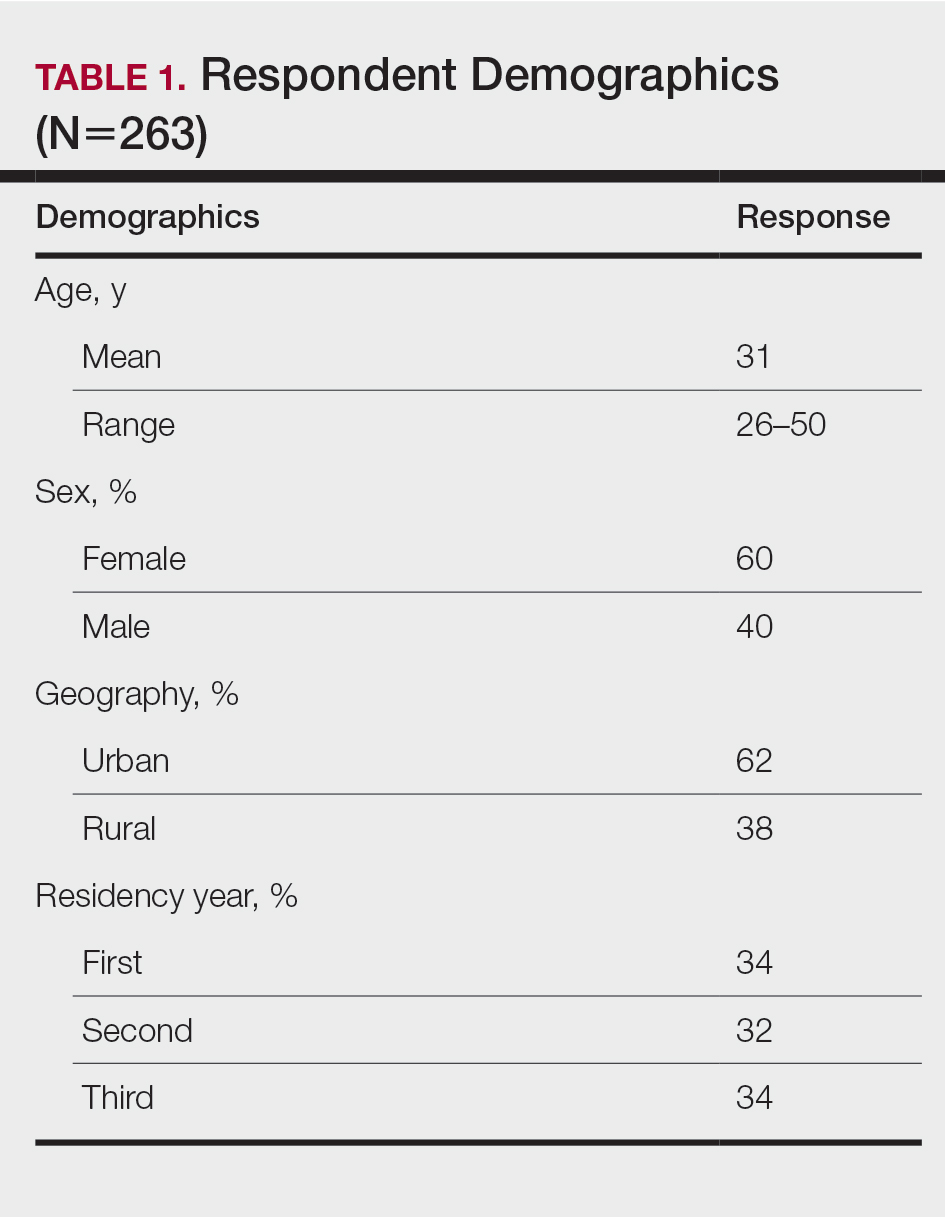
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
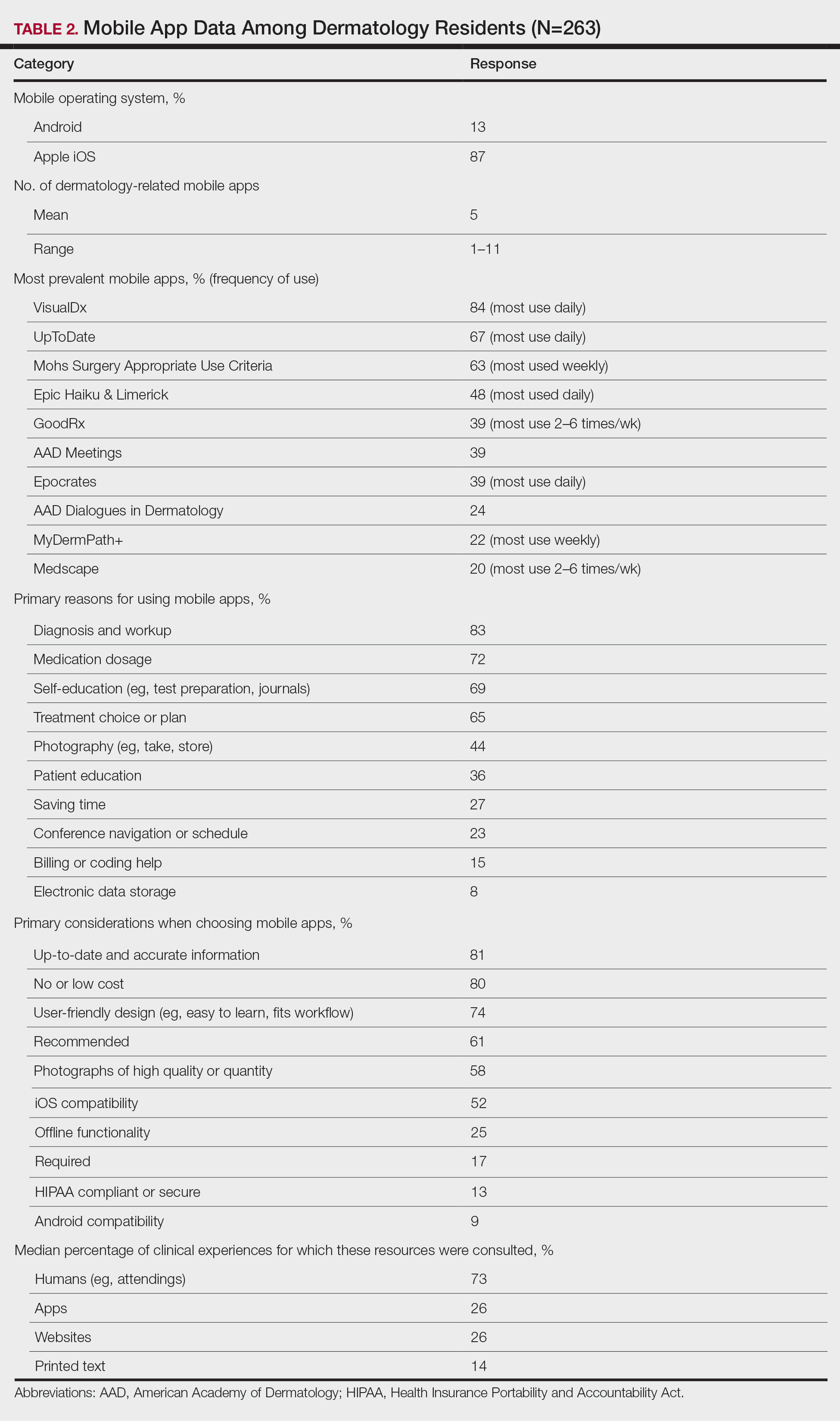
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
Mobile applications (apps) have been a growing part of medicine for the last decade. In 2020, more than 15.5 million apps were available for download,1 and more than 325,000 apps were health related.2 Much of the peer-reviewed literature on health-related apps has focused on apps that target patients. Therefore, we studied apps for health care providers, specifically dermatology residents of different sexes throughout residency. We investigated the role of apps in their training, including how often residents consult apps, which apps they utilize, and why.
Methods
An original online survey regarding mobile apps was emailed to all 1587 dermatology residents in America by the American Academy of Dermatology from summer 2019 to summer 2020. Responses were anonymous, voluntary, unincentivized, and collected over 17 days. To protect respondent privacy, minimal data were collected regarding training programs; geography served as a proxy for how resource rich or resource poor those programs may be. Categorization of urban vs rural was based on the 2010 Census classification, such that Arizona; California; Colorado; Connecticut; Florida; Illinois; Maryland; Massachusetts; New Jersey; New York; Oregon; Puerto Rico; Rhode Island; Texas; Utah; and Washington, DC, were urban, and the remaining states were rural.3
We hypothesized that VisualDx would be 1 of 3 most prevalent apps; “diagnosis and workup” and “self-education” would be top reasons for using apps; “up-to-date and accurate information” would be a top 3 consideration when choosing apps; the most consulted resources for clinical experiences would be providers, followed by websites, apps, and lastly printed text; and the percentage of clinical experiences for which a provider was consulted would be higher for first-year residents than other years and for female residents than male residents.
Fisher exact 2-tailed and Kruskal-Wallis (KW) pairwise tests were used to compare groups. Statistical significance was set at P<.05.
Results
Respondents
The response rate was 16.6% (n=263), which is similar to prior response rates for American Academy of Dermatology surveys. Table 1 contains respondent demographics. The mean age of respondents was 31 years. Sixty percent of respondents were female; 62% of respondents were training in urban states or territories. Regarding the dermatology residency year, 34% of respondents were in their first year, 32% were in their second, and 34% were in their third. Eighty-seven percent of respondents used Apple iOS. Every respondent used at least 1 dermatology-related app (mean, 5; range, 1–11)(Table 2).
Top Dermatology-Related Apps
The 10 most prevalent apps are listed in Table 2. The 3 most prevalent apps were VisualDx (84%, majority of respondents used daily), UpToDate (67%, majority of respondents used daily), and Mohs Surgery Appropriate Use Criteria (63%, majority of respondents used weekly). A higher percentage of third-year residents used GoodRx compared to first- and second-year residents (Fisher exact test: P=.014 and P=.041, respectively). A lower percentage of female respondents used GoodRx compared to male residents (Fisher exact test: P=.003). None of the apps were app versions of printed text, including textbooks or journals.
Reasons for Using Apps
The 10 primary reasons for using apps are listed in Table 2. The top 3 reasons were diagnosis and workup (83%), medication dosage (72%), and self-education (69%). Medication dosage and saving time were both selected by a higher percentage of third-year residents than first-year residents (Fisher exact test: P=.041 and P=.024, respectively). Self-education was selected by a lower percentage of third-year residents than second-year residents (Fisher exact test: P=.025).
Considerations When Choosing Apps
The 10 primary considerations when choosing apps are listed in Table 2. The top 3 considerations were up-to-date and accurate information (81%), no/low cost (80%), and user-friendly design (74%). Up-to-date and accurate information was selected by a lower percentage of third-year residents than first- and second-year residents (Fisher exact test: P=.02 and P=.03, respectively).
Consulted Resources
Apps were the second most consulted resource (26%) during clinical work, behind human guidance (73%). Female respondents consulted both resources more than male respondents (KW: P≤.005 and P≤.003, respectively). First-year residents consulted humans more than second-year and third-year residents (KW: P<.0001).
There were no significant differences by geography or mobile operating system.
Comment
The response rate and demographic results suggest that our study sample is representative of the target population of dermatology residents in America. Overall, the survey results support our hypotheses.
A survey conducted in 2008 before apps were readily available found that dermatology residents felt they learned more successfully when engaging in hands-on, direct experience; talking with experts/consultants; and studying printed materials than when using multimedia programs.4 Our study suggests that the usage of and preference for multimedia programs, including apps, in dermatology resident training has risen substantially, despite the continued availability of guidance from attendings and senior residents.
As residents progress through training, they increasingly turn to virtual resources. According to our survey, junior residents are more likely than third-year residents to use apps for self-education, and up-to-date and accurate information was a more important consideration when choosing apps. Third-year residents are more likely than junior residents to use apps for medication dosage and saving time. Perhaps related, GoodRx, an app that provides prescription discounts, was more prevalent among third-year residents. It is notable that most of the reported apps, including those used for diagnosis and treatment, did not need premarket government approval to ensure patient safety, are not required to contain up-to-date information, and do not reference primary sources. Additionally, only UpToDate has been shown in peer-reviewed literature to improve clinical outcomes.5
Our survey also revealed a few differences by sex. Female respondents consulted resources during clinical work more often than male residents. This finding is similar to the limited existing research on dermatologists’ utilization of information showing higher dermoscopy use among female attendings.6 Use of GoodRx was less prevalent among female vs male respondents. Perhaps related, a 2011 study found that female primary care physicians are less likely to prescribe medications than their male counterparts.7
Our study had several limitations. There may have been selection bias such that the residents who chose to participate were relatively more interested in mobile health. Certain demographic data, such as race, were not captured because prior studies do not suggest disparity by those demographics for mobile health utilization among residents, but those data could be incorporated into future studies. Our survey was intentionally limited in scope. For example, it did not capture the amount of time spent on each consult resource or the motivations for consulting an app instead of a provider.
Conclusion
A main objective of residency is to train new physicians to provide excellent patient care. Our survey highlights the increasing role of apps in dermatology residency, different priorities among years of residency, and different information utilization between sexes. This knowledge should encourage and help guide standardization and quality assurance of virtual residency education and integration of virtual resources into formal curricula. Residency administrators and residents should be aware of the apps used to learn and deliver care, consider the evidence for and regulation of those apps, and evaluate the accessibility and approachability of attendings to residents. Future research should examine the educational and clinical outcomes of app utilization among residents and the impact of residency programs’ unspoken cultures and expectations on relationships among residents of different demographics and their attendings.
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
- Statistica. Number of apps available in leading app stores 2020. Accessed September 21, 2020. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/
- Research2Guidance. mHealth economics 2017—current status and future trends in mobile health. Accessed July 16, 2021. https://research2guidance.com/product/mhealth-economics-2017-current-status-and-future-trends-in-mobile-health/
- United States Census Bureau. 2010 Census Urban and Rural Classification and Urban Area Criteria. Accessed September 21, 2020. https://www.census.gov/programs-surveys/geography/guidance/geo-areas/urban-rural/2010-urban-rural.html
- Stratman EJ, Vogel CA, Reck SJ, et al. Analysis of dermatology resident self-reported successful learning styles and implications for core competency curriculum development. Med Teach. 2008;30:420-425.
- Wolters Kluwer. UpToDate is the only clinical decision support resource associated with improved outcomes. Accessed July 22, 2021. https://www.uptodate.com/home/research
- Engasser HC, Warshaw EM. Dermatoscopy use by US dermatologists: a cross-sectional survey. J Am Acad Dermatol. 2010;63:412-419.
- Smith AW, Borowski LA, Liu B, et al. U.S. primary care physicians’ diet-, physical activity–, and weight-related care of adult patients. Am J Prev Med. 2011;41:33-42. doi:10.1016/j.amepre.2011.03.017
Practice Points
- Virtual resources, including mobile apps, have become critical tools for learning and patient care during dermatology resident training for reasons that should be elucidated.
- Dermatology residents of different years and sexes utilize mobile apps in different amounts and for different purposes.
Acquired Unilateral Nevoid Telangiectasia With Pruritus and Unknown Etiology
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
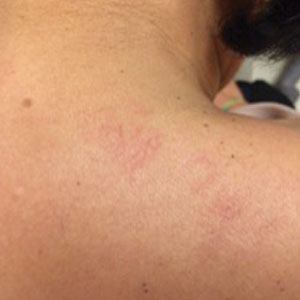
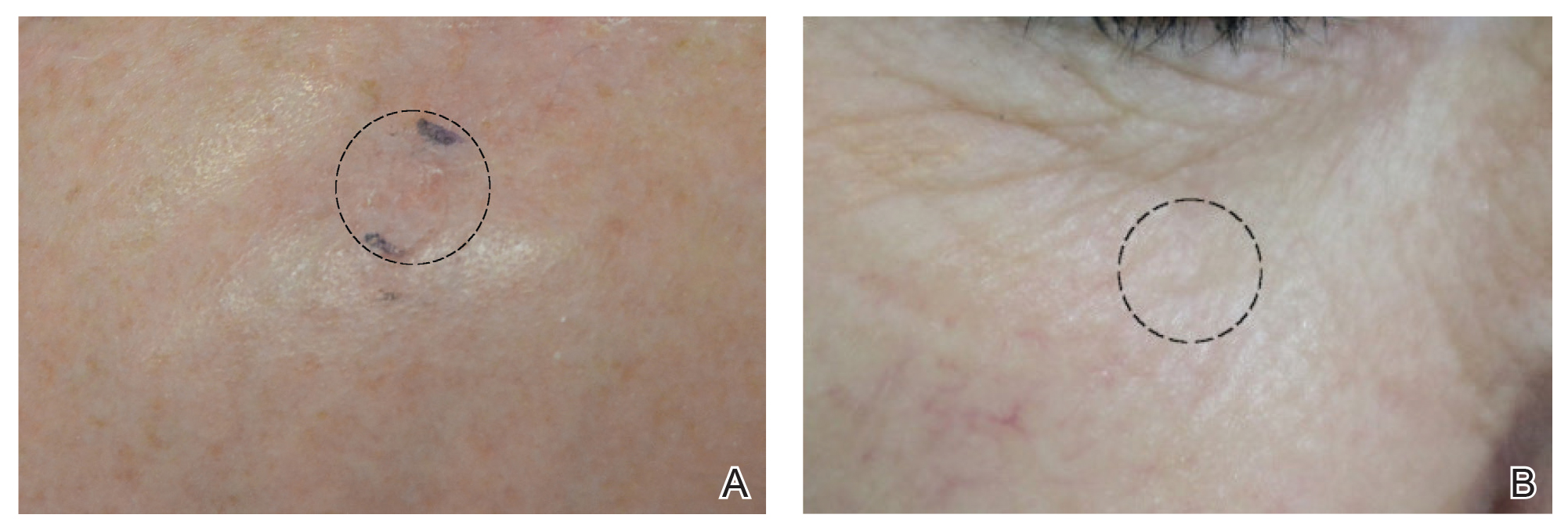
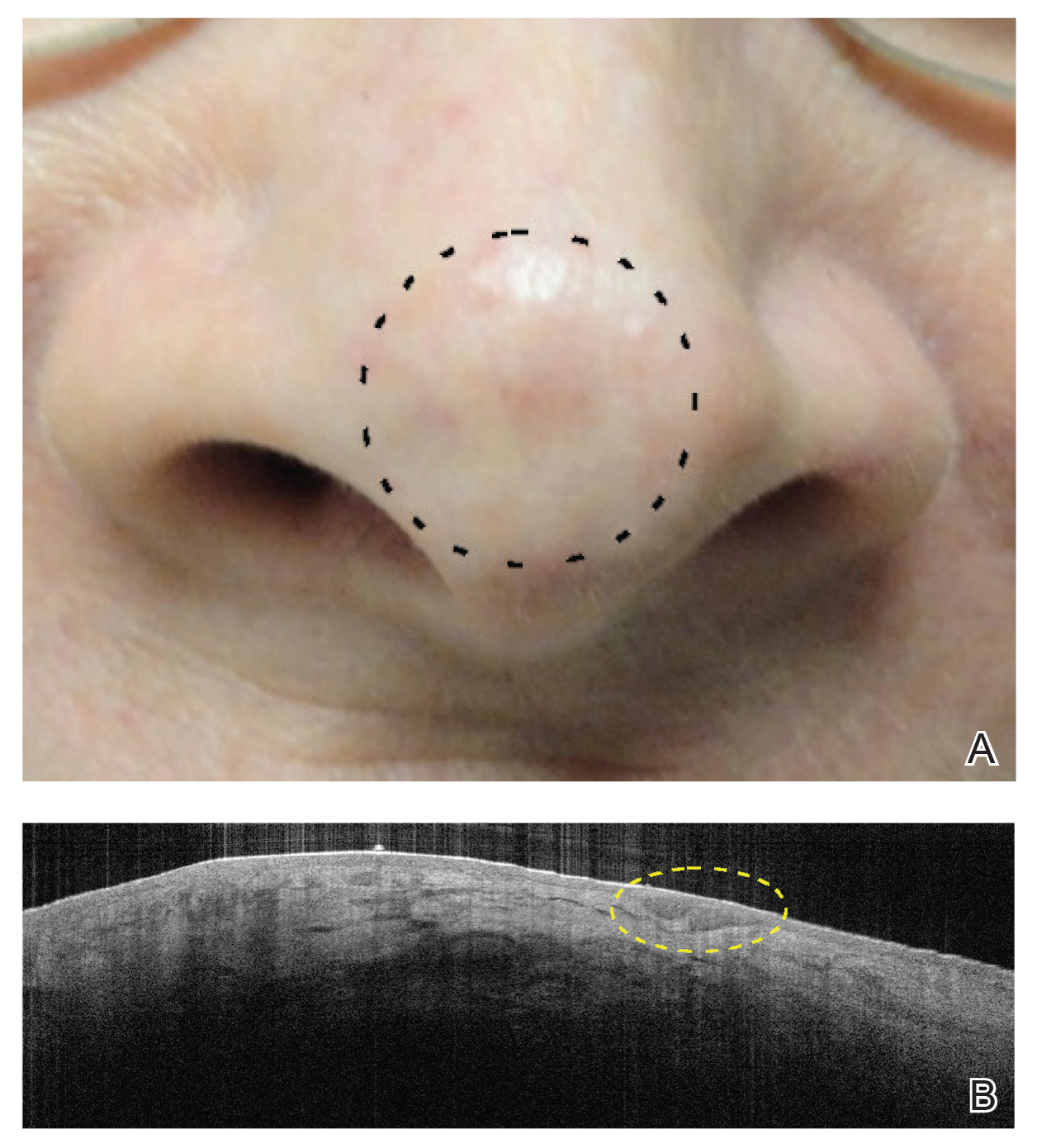
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.
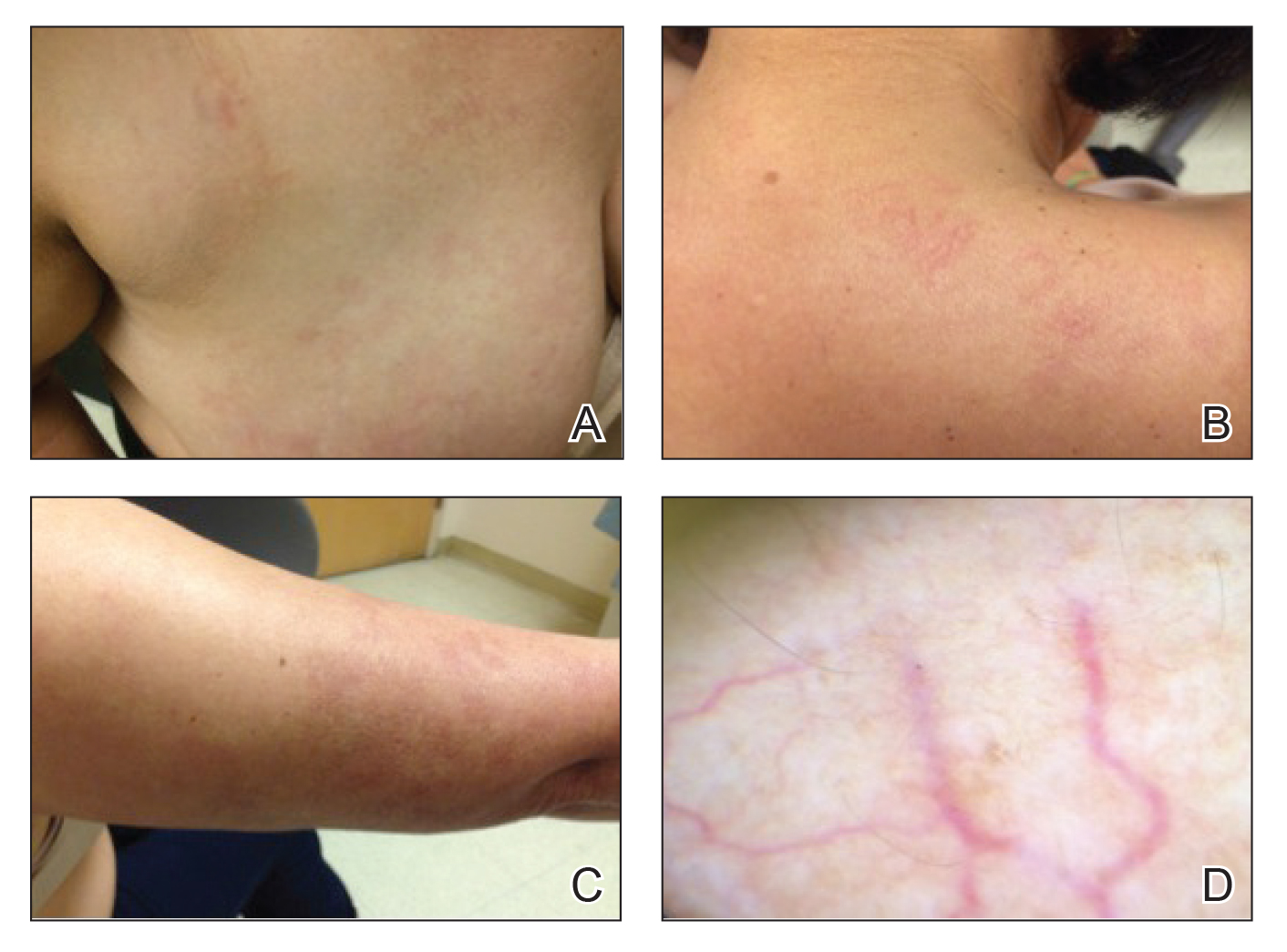
Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.

Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
To the Editor:
Unilateral nevoid telangiectasia (UNT) is a rare cutaneous disease characterized by superficial telangiectases arranged in a unilateral linear pattern. First described by Alfred Blaschko in 1899, this rare disease has been reported in higher frequency in recent years, with approximately 100 cases published in the literature according to a PubMed search of articles indexed for MEDLINE using the term unilateral nevoid telangiectasia.1 Unilateral nevoid telangiectasia can be congenital or acquired; occurs more commonly in women; and typically involves the dermatomal distributions of the trigeminal, cervical, and upper thoracic nerves. Although the pathogenesis of the disease remains unknown, the currently proposed etiology involves hyperestrogenic states, including puberty, pregnancy, and chronic liver disease.2 We report a case of progressively worsening, pruritic, unilateral telangiectases of unknown etiology.
A 55-year-old woman presented to our dermatology clinic with progressive red spots involving the right side of the upper body of 3 years’ duration. She noted pruritus, and the rash was otherwise asymptomatic. Her medical history was notable for hypertension, dyspepsia, sciatica, uterine fibroids, and a hysterectomy. Her medications included lisinopril, hydrochlorothiazide, tramadol, aspirin, and a multivitamin. The patient did not report the use of oral contraceptive pills or hormone replacement therapy. She also denied the use of cigarettes or illicit drugs but reported occasional alcohol consumption. A review of systems was negative for any constitutional symptoms or symptoms of liver disease. Her family history also was noncontributory.
Physical examination revealed multiple, 1- to 3-mm, telangiectatic macules and patches in a blaschkoid distribution on the right side of the upper chest, back, shoulder, and arm (Figure, A–C). Darier sign was negative. There was no evidence of palmar erythema, hepatosplenomegaly, ascites, thyromegaly, or thyroid nodules. Dermoscopy confirmed the presence of telangiectasia (Figure, D). More specifically, dermoscopy revealed plump telangiectasia with faint pigment in the background, consistent with UNT. Additionally, there was no pink-white, shiny, scarlike background, and vessels were not thin or arborized, further supporting our diagnosis vs other entities included in the differential diagnosis.

Laboratory testing for estrogen levels was within normal postmenopausal limits. A complete blood cell count, basic metabolic panel, hepatic panel, and thyroid stimulating hormone levels all were within reference range. Hepatitis B and C virus testing was nonreactive. The diagnosis of UNT was made based on clinical characteristics. The patient then was referred for pulsed dye laser treatment.
Since the first reports of UNT in 1899, it has been described in multiple individually reported cases. The typical description of UNT involves linearly arranged telangiectasia of one side of the body, following either dermatomal or blaschkoid distribution, most commonly along the C3 and C4 dermatome. In 1970, Selmanowitz3 divided the diagnosis into 2 categories: congenital and acquired. The congenital form is less common overall, seen more frequently in males, and occurs in direct relation to the neonatal period.4 The acquired form that is more common overall and seen more frequently in females is suggested to be due to hyperestrogenic states. Most reports of the acquired form involve some underlying pathology that may lead to higher estrogen states. In a review article published in 2011, Wenson et al1 summarized the reported cases to date. The authors found that out of close to 100 cases reported, 26 acquired cases were associated with pregnancy and 23 with puberty. They further found 10 cases associated with hepatic disease, 2 associated with hormonal contraceptive pills, 1 associated with hyperthyroidism, and 1 associated with carcinoid syndrome.1Interestingly, a more varied presentation of disease has been reported, as cases are now being reported in healthy patients with no comorbidities or reasons for hyperestrogenism.5 In fact, presentations in healthy adult men have led some authors to believe that estrogen may not play a major role in the pathogenesis of the disease.5-8 Reports of 16 cases of UNT have indicated no association with hyperestrogenic states.1 Because the etiology remains unknown, individual cases both supporting and refuting the hypothesis of estrogen-driven vessel inflammation may drive the investigation of further explanations.
Because UNT usually is asymptomatic, treatment options are largely based on improvement in appearance of the lesions. The pulsed dye laser (PDL) has shown success in treatment of lesions, as Sharma et al,9 reported resolution of lesions in 9 cases. These cases were not without side effects, as some patients did experience reversible pigmentary changes. Other studies have validated the use of PDL for cosmetic improvement of UNT; however, some studies have noted the recurrence of lesions after treatment.10
Our case provides another unique presentation of UNT. Our patient was a healthy adult woman with no hyperestrogen-based etiology for disease. Importantly, our patient also represented a rare instance of UNT presenting with symptoms such as pruritus, though UNT classically is described as an asymptomatic phenomenon. In our patient, treatment with PDL was suggested and believed to be warranted not only for cosmetic improvement but also in light of the fact that her lesions were symptomatic.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
- Wenson SF, Jan F, Sepehr A. Unilateral nevoid telangiectasia syndrome: a case report and review of the literature. Dermatol Online J. 2011;17:2.
- Wilkin JK. Unilateral nevoid telangiectasia: three new cases and the role of estrogen. Arch Dermatol. 1977;113:486-488.
- Selmanowitz VJ. Unilateral nevoid telangiectasia. Ann Intern Med. 1970;73:87-90.
- Karakas¸ M, Durdu M, Sönmezog˘lu S, et al. Unilateral nevoid telangiectasia. J Dermatol. 2004;31:109-112.
- Jordão JM, Haendchen LC, Berestinas TC, et al. Acquired unilateral nevoid telangiectasia in a healthy men. An Bras Dermatol. 2010;85:912-914.
- Tas¸kapan O, Harmanyeri Y, Sener O, et al. Acquired unilateral nevoid telangiectasia syndrome. Acta Derm Venereol. 1997;77:62-63.
- Karabudak O, Dogan B, Taskapan O, et al. Acquired unilateral nevoid telangiectasia syndrome. J Dermatol. 2006;33:825-826.
- Jucas JJ, Rietschel RL, Lewis CW. Unilateral nevoid telangiectasia. Arch Dermatol. 1979;115:359-360.
- Sharma VK, Khandpur S. Unilateral nevoid telangiectasia—response to pulsed dye laser. Int J Dermatol. 2006;45:960-964.
- Cliff S, Harland CC. Recurrence of unilateral naevoid telangiectatic syndrome following treatment with the pulsed dye laser. J Cutan Laser Ther. 1999;1:105-107.
Practice Points
- Unilateral nevoid telangiectasia may present in patients without an underlying hyperestrogenic state.
- Unilateral nevoid telangiectasia may present with symptoms including pruritus.
APPlying Knowledge: Evidence for and Regulation of Mobile Apps for Dermatologists
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Practice Points
- Physicians who are selecting an app for self-education or patient care should take into consideration the strength of the evidence supporting the app as well as the rigor of any approval process the app had to undergo.
- Only a minority of health-related apps are regulated by the government. This regulation has not kept up with the evolution of app software and may become more indirect.
Virtual Dermatology: A COVID-19 Update
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.
Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
The growing threat of novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), now commonly known as coronavirus disease 2019 (COVID-19), has forced Americans to stay home due to quarantine, especially older individuals and those who are immunocompromised or have an underlying health problem such as pulmonary or cardiac disease. The federal government’s estimated $2 trillion CARES Act (Coronavirus Aid, Relief, and Economic Security Act)1 will provide a much-needed boost to health care and the economy; prior recent legislation approved an $8.6 billion emergency relief bill,2 HR 6074 (Coronavirus Preparedness and Response Supplemental Appropriations Act of 2020), which expands Medicare coverage of telehealth to patients in their home rather than having them travel to a designated site, covers both established and new patients, allows physicians to waive or reduce co-payments and cost-sharing requirements, and reimburses the same as an in-person visit.
Federal emergency legislation temporarily relaxed the Health Insurance Portability and Accountability Act (HIPAA),3,4 allowing physicians to use Facetime and Skype for Medicare patients. In addition, Medicare will reimburse telehealth services for out-of-state-providers; however, cross-state licensure is governed by the patient’s home state.5 As of March 25, 2020, emergency legislation to temporarily allow out-of-state physicians to provide care, whether or not it relates to COVID-19, was enacted in 13 states: California, Colorado, Connecticut, Delaware, Hawaii, Idaho, Indiana, Iowa, Maryland, Minnesota, New York, North Carolina, and North Dakota.6 Ongoing legislation is rapidly changing; for daily updates on licensing laws, refer to the Federation of State Medical Boards website. Check your own institutional policies and malpractice provider prior to offering telehealth, as local laws and regulations may vary. Herein, we offer suggestions for using teledermatology.
Reimbursement
Prior to the COVID-19 pandemic, 16 states—Arkansas, Colorado, Delaware, Hawaii, Kentucky, Maine, Minnesota, Mississippi, Missouri, Montana, Nevada, New Jersey, New Mexico, Tennessee, Utah, and Virginia—had true payment parity laws,7 which reimbursed telehealth as a regular office visit using modifier -95. Several states have enacted emergency telehealth expansion laws to discourage COVID-19 spread8; some states such as New Jersey now prohibit co-payments or out-of-pocket deductibles from all in-network insurance plans (commercial Medicare and Medicaid).9,10 Updated legislation about COVID-19 and telemedicine can be found on the Center for Connected Health Policy website. An interactive map of laws and reimbursement policies also is available on the websites of the American Telehealth Association and the American Academy of Dermatology. The ability to charge a patient directly for telehealth services depends on the insurance provider agreement. If telehealth is a covered service, you cannot charge these patients out-of-pocket.
Teledermatology Options
For many conditions, the effectiveness and quality of teledermatology is comparable to a conventional face-to-face visit.11 There are 3 types of telehealth visits:
• Store and forward: The clinician reviews images or videos and responds asynchronously,12 similar to an email chain.
• Live interactive: The clinician uses 2-way video synchronously.12 In states with parity laws, this method is reimbursed equally to an in-person visit.
• Remote patient monitoring: Health-related data are collected and transmitted to a remote clinician, similar to remote intensive care unit management.12 Dermatologists are unlikely to utilize this modality.
The Virtual Visit
Follow these guidelines for practicing teledermatology: (1) ensure that the image or video is clear and that there is proper lighting, a monochromatic background, and a clear view of the anatomy necessary to evaluate; (2) dress in appropriate attire as if you were in clinic, such as scrubs, a white coat, or other professional attire; (3) begin the telehealth encounter by obtaining informed consent,13 according to state14 or Medicare guidelines; (4) document the location of the patient and provider; (5) for live virtual visits, document similarly to an in-person visit5; (6) for all other virtual care, document minutes spent on each task; and (7) select only 1 billing code per visit.
In some states, regulations for commercial and/or Medicaid plans require that other modifiers be added to billing codes, which vary plan-by-plan:
• Modifier GQ: For asynchronous care (store and forward).
• Modifier GT: For synchronous live telehealth visits.
• Modifier -95: In states where there are equal parity laws or if you are billing a commercial insurance payer (may vary by plan).
Medicare does not require any additional modifiers.15 If the plan reimburses telemedicine equally to a face-to-face visit, use regular office visit codes. The eTable16 lists billing codes and Medicare reimbursement rates.

Secure Software
Several electronic medical record systems already include secure patient communication. Other HIPAA-compliant communication options with a variety of features are available to clinicians:
• Klara allows for HIPAA-secure texting, group messaging, photograph uploads, and telephone calls.
• Doximity offers free calling and faxes.
• G Suite for health care offers HIPAA-compliant texting, emailing, and video calls through Google Voice and Google Hangouts Meet.
• Secure video chat is available on Zoom for Healthcare, VSee, Doxy.me, and other platforms.
• Multiservice platforms such as DermEngine include billing, payments, teledermatology, and teledermoscopy and allow for interprofessional consultation.
The Bottom Line
Telehealth readiness is playing a key role in containing the spread of COVID-19. In-person dermatology visits are now being limited to urgent conditions only, as per institutional guidelines.4
Acknowledgment
We thank Garfunkel Wild, P.C. (Great Neck, New York), for their expertise and assistance.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
- Coronavirus Aid, Relief, and Economic Security Act, 2020. HR 748, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr748. Accessed March 26, 2020.
- Coronavirus Preparedness and Response Supplemental Appropriations Act, 2020. HR 6074, 116th Cong, 2nd Sess (2020). https://www.govtrack.us/congress/bills/116/hr6074/text. Accessed March 22, 2020.
- Azar AM II. Waiver or Modification of Requirements Under Section 1135 of the Social Security Act. Washington, DC: US Department of Health and Human Services; 2020. https://www.phe.gov/emergency/news/healthactions
/section1135/Pages/covid19-13March20.aspx. Accessed March 25, 2020. - American Academy of Dermatology Association. Can dermatologists use telemedicine to mitigate COVID-19 outbreaks? https://www.aad.org/member/practice/telederm/toolkit. Updated March 28, 2020. Accessed March 26, 2020.
- American Medical Association. AMA quick guide to telemedicine in practice. https://www.ama-assn.org/practice-management/digital/ama-quick-guide-telemedicine-practice?utm_source=twitter&utm_medium=social_ama
&utm_term=3207044834&utm_campaign=Public+Health. Updated March 26, 2020. Accessed March 26, 2020. - Federation of State Medical Boards. States waiving licensure requirements in response to COVID-19. http://www.fsmb.org/sitassets/advocacy/pdf/state-emergency-declarations-licensures-requimentscovid-19.pdf. Updated March 30, 2020. Accessed March 30, 2020.
- American Telemedicine Association. 2019 State of the States: coverage & reimbursement. https://cdn2.hubspot.net/hubfs/5096139/Files/Thought Leadership_ATA/2019 State of the States summary_final.pdf. Published July 18, 2019. Accessed March 30, 2020.
- COVID-19 related state actions. Center for Connected Health Policy website. https://www.cchpca.org/resources/covid-19-related-state-actions. Updated March 27, 2020. Accessed March 26, 2020.
- Governor Murphy announces departmental actions to expand access to telehealth and tele-mental health services in response to COVID-19 [news release]. Trenton, NJ: State of New Jersey; March 22, 2020. https://www.nj.gov/governor/news/news/562020/20200322b.shtml. Accessed March 26, 2020.
- Caride M. Use of telemedicine and telehealth to respond to the COVID-19 pandemic. State of New Jersey website. https://www.state.nj.us/dobi/bulletins/blt20_07.pdf. Published March 22, 2020. Accessed March 30, 2020.
- Lee JJ, English JC 3rd. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Tongdee E, Siegel DM, Markowitz O. New diagnostic procedure codes and reimbursement. Cutis. 2019;103:208-211.
- Telemedicine forms. American Telemedicine Association Web site. http://hub.americantelemed.org/thesource/resources/telemedicine-forms. Accessed March 22, 2020.
- State telemedicine laws, simplified. eVisit Web site. https://evisit.com/state-telemedicine-policy/. Accessed March 22, 2020.
- Centers for Medicare & Medicaid Services. Medicare Fee-for-Service (FFS) Response to the Public Health Emergency on the Coronavirus (COVID-19). March 20, 2020. https://www.cms.gov/files/document/se20011.pdf. Accessed March 29, 2020.
- Centers for Medicare & Medicaid Services. Medicare telemedicine health care provider fact sheet. https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Published March 17, 2020. Accessed March 20, 2020.
Emerging Noninvasive Treatments of Nonmelanoma Skin Cancers
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
Nonmelanoma skin cancer (NMSC) is the most common malignancy worldwide, and its incidence continues to increase. More than 5 million NMSCs are estimated to occur annually in the United States alone.1 There are more cases of basal cell carcinoma (BCC) than all other cancers combined, with squamous cell carcinoma (SCC) being the second most common cancer in the United States.1-3 The rising incidence of NMSCs highlights the importance of investigating additional treatment options with fewer side effects, better cosmetic outcomes, and better efficacy.1
Originally, treatment options for NMSCs largely relied on destructive and surgical methods. Basal cell carcinoma and SCC commonly are treated with cryosurgery; electrodesiccation and curettage; or more definitive surgical options, including excision and Mohs micrographic surgery (MMS). Over time, topical agents such as 5-fluorouracil, imiquimod, ingenol mebutate, and various forms of aminolevulinic acid (ALA) for photodynamic therapy (PDT) were included for superficial lesions as well as field treatment. The development of oral hedgehog (Hh) inhibitors, such as vismodegib, offered a promising alternative to patients with advanced disease. Each treatment has its own specific indications and side effects; thus, there is always room for novel therapeutic approaches. We review new and potential treatments from 2018 and beyond. Although only 5% of SCCs become locally advanced, recur, or metastasize, and 0.4% to 0.8% of BCCs progress to advanced disease, many of the newer studies target advanced NMSCs, given their life-threatening and debilitating nature.4,5 Similarly, the incidence of nevoid basal cell carcinoma (NBCC) syndrome is as low as 1 in 57,000 to 1 in 164,000 but continues to be studied because of its morbidity and the potential to contribute new treatment options for BCC in the general population.6
Topical Therapy
Sonidegib
Basal cell carcinoma proliferation is a result of an unregulated Hh pathway that is initiated when the Hh ligand binds to the patched 1 protein (PTCH1).7-11 Patched 1 protein normally inhibits the smoothened (SMO) transmembrane receptor protein, decreasing the signaling cascade. In BCCs, there is a loss of PTCH1 function, effectively increasing the Hh pathway activity. Sonidegib is an Hh inhibitor that in turn prevents inhibition of PTCH1 in an attempt to reregulate the pathway.7-11 Although sonidegib is known for its 2015 approval by the US Food and Drug Administration (FDA) as a systemic therapy for locally advanced BCCs,12 one study investigated a topical formulation on 8 patients with NBCC syndrome.13 Patients were treated twice daily with sonidegib cream 0.75% for 4 weeks in a double-blind, randomized, vehicle-controlled study. A total of 27 BCCs were randomized and treated with either vehicle or sonidegib. A biopsy was taken at the end of the study of 1 sonidegib-treated and 1 vehicle-treated BCC lesion per patient. Of the 13 sonidegib-treated BCC lesions, 3 (23.1%) showed complete response, 9 (69.2%) showed partial response, and 1 (7.7%) showed no response vs 13 of 14 (92.8%) lesions that did not respond to the vehicle. Patients tolerated the treatment well without skin irritation or signs of local or systemic side effects.13 Topical sonidegib should be further investigated as an adjunct or in different vehicles given the successful regression of BCCs and its minimal side-effect profile.
Systemic Therapy
Cemiplimab
Cemiplimab is a human monoclonal antibody against programmed death receptor 1 (PD-1) that was FDA approved in September 2018 for the treatment of metastatic cutaneous SCC.14 Programmed death receptor 1 is found on T lymphocytes, B lymphocytes, and macrophages, which normally assist in the immune response to tumor cells. However, programmed cell death ligand 1 (PD-L1) and programmed cell death ligand 2 (PD-L2) are found on tumor cells and bind to PD-1. Cemiplimab prevents PD-1 from binding to PD-L1 and PD-L2, allowing an appropriate immune response.14,15 A phase 1 clinical trial of cemiplimab showed a 50% (13/26) response rate.16 The phase 2 trial included patients with advanced SCC, but the primary analysis only considered patients with metastatic SCC. Phase 2 results showed a 47.5% (28/59) response rate. Patients received intravenous cemiplimab 3 mg/kg once every 2 weeks for up to 48 weeks in phase 1 and up to 96 weeks in phase 2. Both phases of the trial showed a response to treatment lasting longer than 6 months in more than 50% of patients. The most common adverse events were diarrhea, fatigue, nausea, constipation, and rash.16
Although immune-mediated adverse reactions are rare, they can occur given cemiplimab’s mechanism of action and may range from severe to fatal. Examples of immune-mediated adverse reactions that occurred during the study included pneumonitis, colitis, hepatitis, adrenal insufficiency, hypophysitis, hypothyroidism, hyperthyroidism, type 1 diabetes mellitus, nephritis with renal dysfunction, and immune-mediated dermatologic reactions.14 It is important to monitor for immune-mediated adverse reactions and address them immediately once detected.
Other PD-1 Inhibitors
Although PD-1 inhibitors have been studied in advanced SCCs, their clinical data are limited for BCCs.17 Prior to 2018, there was a small number of case reports of patients with BCC with partial to exceptional response to PD-1 inhibitors. Recently, 2 additional case reports were published with contrasting outcomes using 2 different PD-1 inhibitors. An elderly patient with metastatic non–small cell lung cancer was treated with nivolumab after failing chemotherapy. She subsequently developed a BCC on the nose that was resected but recurred 2 months later despite continuing nivolumab.17 Another case report detailed a patient with a history of BCC on the shoulder excised 5 years prior who presented with recurrence on the sternum and clavicle.18 One year later the patient was found to have BCC metastases to the lung. After progression of disease despite vismodegib and recurrence of BCC with taladegib, the patient was then placed on pembrolizumab. At 6 weeks and 12 months, computed tomography showed resolution of multiple lung lesions. Sixteen weeks after initiation of pembrolizumab treatment, spinal metastases were found, but the treatment was continued because of the improvement in the lung metastases.18
Taladegib
Taladegib is a SMO antagonist that has been through a phase 1 trial in patients with advanced cancer, including treatment-naive and previously treated BCCs.19 Eighty-four patients were treated to examine the safety profile and determine an appropriate phase 2 dose and administration schedule. The maximum tolerable dose was determined to be 400 mg because of dose-limiting toxicities. All clinical responses were in patients with BCCs (47/84 [55.9%] patients), with a response rate of 46.8%. Eleven of 16 (68.8%) Hh-treatment–naive patients and 11 of 31 (35.5%) patients previously treated with Hh responded to taladegib. Common adverse events were dysgeusia, fatigue, nausea, and muscle spasms.19 Although vismodegib is an FDA-approved SMO antagonist since 2012, treatment resistance and tolerability issues have been continuing concerns.20,21 Taladegib is a potential alternative that may be found to have improved pharmacodynamics and pharmacokinetics. Not only did in vitro studies show a preferable protein-binding profile with taladegib, but it also displayed dose proportionality, while vismodegib has been known to have nonlinear pharmacokinetics.19
Posaconazole
Posaconazole is a systemic antifungal agent that is a structural analogue to itraconazole.22 Itraconazole has been found to inhibit the Hh pathway as an SMO antagonist. In a study with mice, posaconazole was found to have strong activity against drug-resistant SMO mutants while inhibiting the growth of Hh-dependent BCCs in vivo. A marked decrease also was seen in the ciliary accumulation of SMO, suggesting a similar mechanism of action to itraconazole. Posaconazole’s use for BCCs currently is limited to basic science studies but may offer a potential alternative to itraconazole, which is known to have many drug-drug interactions and requires dose adjustments in renal and hepatic insufficiency. When used as an antifungal compared to itraconazole, posaconazole has a favorable long-term safety profile due to fewer drug-drug interactions and mild side effects; it also does not require dose adjustments in mild to moderate renal or hepatic insufficiency.22 Thus, posaconazole is a potentially safer alternative to itraconazole for the treatment of BCCs. Although phase 2 studies of itraconazole for BCCs have shown decreased cell proliferation, tumor size, and reduced GLI1 messenger RNA, side effects included fatigue and grade 4 heart failure.23,24
Radiation Therapy
Radiation therapies (RTs), such as superficial RT, have been long-established treatment options.25 However, there also are emerging methods of delivering RT, including electronic brachytherapy (EB). Although there is a low likelihood of residual tumor after RT given the number of sessions involved and the more aggressive nature of the treatment, these factors also can be a substantial burden on the patient. Furthermore, RT may result in subsequent scar tissue, which can hinder the use of other emerging technologies, such as noninvasive imaging devices, following RT.
Superficial RT
Superficial RT is a secondary option for the treatment of NMSC for use in special circumstances, such as when surgical intervention is contraindicated or refused, and after the benefits and risks of treatment alternatives have been discussed.26 However, depending on the tumor type and anatomical location, 6 to 18 treatments may be required, with treatment frequency ranging from 1 to 5 treatments per week.25 Patients may find this treatment regimen difficult to maintain given the length of time and frequency of treatments required. Side effects include radiation dermatitis and postinflammatory hypopigmentation or hyperpigmentation in patients with dark skin, and there is a risk for recurrence.25,27
Electronic Brachytherapy
Brachytherapy is a method of delivering RT via radioactive isotopes, whereas EB uses lower-energy photons that require less shielding.28 As a relatively new therapy, studies on the efficacy of EB on NMSC continue to grow but with limited data comparing EB with established treatments. Furthermore, there are limited long-term follow-up data, and future studies should expand the patient demographic to younger patients before treatment guidelines can be established.28
RT With Concurrent and Adjuvant Vismodegib
Vismodegib is an SMO inhibitor that was FDA approved in 2012 for the treatment of locally advanced BCC in patients who are not candidates for surgery or RT.29 Over time, studies have looked into other indications for vismodegib, such as a neoadjuvant to MMS or in patients with NBCC syndrome.11 Prior to 2018, there were only 2 known case reports of concurrent vismodegib and RT used for recurrent advanced BCC.30 Recently, vismodegib has been further examined in combination with RT in a case report,31 basic science study,32 and phase 2 trials (ClinicalTrials.gov Identifiers NCT02956889 and NCT01835626).
Prior studies showed low cure rates with vismodegib alone after RT (43%) as well as decreasing cure rates with primary RT alone as tumor size increased.33,34 In 2018, vismodegib was used concurrently and as an adjuvant to RT in a patient with advanced multifocal BCC.31 The patient had multiple large BCCs on the trunk that were painful and bleeding. The patient was started on RT and 150 mg/d vismodegib concurrently, which was then continued adjuvantly for 3 months until it was discontinued because of diarrhea. The patient had complete response in all lesions with resolution of symptoms.31 A separate basic science study further supported the potential role of vismodegib in radiation sensitization of both BCCs and head and neck SCCs.32 There presently are 2 phase 2 trials investigating the concurrent use of vismodegib and RT, which could help determine the efficacy of the combined approach for patients with advanced BCCs who are poor surgical candidates (NCT02956889 and NCT01835626).
Photodynamic Therapy
Photodynamic therapy has been in use since the 1970s when Dougherty et al35 performed one of the first studies on its use in skin cancer. Since then, PDT has been used for the treatment of actinic keratoses (AKs) and more recently BCCs. In PDT, a photosensitizer (PS) is applied and activated by a 400-nm blue light or 635-nm red light, depending on the PS used. The PS then produces highly reactive oxygen species, leading to apoptosis of the cancer cells.36 In Europe, red light PDT is licensed for the treatment of AKs as well as superficial and nodular BCCs, though approved indications vary between countries. In the United States, PDT is only FDA approved for the treatment of AKs.37
Aminolevulinic Acid Hydrochloride
Aminolevulinic acid hydrochloride is a red light PS used to treat AKs since 2011 and BCCs since 2017 in Europe in addition to AKs in the United States since 2016.38,39 A phase 3 noninferiority clinical trial in Europe of 281 patients compared the treatment of nonaggressive BCCs with ALA to methyl aminolevulinate (MAL) cream.40 The study found a complete response rate of 93.4% vs 91.8%. Superficial BCCs treated with ALA had a clearance rate of 94.7% vs 96.4% with MAL, while nodular BCCs treated with ALA had a clearance rate of 85.7% vs 76.2% with MAL. A 1-year clinical follow-up showed similar recurrence rates (8.4% for ALA vs 8.5% for MAL).40 The results of this study led to an expanded indication in Europe to include the treatment of BCCs.38 Aminolevulinic acid hydrochloride currently is undergoing phase 3 clinical trials in the United States for approval for the treatment of superficial BCCs (NCT03573401). If similar outcomes are achieved, US patients may have access to an alternative nonsurgical treatment of BCCs. The ongoing US trial is exclusively investigating the efficacy and safety for superficial BCCs, which may limit FDA approval to only superficial BCCs, accounting for only 8.4% to 24.1% of all BCCs.35,41,42
Laser Therapy
Ablative and nonablative lasers have been used to treat NMSCs in the literature. Ablative lasers destroy tumors through vaporization of tissue water, whereas nonablative lasers target the vasculature of tumors while preserving the surrounding tissue.43,44 Nonablative lasers include pulsed dye lasers (PDL) and Nd:YAG lasers. Examples of ablative lasers include CO2 and erbium:YAG lasers. Given the status of lasers as an emerging treatment method, there currently is no standardized laser setting for any of the laser therapies used to treat NMSCs. Although there is the potential for optimal cosmetic outcomes and a limited side-effect profile for nonablative laser therapies, there are limited data on long-term follow-up to study recurrence rates and establish a more standardized treatment protocol.
Pulsed Dye Lasers
Although there were no studies on PDL therapy alone in 2018, a study published in 2019 evaluated a combination laser treatment using a 595-nm PDL and 1927-nm fractional laser for the treatment of 93 BCCs, yielding a 95.7% (89/93) clearance rate and 4.5% (4/89) recurrence rate over a follow-up period of up to 6 years (range, 2.53 months to 6.03 years).45 Studies of PDL prior to 2018 had follow-ups ranging from 2 weeks to 6 months.46-51 Although the majority were biopsy-proven BCCs, reflectance confocal microscopy also was used for same-day diagnoses. Long-term follow-up included clinical examinations, dermoscopy, and optical coherence tomography.45 The clearance rate (95.7%) using noninvasive imaging in conjunction with the combination laser treatment was superior to both histologic and clinical clearance rates of prior PDL-only studies, which ranged from 25% to 95%.46-51 To have long-term follow-up data, the study used noninvasive imaging with clinical follow-up because histology would not be viable for long-term follow-up. This study was retrospective rather than prospective, which was a limitation.45
Nd:YAG Lasers
The majority of studies utilizing Nd:YAG lasers investigated their efficacy in treating BCCs, with the exception of 1 study of facial SCCs. This major study in 2009 of 627 BCCs showed a 2.5% recurrence rate after a follow-up time of 3 months to 5 years.52 Nd:YAG lasers continue to be investigated, including a more recent study of 31 extrafacial, biopsy-proven BCCs that were treated with the 1064-nm Nd:YAG laser, which showed a 90% histologic clearance on 1-month follow-up after a single treatment.53 In 2019, a retrospective review of 16 BCC lesions on the head, neck, trunk, and extremities showed 100% clearance after 1 treatment, with an average follow-up period of 9 months (range, 6–15 months).54 In a retrospective review, Markowitz and Psomadakis55 contributed data supporting the further investigation and use of the 1064-nm Nd:YAG laser for BCC treatment while leveraging noninvasive imaging to demonstrate a same-day management model. Seventeen BCC lesions on the face and body were diagnosed by reflectance confocal microscopy and treated with an Nd:YAG laser, and clearance was monitored clinically, dermoscopically, and by optical coherence tomography. There was 100% clearance of the lesions in the study, with 82.4% (14/17) clearing after 1 treatment; mean follow-up was 103 days (range, 48–371 days).55 These studies were limited by their short follow-up time; long-term data are needed to determine true rates of recurrence.
Ablative Lasers
Ablative lasers also have been used in the treatment of NMSCs. In addition to the potentially increased healing time compared to nonablative lasers, other limitations of ablative laser therapy include residual tumor burden or recurrence that may not be easily visualized in scarred tissue after nonablative management.44
Conclusion
Although MMS remains the gold standard for invasive management of NMSCs, studies from 2018 and beyond (eTable) expanded not only on MMS topics such as increased patient access and improved techniques but also on the increasing potential of noninvasive treatments. Some of the noninvasive therapies were entirely new compounds, whereas others were already in use for a different disease indication. Furthering our knowledge and expanding our repertoire of management options will prepare us as the number of patients affected by NMSCs increases.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the US population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Rubin AI, Chen EH, Ratner D. Basal cell carcinoma. N Engl J Med. 2005;353:2262-2269.
- Kauvar AN, Arpey CJ, Hruza G, et al. Consensus for nonmelanoma skin cancer treatment, part II. Dermatol Surg. 2015;41:1214-1240.
- Ribero S, Stucci LS, Daniels GA, et al. Drug therapy of advanced cutaneous squamous cell carcinoma: is there any evidence? Curr Opin Oncol. 2017;29:129-135.
- Goldenberg G, Karagiannis T, Palmer JB, et al. Incidence and prevalence of basal cell carcinoma (BCC) and locally advanced BCC (LABCC) in a large commercially insured population in the United States: a retrospective cohort study. J Am Acad Dermatol. 2016;75:957.e2-966.e2.
- Kimonis VE, Goldstein AM, Pastakia B, et al. Clinical manifestations in 105 persons with nevoid basal cell carcinoma syndrome. Am J Med Genet. 1997;69:299-308.
- Sekulic A, Migden MR, Oro AE, et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N Engl J Med. 2012;366:2171-2179.
- Peris K, Licitra L, Ascierto PA, et al. Identifying locally advanced basal cell carcinoma eligible for treatment with vismodegib: an expert panel consensus. Futur Oncol. 2015;11:703-712.
- Sekulic A, Migden MR, Basset-Seguin N, et al; ERIVANCE BCC Investigators. Long-term safety and efficacy of vismodegib in patients with advanced basal cell carcinoma: final update of the pivotal ERIVANCE BCC study. BMC Cancer. 2017;17:332.
- Ibrahim O, Gastman B, Zhang A. Advances in diagnosis and treatment of nonmelanoma skin cancer. Ann Plast Surg. 2014;73:615-619.
- Levine A, Siegel DM, Markowitz O. Update on noninvasive diagnostic imaging and management of nonmelanoma skin cancer. Curr Dermatol Rep. 2018;7:1-15.
- Casey D, Demko S, Shord S, et al. FDA approval summary: sonidegib for locally advanced basal cell carcinoma. Clin Cancer Res. 2017;23:2377-2381.
- Skvara H, Kalthoff F, Meingassner JG, et al. Topical treatment of basal cell carcinomas in nevoid basal cell carcinoma syndrome with a smoothened inhibitor. J Invest Dermatol. 2011;131:1735-1744.
- Markham A, Duggan S. Cemiplimab: first global approval. Drugs. 2018;78:1841-1846.
- Chen L, Aria AB, Silapunt S, et al. Emerging nonsurgical therapies for locally advanced and metastatic nonmelanoma skin cancer. Dermatolog Surg. 2019;45:1-16.
- Migden MR, Rischin D, Schmults CD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med. 2018;379:341-351.
- Sabbatino F, Marra A, Liguori L, et al. Resistance to anti-PD-1-based immunotherapy in basal cell carcinoma: a case report and review of the literature. J Immunother Cancer. 2018;6:126.
- Cannon JGD, Russell JS, Kim J, et al. A case of metastatic basal cell carcinoma treated with continuous PD-1 inhibitor exposure even after subsequent initiation of radiotherapy and surgery. JAAD Case Rep. 2018;4:248-250.
- Bendell J, Andre V, Ho A, et al. Phase I study of LY2940680, a Smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082-2091.
- Lear JT, Corner C, Dziewulski P, et al. Challenges and new horizons in the management of advanced basal cell carcinoma: a UK perspective. Br J Cancer. 2014;111:1476-1481.
- Basset-Seguin N, Sharpe HJ, de Sauvage FJ. Efficacy of hedgehog pathway inhibitors in basal cell carcinoma. Mol Cancer Ther. 2015;14:633-641.
- Chen B, Trang V, Lee A, et al. Posaconazole, a second-generation triazole antifungal drug, inhibits the hedgehog signaling pathway and progression of basal cell carcinoma. Mol Cancer Ther. 2016;15:866-876.
- Kim DJ, Kim J, Spaunhurst K, et al. Open-label, exploratory phase II trial of oral itraconazole for the treatment of basal cell carcinoma. J Clin Oncol. 2014;32:745-751.
- Ally MS, Ransohoff K, Sarin K, et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 2016;152:452-456.
- Nestor MS, Berman B, Goldberg D, et al. Consensus guidelines on the use of superficial radiation therapy for treating nonmelanoma skin cancers and keloids. J Clin Aesthet Dermatol. 2019;12:12-18.
- American Academy of Dermatology and AAD Association. Position statement on superficial radiation therapy for basal cell carcinoma (BCC) and squamous cell carcinomas (SCC). https://server.aad.org/Forms/Policies/Uploads/PS/PS%20Superficial%20Radiation%20Therapy.pdf?. Updated August 9, 2014. Accessed February 26, 2020.
- Skiveren J, Mikkelsen MR, Daugbjerg H, et al. Skin reactions and quality of life after X-ray therapy of basal cell carcinoma. J Skin Cancer. 2012;2012:825095.
- Tom MC, Hepel JT, Patel R, et al. The American Brachytherapy Society consensus statement for electronic brachytherapy. Brachytherapy. 2019;18:292-298.
- Axelson M, Liu K, Jiang X, et al. US Food and Drug Administration approval: vismodegib for recurrent, locally advanced, or metastatic basal cell carcinoma. Clin Cancer Res. 2013;19:2289-2293.
- Pollom EL, Bui TT, Chang AL, et al. Concurrent vismodegib and radiotherapy for recurrent, advanced basal cell carcinoma. JAMA Dermatol. 2015;151:998-1001.
- Franco AI, Eastwick G, Farah R, et al. Upfront radiotherapy with concurrent and adjuvant vismodegib is effective and well-tolerated in a patient with advanced, multifocal basal cell carcinoma. Case Rep Dermatol Med. 2018;2018:2354146.
- Hehlgans S, Booms P, Güllülü Ö, et al. Radiation sensitization of basal cell and head and neck squamous cell carcinoma by the hedgehog pathway inhibitor vismodegib. Int J Mol Sci. 2018;19:E2485.
- Piccinno R, Benardon S, Gaiani FM, et al. Dermatologic radiotherapy in the treatment of extensive basal cell carcinomas: a retrospective study. J Dermatolog Treat. 2017;28:426-430.
- Locke J, Karimpour S, Young G, et al. Radiotherapy for epithelial skin cancer. Int J Radiat Oncol. 2001;51:748-755.
- Dougherty TJ, Kaufman JE, Goldfarb A, et al. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978;38:2628-2635.
- Ding H, Yu H, Dong Y, et al. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J Control Release. 2011;156:276-280.
- Maytin EV, Kaw U, Ilyas M, et al. Blue light versus red light for photodynamic therapy of basal cell carcinoma in patients with Gorlin syndrome: a bilaterally controlled comparison study. Photodiagnosis Photodyn Ther. 2018;22:7-13.
- European Medicines Agency. Ameluz 5-aminolevulinic acid hydrochloride. https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz. Updated May 13, 2019. Accessed February 25, 2020.
- Center for Drug Evaluation and Research. Approval package for Ameluz (aminolevulinic acid hydrochloride) gel, 10%. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208081Orig1s000Approv.pdf. Published May 10, 2016. Accessed February 25, 2020.
- Morton CA, Dominicus R, Radny P, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. 2018;179:309-319.
- Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681-690.
- Kamyab-Hesari K, Seirafi H, Naraghi ZS, et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol. 2014;28:250-253.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Markowitz O, Tongdee E, Levine A. Optimal cosmetic outcomes for basal cell carcinoma: a retrospective study of nonablative laser management. Cutis. 2019;103:292-297, E1-E3.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Moskalik K, Kozlov A, Demin E, et al. The efficacy of facial skin cancer treatment with high-energy pulsed neodymium and Nd:YAG lasers. Photomed Laser Surg. 2009;27:345-349.
- Ortiz AE, Anderson RR, DiGiorgio C, et al. An expanded study of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma. Lasers Surg Med. 2018;50:727-731.
- Ahluwalia J, Avram MM, Ortiz AE. Outcomes of long-pulsed 1064 nm Nd:YAG laser treatment of basal cell carcinoma: a retrospective review. Lasers Surg Med. 2019;51:34-39.
- Markowitz O, Psomadakis CE. Patient-driven management using same-day noninvasive diagnosis and complete laser treatment of basal cell carcinomas: a pilot study. Cutis. 2019;104:345-348.
Practice Points
- As of 2018, there has been an increase in options for the noninvasive management of nonmelanoma skin cancers that should be considered.
- Recently, approved advances in treatment options have included not only advanced basal cell carcinoma but also advanced squamous cell carcinoma such as cemiplimab.
Is Artificial Intelligence Going to Replace Dermatologists?
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
Artificial intelligence (AI) is a loosely defined term that refers to machines (ie, algorithms) simulating facets of human intelligence. Some examples of AI are seen in natural language-processing algorithms, including autocorrect and search engine autocomplete functions; voice recognition in virtual assistants; autopilot systems in airplanes and self-driving cars; and computer vision in image and object recognition. Since the dawn of the century, various forms of AI have been tested and introduced in health care. However, a gap exists between clinician viewpoints on AI and the engineering world’s assumptions of what can be automated in medicine.
In this article, we review the history and evolution of AI in medicine, focusing on radiology and dermatology; current capabilities of AI; challenges to clinical integration; and future directions. Our aim is to provide realistic expectations of current technologies in solving complex problems and to empower dermatologists in planning for a future that likely includes various forms of AI.
Early Stages of AI in Medical Decision-making
Some of the earliest forms of clinical decision-support software in medicine were computer-aided detection and computer-aided diagnosis (CAD) used in screening for breast and lung cancer on mammography and computed tomography.1-3 Early research on the use of CAD systems in radiology date to the 1960s (Figure), with the first US Food and Drug Administration–approved CAD system in mammography in 1998 and for Centers for Medicare & Medicaid Services reimbursement in 2002.1,2
Early CAD systems relied on rule-based classifiers, which use predefined features to classify images into desired categories. For example, to classify an image as a high-risk or benign mass, features such as contour and texture had to be explicitly defined. Although these systems showed on par with, or higher, accuracy vs a radiologist in validation studies, early CAD systems never achieved wide adoption because of an increased rate of false positives as well as added work burden on a radiologist, who had to silence overcalling by the software.1,2,4,5
Computer-aided diagnosis–based melanoma diagnosis was introduced in early 2000 in dermatology (Figure) using the same feature-based classifiers. These systems claimed expert-level accuracy in proof-of-concept studies and prospective uncontrolled trials on proprietary devices using these classifiers.6,7 Similar to radiology, however, real-world adoption did not happen; in fact, the last of these devices was taken off the market in 2017. A recent meta-analysis of studies using CAD-based melanoma diagnosis point to study bias; data overfitting; and lack of large controlled, prospective trials as possible reasons why results could not be replicated in a clinical setting.8
Beyond 2010: Deep Learning
New techniques in machine learning (ML), called deep learning, began to emerge after 2010 (Figure). In deep learning, instead of directing the computer to look for certain discriminative features, the machine learns those features from the large amount of data without being explicitly programed to do so. In other words, compared to predecessor forms of computing, there is less human supervision in the learning process (Table). The concept of ML has existed since the 1980s. The field saw exponential growth in the last decade with the improvement of algorithms; an increase in computing power; and emergence of large training data sets, such as open-source platforms on the Web.9,10
Most ML methods today incorporate artificial neural networks (ANN), computer programs that imitate the architecture of biological neural networks and form dynamically changing systems that improve with continuous data exposure. The performance of an ANN is dependent on the number and architecture of its neural layers and (similar to CAD systems) the size, quality, and generalizability of the training data set.9-12
In medicine, images (eg, clinical or dermoscopic images and imaging scans) are the most commonly used form of data for AI development. Convolutional neural networks (CNN), a subtype of ANN, are frequently used for this purpose. These networks use a hierarchical neural network architecture, similar to the visual cortex, that allows for composition of complex features (eg, shapes) from simpler features (eg, image intensities), which leads to more efficient data processing.10-12
In recent years, CNNs have been applied in a number of image-based medical fields, including radiology, dermatology, and pathology. Initially, studies were largely led by computer scientists trying to match clinician performance in detection of disease categories. However, there has been a shift toward more physicians getting involved, which has motivated development of large curated (ie, expert-labeled) and standardized clinical data sets in training the CNN. Although training on quality-controlled data is a work in progress across medical disciplines, it has led to improved machine performance.11,12
Recent Advances in AI
In recent years, the number of studies covering CNN in diagnosis has increased exponentially in several medical specialties. The goal is to improve software to close the gap between experts and the machine in live clinical settings. The current literature focuses on a comparison of experts with the machine in simulated settings; prospective clinical trials are still lagging in the real world.9,11,13
We look at radiology to explore recent advances in AI diagnosis for 3 reasons: (1) radiology has the largest repository of digital data (using a picture archiving and communication system) among medical specialties; (2) radiology has well-defined, image-acquisition protocols in its clinical workflow14; and (3) gray-scale images are easier to standardize because they are impervious to environmental variables that are difficult to control (eg, recent sun exposure, rosacea flare, lighting, sweating). These are some of the reasons we think radiology is, and will be, ahead in training AI algorithms and integrating them into clinical practice. However, even radiology AI studies have limitations, including a lack of prospective, real-world clinical setting, generalizable studies, and a lack of large standardized available databases for training algorithms.
Narrowing our discussion to studies of mammography—given the repetitive nature and binary output of this modality, which has made it one of the first targets of automation in diagnostic imaging1,2,5,13—AI-based CAD in mammography, much like its predecessor feature-based CAD, has shown promising results in artificial settings. Five key mammography CNN studies have reported a wide range of diagnostic accuracy (area under the curve, 69.2 to 97.8 [mean, 88.2]) compared to radiologists.15-19
In the most recent study (2019), Rodriguez-Ruiz et al15 compared machines and a cohort of 101 radiologists, in which AI showed performance comparability. However, results in this artificial setting were not followed up with prospective analysis of the technology in a clinical setting. First-generation, feature-based CADs in mammography also showed expert-level performance in artificial settings, but the technology became extinct because these results were not generalizable to real-world in prospective trials. To our knowledge, a limitation of radiology AI is that all current CNNs have not yet been tested in a live clinical setting.13-19
The second limitation of radiology AI is lack of standardization, which also applies to mammography, despite this subset having the largest and oldest publicly available data set. In a recent review of 23 studies on AI-based algorithms in mammography (2010-2019), clinicians point to one of the biggest flaws: the use of small, nonstandardized, and skewed public databases (often enriched for malignancy) as training algorithms.13
Standardization refers to quality-control measures in acquisition, processing, and image labeling that need to be met for images to be included in the training data set. At present, large stores of radiologic data that are standardized within each institution are not publicly accessible through a unified reference platform. Lack of large standardized training data sets leads to selection bias and increases the risk for overfitting, which occurs when algorithm models incorporate background noise in the data into its prediction scheme. Overfitting has been noted in several AI-based studies in mammography,13 which limits the generalizability of algorithm performance in the real-world setting.
To overcome this limitation, the American College of Radiology Data Science Institute recently took the lead on creating a reference platform for quality control and standardized data generation for AI integration in radiology. The goal of the institute is for radiologists to work collaboratively with industry to ensure that algorithms are trained on quality data that produces clinically useable output for the clinician and patient.11,20
Similar to initial radiology studies utilizing AI mainly as a screening tool, AI-driven studies in dermatology are focused on classification of melanocytic lesions; the goal is to aid in melanoma screening. Two of the most-recent, most-cited articles on this topic are by Esteva et al21 and Tschandl et al.22 Esteva et al21 matched the performance of 21 dermatologists in binary classification (malignant or nonmalignant) of clinical and dermoscopic images in pigmented and nonpigmented categories. A CNN developed by Google was trained on 130,000 clinical images encompassing more than 2000 dermatologist-labeled diagnoses from 18 sites. Despite promising results, the question remains whether these findings are transferrable to the clinical setting. In addition to the limitation on generalizability, the authors do not elaborate on standardization of training image data sets. For example, it is unclear what percentage of the training data set’s image labels were based on biopsy results vs clinical diagnosis.21
The second study was the largest Web-based study to compare the performance of more than 500 dermatologists worldwide.22 The top 3–performing algorithms (among a pool of 139) were at least as good as the performance of 27 expert dermatologists (defined as having more than 10 years’ experience) in the classification of pigmented lesions into 7 predefined categories.22 However, images came from nonstandardized sources gathered from a 20-year period at one European academic center and a private practice in Australia. Tschandl et al22 looked at external validation with an independent data set, outside the training data set. Although not generalizable to a real-world setting, looking at external data sets helps correct for overfitting and is a good first step in understanding transferability of results. However, the external data set was chosen by the authors and therefore might be tainted by selection bias. Although only a 10% drop in algorithmic accuracy was noted using the external data set chosen by the authors, this drop does not apply to other data sets or more importantly to a real-world setting.22
Current limitations and future goals of radiology also will most likely apply to dermatology AI research. In medicine and radiology, the goal of AI is to first help users by prioritizing what they should focus on. The concept of comparing AI to a radiologist or dermatologist is potentially shortsighted. Shortcomings of the current supervised or semisupervised algorithms used in medicine underscore the points that, first, to make their outputs clinically usable, it should be clinicians who procure and standardize training data sets and, second, it appears logical that the performance of these category of algorithms requires constant monitoring for bias. Therefore, these algorithms cannot operate as stand-alone diagnostic machines but as an aid to the clinician—if the performance of the algorithms is proved in large trials.
Near-Future Directions and Projections
Almost all recent state-of-the-art AI systems tested in medical disciplines fall under the engineering terminology of narrow or weak AI, meaning any given algorithm is trained to do only one specific task.9 An example of a task is classification of images into multiple categories (ie, benign or malignant). However, task classification only works with preselected images that will need substantial improvements in standardization.
Although it has been demonstrated that AI systems can excel at one task at a time, such as classification, better than a human cohort in simulated settings, these literal machines lack the ability to incorporate context; integrate various forms of sensory input such as visual, voice, or text; or make associations the way humans do.9 Multiple tasks and clinical context integration are required for predictive diagnosis or clinical decision-making, even in a simulated environment. In this sense, CNN is still similar to its antiquated linear CAD predecessor: It cannot make a diagnosis or a clinical decision but might be appropriate for triaging cases that are referred for evaluation by a dermatologist.
Medical AI also may use electronic health records or patient-gathered data (eg, apps). However, clinical images are more structured and less noisy and are more easily incorporated in AI training. Therefore, as we are already witnessing, earlier validation and adoption of AI will occur in image-based disciplines, beginning with radiology; then pathology; and eventually dermatology, which will be the most challenging of the 3 medical specialties to standardize.
Final Thoughts
Artificial intelligence in health care is in its infancy; specific task-driven algorithms are only beginning to be introduced. We project that in the next 5 to 10 years, clinicians will become increasingly involved in training and testing large-scale validation as well as monitoring narrow AI in clinical trials. Radiology has served as the pioneering area in medicine and is just beginning to utilize narrow AI to help specialists with very specific tasks. For example, a task would be to triage which scans to look at first for a radiologist or which pigmented lesion might need prompt evaluation by a dermatologist. Artificial intelligence in medicine is not replacing specialists or placing decision-making in the hands of a nonexpert. At this point, CNNs have not proven that they make us better at diagnosing because real-world clinical data are lacking, which may change in the future with large standardized training data sets and validation with prospective clinical trials. The near future for dermatology and pathology will follow what is already happening in radiology, with AI substantially increasing workflow efficiency by prioritizing tasks.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
- Kohli A, Jha S. Why CAD failed in mammography. J Am Coll Radiol. 2018;15:535-537.
- Gao Y, Geras KJ, Lewin AA, Moy L. New frontiers: an update on computer-aided diagnosis for breast imaging in the age of artificial intelligence. Am J Roentgenol. 2019;212:300-307.
- Ardila D, Kiraly AP, Bharadwaj S, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med. 2019;25:954-961.
- Le EPV, Wang Y, Huang Y, et al. Artificial intelligence in breast imaging. Clin Radiol. 2019;74:357-366.
- Houssami N, Lee CI, Buist DSM, et al. Artificial intelligence for breast cancer screening: opportunity or hype? Breast. 2017;36:31-33.
- Cukras AR. On the comparison of diagnosis and management of melanoma between dermatologists and MelaFind. JAMA Dermatol. 2013;149:622-623.
- Gutkowicz-Krusin D, Elbaum M, Jacobs A, et al. Precision of automatic measurements of pigmented skin lesion parameters with a MelaFindTM multispectral digital dermoscope. Melanoma Res. 2000;10:563-570.
- Dick V, Sinz C, Mittlböck M, et al. Accuracy of computer-aided diagnosis of melanoma: a meta-analysis [published online June 19, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1375.
- Hosny A, Parmar C, Quackenbush J, et al. Artificial intelligence in radiology. Nat Rev Cancer. 2018;18:500-510.
- Gyftopoulos S, Lin D, Knoll F, et al. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506-513.
- Chan S, Siegel EL. Will machine learning end the viability of radiology as a thriving medical specialty? Br J Radiol. 2019;92:20180416.
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep learning in radiology: does one size fit all? J Am Coll Radiol. 2018;15:521-526.
- Houssami N, Kirkpatrick-Jones G, Noguchi N, et al. Artificial Intelligence (AI) for the early detection of breast cancer: a scoping review to assess AI’s potential in breast screening practice. Expert Rev Med Devices. 2019;16:351-362.
- Pesapane F, Codari M, Sardanelli F. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp. 2018;2:35.
- Rodriguez-Ruiz A, Lång K, Gubern-Merida A, et al. Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. J Natl Cancer Inst. 2019;111:916-922.
- Becker AS, Mueller M, Stoffel E, et al. Classification of breast cancer in ultrasound imaging using a generic deep learning analysis software: a pilot study. Br J Radiol. 2018;91:20170576.
- Becker AS, Marcon M, Ghafoor S, et al. Deep learning in mammography: diagnostic accuracy of a multipurpose image analysis software in the detection of breast cancer. Invest Radiol. 2017;52:434-440.
- Kooi T, Litjens G, van Ginneken B, et al. Large scale deep learning for computer aided detection of mammographic lesions. Med Image Anal. 2017;35:303-312.
- Ayer T, Alagoz O, Chhatwal J, et al. Breast cancer risk estimation with artificial neural networks revisited: discrimination and calibration. Cancer. 2010;116:3310-3321.
- American College of Radiology Data Science Institute. Dataset directory. https://www.acrdsi.org/DSI-Services/Dataset-Directory. Accessed December 17, 2019.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Tschandl P, Codella N, Akay BN, et al. Comparison of the accuracy of human readers versus machine-learning algorithms for pigmented skin lesion classification: an open, web-based, international, diagnostic study. Lancet Oncol. 2019;20:938-947.
Practice Points
- The use of computer-assisted diagnosis in medicine dates back to the 1960s in radiology.
- New techniques in machine learning, also known as deep learning, were introduced around 2010. Compared to the predecessor forms of computing, these new methods are dynamically changing systems that improve with continuous data exposure and therefore performance is dependent on the quality and generalizability of the training data sets.
- Standardized large data sets and prospective real-life clinical trials are lacking in radiology and subsequently dermatology for diagnosis.
- Artificial intelligence is helpful with triaging and is improving workflow efficiency for radiologists by helping prioritize tasks, which is the current direction for dermatology.
Optimal Cosmetic Outcomes for Basal Cell Carcinoma: A Retrospective Study of Nonablative Laser Management
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
Nonablative laser therapy is emerging as an effective noninvasive treatment option for basal cell carcinoma (BCC) with reduced adverse effects and good cosmetic outcomes compared to surgery. Vascular lasers, such as the pulsed dye laser (PDL), are thought to work by selectively targeting the tumor’s vascular network while preserving normal surrounding tissue.1,2 Although high energy and multiple passes might be required, adjunctive use of dynamic cooling reduces the risk for nonselective thermal injury vs ablative lasers, which destroy the tumor itself through vaporization of tissue water.2
With no established laser management guidelines for the treatment of BCC, earlier studies using a 595-nm PDL varied highly in their protocol.3-8 Pulsed dye laser parameters ranged from a spot size of 7 to 10 mm, fluence of 7.5 to 15 J/cm2, and pulse duration of 0.5 to 3 milliseconds. Follow-up ranged from 12 days to 25 months after the final laser treatment. The number of lesions in prior studies ranged from 7 to 100 BCCs, with the clinical clearance rate ranging from 71.4% to 75% for facial BCC and 78.6% to 95% for nonfacial BCC.3-8 Studies with histologic confirmation had a clearance rate of 66.6% for facial BCC and 25% to 92.3% for nonfacial BCC.3-5,7,8 Most studies examined BCCs on the trunk and extremities with few investigating facial BCC,3-8 which is especially important given that the head and neck are the most common and cosmetically sensitive anatomic locations.9-13
Noninvasive imaging devices, such as reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) can assist with the diagnosis and treatment monitoring of BCC. These devices enable in vivo visualization of tissue in both cross-sectional and en face views and therefore can reduce the need for diagnostic biopsy. Reflectance confocal microscopy enables near-histologic visualization of the epidermis and superficial dermis with a resolution of 0.5 to 1 μm.14 Optical coherence tomography uses an infrared broadband light source that allows users to view skin architecture as deep as 1.5 to 2 mm with a resolution of 5 μm.15
When used synergistically, both devices can enhance the efficacy of nonablative laser treatment. With its increased depth and wider field of view, OCT is an optimal tool for repetitive evaluation of the same site over time and for following biopsy-confirmed tumors undergoing management.16 In addition to delineating tumor margins before treatment, imaging improves the detection of residual skin cancers, despite clearance on clinical and dermoscopic examination. Noninvasive imaging and nonsurgical management with laser therapy allow the physician to leave the skin intact and avoid scar tissue that might otherwise make it more difficult to detect and manage recurrence. The ability of OCT and RCM to monitor the efficacy of nonsurgical therapies for skin cancer has been demonstrated with imiquimod, photodynamic therapy, vismodegib, and ablative laser therapy.17-20
With limited data on nonablative laser management of BCC, several gaps in the literature exist. First, in previously published studies the number of treatments was either determined to be an arbitrary set number or based on clinical clearance, which has the potential to miss residual tumor. Second, many follow-ups were limited to shortly after the final treatment, which limits the accuracy of the clearance rate, given that inflammation and scars can hide residual tumor.21-23 Third, because many studies excised the treated area, long-term follow-up for recurrence was obscured. Last, only a few studies involved facial BCC, which is the most common and cosmetically concerning anatomic location.13
Our study attempted to address these gaps by evaluating the use of noninvasive imaging to guide management of primarily facial BCC. The objective was to perform a retrospective chart review on a subgroup of patients with BCC who were treated with combined nonablative PDL and fractional laser treatment with an extended follow-up period.
Methods
Study Design
We performed a retrospective chart review of 68 patients with 93 BCCs who had been treated with nonablative laser therapy as an alternative to surgery at the Mount Sinai Faculty Practice Associates between February 2011 and December 2018. Patients were followed throughout this period for assessment of clinical and subclinical recurrence. The Icahn School of Medicine at Mount Sinai Program for the Protection of Human Subjects provided institutional review board approval.
Patients
Inclusion criteria included the following: (1) BCC diagnosed by biopsy (see eTable 1 for subtypes) and (2) treated with a nonablative laser due to patient preference and eligibility by the principal investigator (PI). As a retrospective study, lesions were included irrespective of tumor subtype or size. Although the risk for perineural invasion (PNI) is extremely low with BCC (<0.2%), none of the cases demonstrated PNI on diagnostic biopsy and none exhibited clinical evidence of PNI, such as paresthesia, pain, facial paralysis, or diplopia.24
Eligibility determined by the PI included limited clinical ulceration or bleeding, or both, and a safe distance from the eye when wearing an external eye shield (ie, outside the orbital rim). Patients who had Mohs micrographic surgery (MMS) or excision (or both) with recurrence at the treatment site were included. Detailed and thorough clinical and dermoscopic skin examination was critical in early detection of these cancers, allowing for treatment of less advanced tumors. The PI’s diagnostic approach utilized the published diagnostic color wheel algorithm,25 which encompasses both clinical and dermoscopic colors and patterns for early diagnosis (ie, ulceration, pink-white to white shiny areas, absence of pigmented network, leaflike structures, large blue-gray ovoid nests or globular structures, spoke wheel structures, a crystalline pattern, a singular vascular pattern of arborizing vessels), combined with OCT or RCM, when necessary.26 All lesions were imaged with OCT prior to laser treatment to confirm residual tumor following biopsy.
Although postsurgical patients were included, lesions receiving concurrent or prior nonsurgical therapy, such as a topical immunomodulator or oral hedgehog inhibitor (eg, vismodegib), were excluded.
Treatment Protocol
All patients received thorough information about the treatment, treatment alternatives, and potential adverse effects and complications. Lesions were selected based on clinical and dermoscopic findings and were biopsy confirmed. Clinical and dermoscopic photographs were taken at every visit. A camera was used for clinical photographs and a dermatoscope was attached for all contact polarized dermoscopic images. All lesions were imaged with OCT prior to laser therapy to delineate tumor margins and to confirm residual disease following biopsy to preclude biopsy-mediated regression.
Laser treatment consisted of a 595-nm PDL followed by fractional laser treatment with the 1927-nm setting. The range of PDL settings was similar to published studies of PDL for BCC (spot size, 7–10 mm; fluence, 6–15 J/cm2; pulse duration, 0.45–3 milliseconds).3-8 The fractional laser also was used at settings similar to earlier studies for actinic keratosis (fluence, 5–20 mJ; treatment density, 40%–70%).27 Laser treatment was performed by 1 of 5 medically trained providers who were fellows supervised by the PI.
All tumors received 1 to 7 treatments (average, 2.89) at 1- to 2-month intervals. Treatment end point (complete clearance) was judged on the absence of skin cancer clinically, dermoscopically on OCT, or histologically by biopsy, or a combination of these modalities. Recurrence was defined as a new histologically confirmed BCC occurring in an area that was previously documented as clear. Patients returned for follow-up 1 to 2 months after the final treatment to monitor tumor clearance and subsequently every 6 to 12 months for tumor recurrence. Posttreatment care included application of a thick emollient, such as a petrolatum-based product, until the area completely healed.
Data Collection
Clinical photographs, dermoscopic photographs, OCT scans, RCM scans, and biopsy reports were reviewed for each patient, as applicable. All patients were given an unidentifiable number; no protected health information was recorded. Data recorded for each patient included age, tumor subtype and location, tumor size, classification of the tumor as primary or a recurrence, number of treatments, treatment duration, lesion clearance, and length of follow-up.
Results
Patient and Lesion Characteristics
Sixty-eight patients with 93 BCCs (77 facial; 16 nonfacial) were included. The median age of patients was 70 years (range, 31–91 years). All 93 BCCs demonstrated residual tumor on OCT after diagnostic biopsy. Four BCCs had been treated earlier with MMS and were biopsy-proven recurrences. Most BCCs were of the nodular subtype; however, sclerosing, superficial, pigmented, morpheaform, and infiltrative subtypes also were included (eTable 1). Eight BCCs were obtained at outside institutions with no subtype provided. Facial BCCs had a mean (SD) clinical and dermoscopic diameter of 6.75 (4.71) mm (range, 2–24 mm). Patients were followed for 2.53 months to 6.03 years (mean follow-up, 2.43 years) and assessed for clinical and subclinical recurrence.
Tumor Clearance
Most lesions were effectively treated, with 89 of 93 BCCs (95.70%) demonstrating complete tumor clearance. Complete tumor clearance following laser therapy was reported in 74 of 77 facial BCCs (96.10%) and 15 of 16 nonfacial BCCs (93.75%)(eTable 2). Successfully treated BCCs underwent an average of 2.88 laser treatments over a mean duration of 3.54 months (range, 1 week to 1.92 years). Four incomplete responders underwent an average of 3.25 laser treatments over a mean duration of 3.44 months (range, 1.13–6.87 months). Of the 4 lesions that did not clear, 2 were nodular, 1 was pigmented, and 1 was sclerosing.
Number of Treatments
When the clearance rate is divided into lesions that received 3 or fewer laser treatments and those that received more than 3 laser treatments, the following results were determined:
• Lesions receiving 3 or fewer treatments had a clearance rate of 96.05% (73/76) for all BCCs, 96.72% (59/61) for facial BCCs, and 93.33% (14/15) for nonfacial BCCs.
• Lesions receiving more than 3 laser treatments had a clearance rate of 94.12% (16/17) for all BCCs, 93.75% (15/16) for facial BCCs, and 100% (1/1) for nonfacial BCCs.
The relationship between facial BCC tumor diameter and number of treatments required for clearance had a positive correlation coefficient (Pearson r=0.319), indicating that larger BCCs required more laser treatments (eTable 3).
Tumor Recurrence
Four of 89 BCCs (4.49%)(4 of 74 facial BCCs [5.41%]) showed tumor recurrence following laser treatment, as assessed by OCT and dermoscopy. Of them, all were nodular BCCs. Prior to laser treatment, there were 4 additional patients each diagnosed with a recurrence from prior treatment with MMS; all were successfully treated with laser therapy without recurrence post–laser treatment (eFigure 1). Most of the recurrences from prior MMS required more than 3 laser treatments before clearing: 1 required 3 treatments, 2 required 4 treatments, and 1 required 6 treatments.
Of 93 lesions included in this study, 2 BCCs were deemed not clear on histologic analysis, which corresponded with residual tumor seen on OCT. Two additional lesions were determined to be not clear on OCT but were not confirmed as such on biopsy; both lesions were confirmed not clear, however, by histologic analysis on the first layer of MMS
Follow-up
All cleared lesions (89/93) showed complete clinical response to laser treatment for 6 months or more (median follow-up, 2–3 years; mode, 1–2 years; mean, 2.66 years)(eTable 4). Although 45% of patients (40/89) have been followed clinically and/or dermoscopically (as is done for MMS follow-ups) for 3 years to more than 5 years, only 20% of patients (18/89) were followed up with OCT in combination with clinical and/or dermoscopic examination between 3 years and more than 5 years. Follow-up took on a bimodal distribution, with a peak follow-up period at 1 to 2 years and again at 3 to 4 years. Half of the lesions (45/89) were followed up with OCT in combination with clinical and dermoscopic examination at 1 to 6 months (eTable 5). Of the 2 patients with 1-month OCT follow-up, 1 died from other medical causes and the other was unable to return for further follow-up scans.
Comment
High Tumor Clearance Rates With OCT
This study yielded a clearance rate of 95.70% for all BCCs, 96.10% for facial BCCs, and 93.75% for nonfacial BCCs. This rate is higher than the clinical or histologic clearance rate (or both) of earlier studies on facial and nonfacial BCCs, which ranged from 25% to 95%.8-11 In this study, we were able to utilize OCT and histology to confirm clearance. Optical coherence tomography, which has been shown to have a high sensitivity ranging from 86% to 95.7%, is therefore optimally used in treatment monitoring.19,26,28 Optical coherence tomography has a broader specificity range of 75.3% to 98% and was not utilized for diagnostic purposes in this study. Combining OCT with a color wheel dermoscopic approach was helpful in confirming treatment efficacy of nonsurgical therapies and is significantly more accurate than clinical analysis alone (P<.01).19,26,28
We suspect that the higher clearance rates observed in our study were due to the OCT-guided treatment protocol. Optical coherence tomography was used for margination while providing a modality for tailored treatment through visualization of residual tumor on clinically and dermoscopically clear follow-ups, given that several studies found residual tumor at the lateral edge of the tumor margin on histopathologic analysis.5 Utilizing noninvasive imaging technology to delineate tumor margins before treatment can improve efficacy and limit unnecessary treatment to the surrounding normal skin (eFigure 2).29
After grouping lesions by number of laser treatments, the clearance rate remained similar among facial BCCs with 3 or fewer treatments (59/61 [96.72%]), but there was a slightly decreased clearance rate for facial BCCs with more than 3 treatments (15/16 [93.75%]), which may be explained by the need for more laser treatments for larger BCCs (eTable 3). The relationship between facial BCC size and number of laser treatments was found to correlate positively (Pearson r=0.319). The largest lesion (24 mm) was successfully treated with 5 treatments (Figure). The number of nonfacial lesions was limited in this study and was not statistically significant.
there was no clinical evidence of residual BCC.
Cosmetic Outcome
Adverse effects, including erythema, purpura, blistering, and crusting, were short-term and well tolerated. Few patients had subsequent hypopigmentation in the initial months after treatment, which we consider an optimal cosmetic outcome. For example, the patient shown in the Figure would have required extensive reconstruction of the defect using bilateral rotation flaps with incisions along the hairline, grafting, or second-intention healing with partial closure to avoid brow-lifting.30 Given the relatively young age of this patient (a 45-year-old woman) and therefore limited skin laxity, secondary intention or even attempting to match grafted tissue could have resulted in a less than optimal cosmetic outcome. None of the patients experienced clinical or dermoscopic evidence of scarring from the laser treatment.
A few lesions were found to have subclinical inflammation on OCT, which might have obscured residual tumor on the 1-month follow-up scan. This condition may be similar to how pre-MMS diagnostic biopsy scars mask skin cancer during surgery, making it necessary to obtain additional layers beyond the biopsy scar tissue. This scar tissue would otherwise obscure tumor on histology during MMS, similar to subclinical inflammation obscuring residual tumor on OCT.21-23,31 Invasive and noninvasive management of skin cancers will have different healing times and therefore different optimal times to confirm clearance by histology compared to noninvasive imaging. All of the lesions in which inflammation was obscured on OCT 1-month posttreatment remained cleared. However, 1 lesion was found to be clear at a 4-week clearance scan after only 2 nonablative laser treatments and was confirmed as scar tissue on histology. Scar tissue on histology might have obscured any residual tumor. The patient appeared clinically and dermoscopically to have a milia in the same location only 5 months later; however, on OCT and histology, the lesion was confirmed to be a BCC.
Treatment Intervals
Several other studies either used a set number of treatments or determined the number of treatments based on clinical clearance.3-8 When determining the best treatment interval, we considered the period for patients to be clinically and dermoscopically healed to be 1 month. Patients came for their final follow-up scan an additional month after the final treatment in case there was any obscuring inflammation on OCT at 1 month. Given that patients responded well to nonablative laser treatment once skin clinically healed and most patients required 3 treatments, the PI began recommending a total of 3 treatments performed 4 to 6 weeks apart in clinical practice, followed by a final clearance scan 2 months after the third treatment. A period of 2 months was considered ideal for the final clearance scan because no inflammation was seen at the 2-month follow-up in the group of patients who had inflammation at the 1-month follow-up on OCT in our study. Some patients had an extended treatment duration because of noncompliance with the 4- to 6-week follow-up regimen. Although this extension of treatment duration potentially skews the clearance rate, we still included these patients, given the retrospective design of this study.
Lesions That Did Not Clear
Four BCCs did not clear, 3 of which were facial BCCs. All 4 lesions demonstrated residual tumor on OCT. Of the 3 facial lesions that did not clear:
• One was the patient who had obscuring inflammation at the 1-month follow-up and only scar tissue on histologic confirmation.
• Another was a pigmented BCC on the right cheek of a patient with Fitzpatrick skin type IV. This patient received 3 treatments without a response clinically or on OCT. (Most patients who showed complete clearance also showed reduction in tumor size after the first laser treatment. Of note, there were other patients who had lighter skin types with pigmented BCCs and all of these patients had complete response to this treatment regimen; therefore, we do not think that a pigmented BCC is an exclusion to this therapy.)
• The third was a BCC on the nose of a nonadherent patient, which may have contributed to the lack of clearance. We defined nonadherent patients as those who did not follow-up within the appropriate periods and who therefore ran the risk for tumor growth in between treatments.
The nonfacial BCC that did not clear had histologic features of focal sclerosing BCC, a more aggressive subtype of basal cell skin cancer.
Tumor Recurrence
Only 4 of 89 BCCs (4.49%) recurred, with a 5.41% (4/74) recurrence rate among facial BCCs. All recurrences lacked clinical and dermoscopic evidence of BCC but were found on follow-up OCT scan and confirmed with RCM. All recurrences were found 1.5 to 3.9 years posttreatment.
Recurrent tumors following MMS required, on average, more laser treatments than primary tumors to achieve successful tumor clearance, which we attribute to scar tissue from prior therapy obscuring recurrence, resulting in delayed diagnosis, and to inflammation and fibrosis masking residual tumors (eFigure 1). An added benefit of laser treatment is that all 4 recurrent tumors demonstrated improved cosmetic appearance of the original MMS scar.
The benefit of using OCT scans to check for recurrences is that OCT can find residual skin cancers despite the area looking clinically clear, which is especially important during clinical evaluation of a healed postsurgical scar for recurrence because OCT imaging allows us to look as deep as 2 mm under the skin. Nonsurgical treatments also enable us to leave skin intact and avoid creating scar tissue, which makes it easier to detect and manage recurrence.
Limitations
There were several important limitations of this retrospective study:
• Patients were treated by 1 of 5 medically trained fellows. Although the fellows worked under the supervision of the PI, variation in their work from one to another might have led to different end points.
• All patients who appeared clinically clear were offered biopsy to confirm clearance on histology. Some patients agreed to biopsy, but many did not because they were pleased with the cosmetic outcome, which is similar to other studies exhibiting only clinical clearance rates without providing histologic clearance following nonsurgical therapy.6 We believe that imaging with OCT circumvents this problem and offers more accurate confirmation than clinical or dermoscopic correlation alone, or the combination of the 2 modalities.
• Lack of treatment standardization and short length of follow-up can result in underestimation of the recurrence rate. In particular, most patients were followed up with OCT in less than 6 months. These are unavoidable features in a retrospective study and we are currently addressing this problem in a new prospective study.
Extended Follow-up
Although this study is not a prospective design, it does provide recurrence data over extended follow-up for the nonablative laser management of BCCs (eTables 4 and 5). Studies have demonstrated that MMS has a 5-year cure rate as high as 99% for BCC.32 Given the limited follow-up period of prior nonablative laser management studies, recurrences might not have been fully evaluated. Our study had a 4.49% recurrence rate for all BCCs and a 5.41% recurrence rate for facial BCCs but was not detectable by clinical examination combined with dermoscopic findings alone. All recurrences required the utilization of OCT or RCM or a combination of these modalities to be diagnosed. In 1 patient with recurrence, we were able to see residual tumor on both OCT and RCM without any inflammation obscuring the scan, given that 3 years had passed. Although 2 months is an optimal follow-up time for OCT, we have not found an optimal follow-up time for RCM, which is another reason why OCT might be preferable to other imaging modalities, such as RCM and high-definition OCT, that have higher resolution but provide less depth on imaging. Although only 40 of 89 patients (4.49%) had follow-up ranging from 3 years to greater than 5 years, long-term follow-up to date has been limited in prior studies.
We believe the high clearance rates and limited recurrence are secondary to the utilization of noninvasive imaging, as the majority of these recurrences would not have been diagnosed based on clinical and/or dermoscopic information alone. Additionally, the 4 biopsy-proven post-MMS recurrence patients that were treated in this study also may not have been diagnosed this early without the use of additional noninvasive imaging. In our opinion, although laser management can be used without noninvasive imaging guidance—dermoscopy, OCT, and/or RCM—this technology is critical not only for early detection but also for proper management of patients.
Conclusion
This study showed a 95.70% clearance rate for all BCCs and a 96.10% clearance rate for facial BCCs. Although we had a zero clinical recurrence rate, 4.49% of all BCCs and 5.41% of facial BCCs had recurred on subsequent monitoring with noninvasive imaging. Given the large size of the study and extended follow-up, we found nonablative laser management to be a reliable treatment alternative with improved cosmetic outcome (Figure) and minimal short-term adverse effects compared to surgery.
Tailored care for the individual patient is based on a variety of options and patient preference, including ease of compliance, number of follow-up visits, invasive vs noninvasive diagnosis and monitoring, and downtime for healing. The use of noninvasive imaging also allowed us to find a more standardized treatment regimen using this nonablative laser combination. We found that 3 or fewer and more than 3 treatments had similar efficacy in tumor clearance. We recommend a standard laser protocol of 3 treatments every 4 to 6 weeks with follow-up 2 months after the final treatment to assess for clearance with OCT.
Larger BCCs might require additional treatments; therefore, we caution against laser therapy without concomitant use of OCT imaging to visualize residual tumor. Utilizing other noninvasive modalities, such as dermoscopy, in combination with thorough skin examination also is critical in the early detection of skin cancers to improve the efficacy of this less-aggressive, nonablative, and cosmetically optimal treatment protocol.
Acknowledgement—We would like to acknowledge Dimitrios Karponis, BSc, from the Impirial College London, England, for his assistance with a portion of the statistical analysis.
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
- Campolmi P, Troiano M, Bonan P, et al. Vascular based non conventional dye laser treatment for basal cell carcinoma. Dermatol Ther. 2008;21:402-405.
- Soleymani T, Abrouk M, Kelly KM. An analysis of laser therapy for the treatment of nonmelanoma skin cancer. Dermatol Surg. 2017;43:615-624.
- Alonso-Castro L, Ríos-Buceta L, Boixeda P, et al. The effect of pulsed dye laser on high-risk basal cell carcinomas with response control by Mohs micrographic surgery. Lasers Med Sci. 2015;30:2009-2014.
- Karsai S, Friedl H, Buhck H, et al. The role of the 595-nm pulsed dye laser in treating superficial basal cell carcinoma: outcome of a double-blind randomized placebo-controlled trial. Br J Dermatol. 2015;172:677-683.
- Konnikov N, Avram M, Jarell A, et al. Pulsed dye laser as a novel non-surgical treatment for basal cell carcinomas: response and follow up 12-21 months after treatment. Lasers Surg Med. 2011;43:72-78.
- Minars N, Blyumin-Karasik M. Treatment of basal cell carcinomas with pulsed dye laser: a case series. J Skin Cancer. 2012;2012:286480.
- Shah SM, Konnikov N, Duncan LM, et al. The effect of 595 nm pulsed dye laser on superficial and nodular basal cell carcinomas. Lasers Surg Med. 2009;41:417-422.
- Tran HT, Lee RA, Oganesyan G, et al. Single treatment of non-melanoma skin cancers using a pulsed-dye laser with stacked pulses. Lasers Surg Med. 2012;44:459-467.
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317.
- Silverman MK, Kopf AW, Bart RS, et al. Recurrence rates of treated basal cell carcinomas. part 3: surgical excision. J Dermatol Surg Oncol. 1992;18:471-476.
- Silverman MK, Kopf AW, Grin CM, et al. Recurrence rates of treated basal cell carcinomas. part 2: curettage-electrodesiccation. J Dermatol Surg Oncol. 1991;17:720-726.
- Dubin N, Kopf AW. Multivariate risk score for recurrence of cutaneous basal cell carcinomas. Arch Dermatol. 1983;119:373-377.
- Subramaniam P, Olsen CM, Thompson BS, et al. Anatomical distributions of basal cell carcinoma and squamous cell carcinoma in a population-based study in Queensland, Australia. JAMA Dermatol. 2017;153:175-182.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast.J Invest Dermatol. 1995;104:946-952.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Sattler E, Kästle R, Welzel J. Optical coherence tomography in dermatology. J Biomed Opt. 2013;18:061224.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Ski Res Technol. 2014;20:170-176.
- Segura S, Puig S, Carrera C, et al. Non-invasive management of non-melanoma skin cancer in patients with cancer predisposition genodermatosis: a role for confocal microscopy and photodynamic therapy. J Eur Acad Dermatol Venereol. 2011;25:819-827.
- Ulrich M, Lange-Asschenfeldt S, Gonzalez S. The use of reflectance confocal microscopy for monitoring response to therapy of skin malignancies. Dermatol Pract Concept. 2012;2:43-52.
- Couzan C, Cinotti E, Labeille B, et al. Reflectance confocal microscopy identification of subclinical basal cell carcinomas during and after vismodegib treatment. J Eur Acad Dermatol Venereol. 2018;32:763-767.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Wagner RF Jr, Cottel WI. Multifocal recurrent basal cell carcinoma following primary tumor treatment by electrodesiccation and curettage. J Am Acad Dermatol. 1987;17:1047-1049.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. Dermatol Surg. 2012;38:1582-1603.
- Lewin JM, Carucci JA. Advances in the management of basal cell carcinoma. F1000Prime Rep. 2015;7:53.
- Markowitz O. A Practical Guide to Dermoscopy. Philadelphia, PA: Wolters Kluwer; 2017.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal cell carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Weiss ET, Brauer JA, Anolik R, et al. 1927-nm fractional resurfacing of facial actinic keratoses: a promising new therapeutic option. J Am Acad Dermatol. 2013;68:98-102.
- Olsen J, Themstrup L, De Carvalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Levine A, Siegel D, Markowitz O. Imaging in cutaneous surgery. Future Oncol. 2017;13:2329-2340.
- Gross K, Steinman H, Rapini R. Mohs Surgery: Fundamentals and Techniques. St. Louis, MO: Mosby; 1998.
- Suzuki HS, Serafini SZ, Sato MS. Utility of dermoscopy for demarcation of surgical margins in Mohs micrographic surgery. An Bras Dermatol. 2014;89:38-43.
- Rowe DE, Carroll RJ, Day CL Jr. Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424-431
Practice Points
- A major benefit of nonablative laser therapy over more invasive options in the management of basal cell carcinoma (BCC) is minimal scarring.
- When patients are managed with nonablative laser therapy, follow-up with clinical, dermoscopic, and/or noninvasive imaging is more efficient during treatment as well as when assessing for recurrences.
- Optical coherence tomography in combination with nonablative laser therapy allows for detection of residual skin cancers that would not be evident on clinical and/or dermoscopic examination.
- Utilizing early detection techniques, such as a color wheel dermoscopic approach, along with other noninvasive imaging modalities facilitates the use of less invasive treatment options for primary and/or recurrent BCCs.
New Diagnostic Procedure Codes and Reimbursement
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.
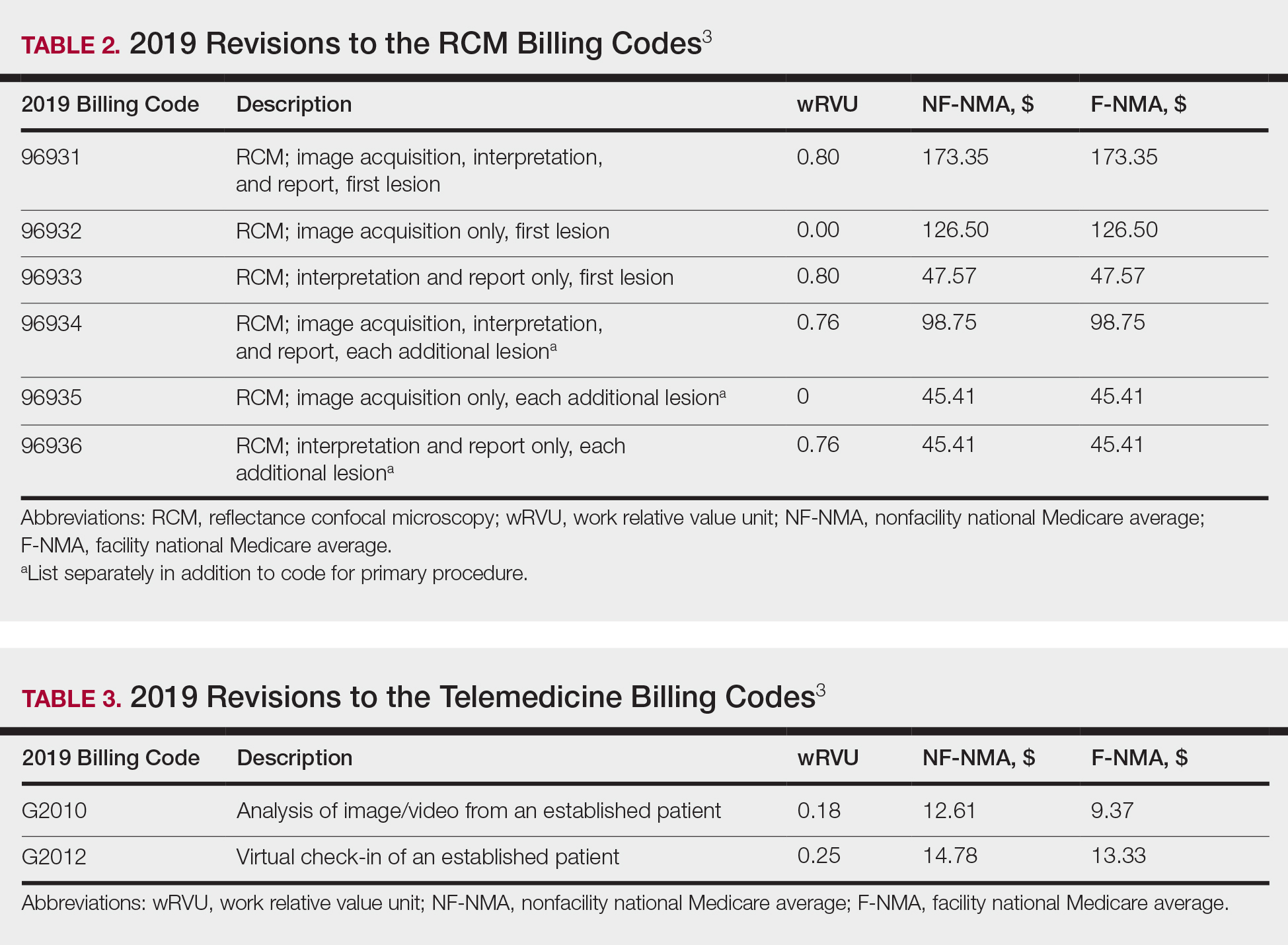
Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.

Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
As the US population continues to grow and patients become more aware of their health needs, payers are beginning to recognize the benefits of more efficient and cost-effective health care. With the implementation of the new Medicare Physician Fee Schedule on January 1, 2019, some old billing codes were revalued while others were replaced entirely with new codes.1 The restructuring of the standard biopsy codes now takes the complexity of different sampling techniques into consideration. Furthermore, Current Procedural Terminology (CPT) Category III tracking codes for some imaging devices (eg, optical coherence tomography) added in 2017 require more data before obtaining a Category I reimbursable code, while codes for other imaging devices such as reflectance confocal microscopy (RCM) remain relatively the same.2-4 Notably, the majority of the new 2019 telemedicine codes are applicable to dermatology.2,3 In this article, we discuss the new CPT codes for reporting diagnostic procedures, including biopsy, noninvasive imaging, and telemedicine services. We also provide a summary of the national average reimbursement rates for these procedures.
Background on Reimbursement
To better understand how reimbursement works, it is important to know that all billing codes are provided a relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.5 The total RVU consists of the work RVU (wRVU), practice expense RVU (peRVU), and malpractice expense RVU (mRVU). The wRVU represents the time, effort, and complexity involved in performing the service. The peRVU reflects the direct cost of supplies, personnel, and durable equipment involved in providing the service, excluding typical office overhead costs such as rent, utilities, and administrative staff. The mRVU is to cover the cost of malpractice insurance.5 The peRVU can be further specified as facility versus nonfacility services depending on where the service is performed.6 A facility peRVU is for services completed in a facility such as a hospital, outpatient hospital setting, or nursing home. The facility provides some of the involved supplies, personnel, and equipment for which they can recapture costs by separate reporting, resulting in a lower total RVU for the provider charges compared with nonfacility locations where the physician must provide these items.6 Many physicians may not be aware of how critical their role is in determining their own reimbursement rates by understanding RVUs and properly filling out Relative Value Scale Update Committee (RUC) surveys. If surveys sent to practitioners are accurately completed, RVUs have the potential to be fairly valued; however, if respondents are unaware of all of the components that are inherent to a procedure, they may end up minimizing the effort or time involved, which would skew the results and hurt those who perform the procedure. Rather than inputting appropriate preoperative and postoperative service times, many respondents often put 0s and 1s throughout the survey, which misrepresents the amount of time involved for a procedure. For example, inputting a preoperative time as 0 or 1 minute may severely underestimate the work involved for a procedure if the true preoperative time is 5 minutes. Such survey responses affect whether or not RVUs are valued appropriately.
The billing codes and their RVUs as well as Medicare payment values in your area can be found on the Centers for Medicare & Medicaid Services website.2,3 Table 1 provides a comparison of the old and new biopsy codes, and Table 2 shows the new RCM codes.

Biopsy Codes
Prior to 2019, biopsies were reimbursed using CPT code 11100 for the initial biopsy and 11101 for each additional biopsy.2 Called up for refinement in the RUC process, initial data from the Physician Practice Expense Information Survey pointed to the likelihood of different sampling techniques having different amounts of work being supplied by different techniques.1 Imaging modalities such as dermoscopy or RCM could help minimize the need for surgical biopsies. Dermoscopy, which has been proven to allow for more efficient and accurate diagnoses in dermatology, is reimbursed in Europe but not in the United States.7-9 In 2016, CPT codes 96931 through 96936 were created for RCM and are covered by most insurances.10 Optical coherence tomography, another noninvasive imaging technology, currently is not reimbursed but did receive Category III codes (0470T-0471T), also known as a tracking codes, in 2017.4 Category III codes are used for emerging technologies that have future potential but do not have enough US-based evidence to support receiving Category I CPT codes. The use of Category III codes allows for data collection on emerging technologies and services, with the potential to convert the Category III codes to Category I codes once certain criteria are met.11
Beginning in 2019, the standard biopsy codes 11100 and 11101 were replaced with 6 new codes to represent primary (11102, 11104, 11106) and add-on biopsies (11103, 11105, 11107) based on the sampling technique utilized and the thickness of the sample (Table 1). Previously, the biopsy codes did not reflect the complexity of the different biopsy techniques, whereas the new codes provide differentiation of the method of removal (ie, tangential, punch, incisional).2,3 The base code is dependent on whichever biopsy performed has the highest complexity, with incisional biopsy--a partial excision--being considered the most complex.3 Punch biopsy is considered the next level of complexity, followed by tangential biopsy. Each of the 6 new biopsy codes also received a new wRVU, which determines reimbursement under Medicare and most other insurers when combined with direct peRVU and mRVU. Additional biopsies, reported using the add-on codes, are reimbursed at a lower level than the base codes because of removal of duplicate inputs for preservice and postservice care.3
Telehealth Codes
Telemedicine services offer another form of imaging that providers can use to communicate remotely with patients through a live interactive video stream (with audio), a store-and-forward system with photographs or videos shared asynchronously, or remote patient monitoring.12 Although live video streaming uses a webcam, store-and-forward services involve sending photographs or videos electronically for later evaluation.12,13 Remote patient monitoring allows the collection of health-related data and transmission to a physician without the need for an office visit.13 Most states require physicians to have a license in the state in which the patient is located at the time of the encounter. Given the difficulty of applying for licensure in multiple states, several states started creating their own special licenses to allow out-of-state providers to offer services through telemedicine.14 The Federation of State Medical Boards then created the Interstate Medical Licensure Compact (IMLC) for an expedited process to apply for medical licensure in other states. The IMLC was formed to increase access to health care in underserved or rural areas including but not limited to the use of telemedicine.15 To qualify for IMLC, a physician must have a medical license in a state registered with the IMLC (ie, state of principal license) and have at least one of the following in their state of principal license: primary residence, 25% of their medical practice, a current employer, or US federal income taxes filed.15 The remaining states that do not have a licensing process for telemedicine allow practice in contiguous states or may provide temporary licenses dependent on the situation.14
Since 2017, billing codes for telemedicine have been the same as those used for in-person evaluation and management services with modifiers -95 or GQ added to the end of the code. Modifier -95 has been used for real-time telemedicine services, while modifier GQ has been used for store-and-forward services.16 For example, the code 99201, which is used to bill for new patients at outpatient visits, would become 99201-95 if performed using a live audio and video feed or 99201-GQ if information was sent electronically for later analysis. To receive reimbursement from Medicare, modifier -95 requires real-time communication using both audio and video; however, modifier GQ is only reimbursable in federal telemedicine demonstration programs in Alaska or Hawaii.12 Note that reimbursement is up to the discretion of private providers, and even Medicare reimbursement can vary from state to state.
In 2019, new Healthcare Common Procedure Coding System telemedicine codes were introduced to include virtual check-ins (G2012) and evaluation of patient-transmitted images and videos (G2010). G2010 is the first store-and-forward code that has the potential to be reimbursed outside of Alaska or Hawaii.3,12 G2012 allows providers to monitor the patients' well-being outside of the office setting, a cost-effective alternative if patients do not require a full visit. More detailed descriptions of the new codes can be found in Table 3.1
Final Thoughts
As insurance providers continue to better monitor health care costs, it is of utmost importance that physicians become more involved in accurately assessing their services and procedures, given that the changes in RVUs mirror the Centers for Medicare & Medicaid Services' utilization of the RUC's interpretation of our survey responses.1 The current billing codes attempt to better represent the work involved for each service, one example being the modification to more specific biopsy codes in 2019.
With the growth of technology, CPT and Healthcare Common Procedure Coding System codes also reflect a push toward more efficient health care delivery and broader coverage for provider services, as demonstrated by the introduction of new telemedicine codes as well as recent additions of noninvasive imaging codes. Although technology makes health care more cost-effective for patients, clinicians can still maintain their overall reimbursements by efficiently seeing an increasing number of patients; for example, a patient diagnosed noninvasively using RCM can then receive same-day care, which impacts patients' quality of life by minimizing travel time, number of office visits, and time taken off from work, while allowing providers to manage a higher patient volume more productively. The new CPT codes discussed here reflect the growth of medical technology potential, which increases our diagnostic capability, making it even more critical for physicians to engage with these developments.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
- Centers for Medicare & Medicaid Services. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule and Other Revisions to Part B for CY 2019; Medicare Shared Savings Program Requirements; Quality Payment Program; Medicaid Promoting Interoperability Program; Quality Payment Program--Extreme and Uncontrollable Circumstance Policy for the 2019 MIPS Payment Year; Provisions From the Medicare Shared Savings Program-- Accountable Care Organizations--Pathways to Success; and Expanding the Use of Telehealth Services for the Treatment of Opioid Use Disorder Under the Substance Use-Disorder Prevention That Promotes Opioid Recovery and Treatment (SUPPORT) for Patients and Communities Act. Fed Registr. 2018;83(226):59452-60303. To be codified at 42 CFR §405, 410, 411, 414, 415, 425, and 495.
- Centers for Medicare & Medicaid Services. CY 2018 PFS Final Rule Addenda. https://www.cms.gov/Medicare/Medicare-Fee-for-Service Payment/PhysicianFeeSched/Downloads/CY2018-PFS-FR-Addenda.zip. Published 2018. Accessed March 28, 2019.
- Overview: Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed March 28, 2019.
- Medicare Learning Network. July 2017 update of the hospital outpatient prospective payment system (OPPS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/MM10122.pdf. Published 2017. Accessed March 21, 2019.
- Medicare Learning Network. Medicare Physician Fee Schedule. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/medcrephysfeeschedfctsht.pdf. Published February 2017. Accessed March 19, 2019.
- Medicare Learning Network. How to use the searchable Medicare Physician Fee Schedule (MPFS). Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/How_to_MPFS_Booklet_ICN901344.pdf. Published September 2017. Accessed March 19, 2019.
- Fox GN. Dermoscopy: an invaluable tool for evaluating skin lesions. Am Fam Physician. 2008;78:704, 706.
- Soyer HP, Argenziano G, Talamini R, et al. Is dermoscopy useful for the diagnosis of melanoma? Arch Dermatol. 2001;137:1361-1363.
- Kornek T, Schäfer I, Reusch M, et al. Routine skin cancer screening in Germany: four years of experience from the dermatologists' perspective. Dermatology. 2012;225:289-293.
- American Academy of Dermatology Association. New CPT coding updates for 2016. Derm Coding Consult. 2015;19:1-2. https://www.aad.org/File Library/Main navigation/Member resources and programs/Publications/DCC/DCC_Winter_2015.pdf. Published 2014. Accessed March 21, 2019.
- American Medical Association. CPT Category III codes. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/physicians/cpt/cpt-category3-codes-long-descriptors.pdf. Updated July 26, 2018. Accessed March 21, 2019.
- Medicare Learning Network. Telehealth services. Centers for Medicare & Medicaid Services website. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/TelehealthSrvcsfctsht.pdf. Accessed March 19, 2019.
- Final policy, payment, and quality provisions in the Medicare Physician Fee Schedule for calendar year 2018. Centers for Medicare & Medicaid Services website. https://www.cms.gov/newsroom/fact-sheets/final-policy-payment-and-quality-provisions-medicare-physician-fee-schedule-calendar-year-2018. Published November 2, 2017. Accessed March 19, 2019.
- State Telehealth Laws & Reimbursement Policies. Sacramento, CA: Center for Connected Health Policy; 2018. https://www.cchpca.org/sites/default/files/2018-10/CCHP_50_State_Report_Fall_2018.pdf. Accessed March 19, 2019.
- The IMLC. Interstate Medical Licensure Compact website. https://imlcc.org/. Accessed March 19, 2019.
- Current Procedural Terminology 2018, Professional Edition. Chicago, IL: American Medical Association; 2018.
PRACTICE POINTS
- Reimbursement typically is proportional to the relative value unit (RVU), a number representing the value of the work involved and cost of providing a service relative to other services.
- The total RVU consists of the work RVU, practice expense RVU, and malpractice expense RVU.
- The new 2019 biopsy codes reflect the complexity of the sampling technique (ie, whether the biopsy is tangential, punch, or incisional).
- Accurate completion of Relative Value Scale Update Committee surveys sent to practitioners will allow RVUs to be valued appropriately.
Mobile App Rankings in Dermatology
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).
Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).
Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
As technology continues to advance, so too does its accessibility to the general population. In 2013, 56% of Americans owned a smartphone versus 77% in 2017.1With the increase in mobile applications (apps) available, it is no surprise that the market has extended into the medical field, with dermatology being no exception.2 The majority of dermatology apps can be classified as teledermatology apps, followed by self-surveillance, disease guide, and reference apps. Additional types of dermatology apps include dermoscopy, conference, education, photograph storage and sharing, and journal apps, and others.2 In this study, we examined Apple App Store rankings to determine the types of dermatology apps that are most popular among patients and physicians.
METHODS
A popular app rankings analyzer (App Annie) was used to search for dermatology apps along with their App Store rankings.3 Although iOS is not the most popular mobile device operating system, we chose to evaluate app rankings via the App Store because iPhones are the top-selling individual phones of any kind in the United States.4
We performed our analysis on a single day (July 14, 2018) given that app rankings can change daily. We incorporated the following keywords, which were commonly used in other dermatology app studies: dermatology, psoriasis, rosacea, acne, skin cancer, melanoma, eczema, and teledermatology. The category ranking was defined as the rank of a free or paid app in the App Store’s top charts for the selected country (United States), market (Apple), and device (iPhone) within their app category (Medical). Inclusion criteria required a ranking in the top 1500 Medical apps and being categorized in the App Store as a Medical app. Exclusion criteria included apps that focused on cosmetics, private practice, direct advertisements, photograph editing, or claims to cure skin disease, as well as non–English-language apps. The App Store descriptions were assessed to determine the type of each app (eg, teledermatology, disease guide) and target audience (patient, physician, or both).
Another search was performed using the same keywords but within the Health and Fitness category to capture potentially more highly ranked apps among patients. We also conducted separate searches within the Medical category using the keywords billing, coding, and ICD (International Classification of Diseases) to evaluate rankings for billing/coding apps, as well as EMR and electronic medical records for electronic medical record (EMR) apps.
RESULTS
The initial search yielded 851 results, which was narrowed down to 29 apps after applying the exclusion criteria. Of note, prior to application of the exclusion criteria, one dermatology app that was considered to be a direct advertisement app claiming to cure acne was ranked fourth of 1500 apps in the Medical category. However, the majority of the search results were excluded because they were not popular enough to be ranked among the top 1500 apps. There were more ranked dermatology apps in the Medical category targeting patients than physicians; 18 of 29 (62%) qualifying apps targeted patients and 11 (38%) targeted physicians (Tables 1 and 2). No apps targeted both groups. The most common type of ranked app targeting patients was self-surveillance (11/18), and the most common type targeting physicians was reference (8/11). The highest ranked app targeting patients was a teledermatology app with a ranking of 184, and the highest ranked app targeting physicians was educational, ranked 353. The least common type of ranked apps targeting patients were “other” (2/18 [11%]; 1 prescription and 1 UV monitor app) and conference (1/18 [6%]). The least common type of ranked apps targeting physicians were education (2/11 [18%]) and dermoscopy (1/11 [9%]).
Our search of the Health and Fitness category yielded 6 apps, all targeting patients; 3 (50%) were self-surveillance apps, and 3 (50%) were classified as other (2 UV monitors and a conferencing app for cancer emotional support)(Table 3).
Our search of the Medical category for billing/coding and EMR apps yielded 232 and 164 apps, respectively; of them, 49 (21%) and 54 (33%) apps were ranked. These apps did not overlap with the dermatology-related search criteria; thus, we were not able to ascertain how many of these apps were used specifically by health care providers in dermatology.
COMMENT
Patient Apps
The most common apps used by patients are fitness and nutrition tracker apps categorized as Health and Fitness5,6; however, the majority of ranked dermatology apps are categorized as Medical per our findings. In a study of 557 dermatology patients, it was found that among the health-related apps they used, the most common apps after fitness/nutrition were references, followed by patient portals, self-surveillance, and emotional assistance apps.6 Our search was consistent with these findings, suggesting that the most desired dermatology apps by patients are those that allow them to be proactive with their health. It is no surprise that the top-ranked app targeting patients was a teledermatology app, followed by multiple self-surveillance apps. The highest ranked self-surveillance app in the Health and Fitness category focused on monitoring the effects of nutrition on symptoms of diseases including skin disorders, while the highest ranked (as well as the majority of) self-surveillance apps in the Medical category encompassed mole monitoring and cancer risk calculators.
Benefits of the ranked dermatology apps in the Medical and Health and Fitness categories targeting patients include more immediate access to health care and education. Despite this popularity among patients, Masud et al7 demonstrated that only 20.5% (9/44) of dermatology apps targeting patients may be reliable resources based on a rubric created by the investigators. Overall, there remains a research gap for a standardized scientific approach to evaluating app validity and reliability.
Teledermatology
Teledermatology apps are the most common dermatology apps,2 allowing for remote evaluation of patients through either live consultations or transmittance of medical information for later review by board-certified physicians.8 Features common to many teledermatology apps include accessibility on Android (Google Inc) and iOS as well as a web version. Security and Health Insurance Portability and Accountability Act compliance is especially important and is enforced through user authentications, data encryption, and automatic logout features. Data is not stored locally and is secured on a private server with backup. Referring providers and consultants often can communicate within the app. Insurance providers also may cover teledermatology services, and if not, the out-of-pocket costs often are affordable.
The highest-ranked patient app (ranked 184 in the Medical category) was a teledermatology app that did not meet the American Telemedicine Association standards for teledermatology apps.9 The popularity of this app among patients may have been attributable to multiple ease-of-use and turnaround time features. The user interface was simplistic, and the design was appealing to the eye. The entry field options were minimal to avoid confusion. The turnaround time to receive a diagnosis depended on 1 of 3 options, including a more rapid response for an increased cost. Ease of use was the highlight of this app at the cost of accuracy, as the limited amount of information that users were required to provide physicians compromised diagnostic accuracy in this app.
For comparison, we chose a nonranked (and thus less frequently used) teledermatology app that had previously undergone scientific evaluation using 13 evaluation criteria specific to teledermatology.10 The app also met the American Telemedicine Association standard for teledermatology apps.9 The app was originally a broader telemedicine app but featured a section specific to teledermatology. The user interface was simple but professional, almost resembling an EMR. The input fields included a comprehensive history that permitted a better evaluation of a lesion but might be tedious for users. This app boasted professionalism and accuracy, but from a user standpoint, it may have been too time-consuming.
Striking a balance between ensuring proper care versus appealing to patients is a difficult but important task. Based on this study, it appears that popular patient apps may in fact have less scientific rationale and therefore potentially less accuracy.
Self-surveillance
Although self-surveillance apps did not account for the highest-ranked app, they were the most frequently ranked app type in our study. Most of the ranked self-surveillance apps in the Medical category were for monitoring lesions over time to assess for changes. These apps help users take photographs that are well organized in a single, easy-to-find location. Some apps were risk calculators that assessed the risk for malignancies using a questionnaire. The majority of these self-surveillance apps were specific to skin cancer detection. Of note, one of the ranked self-surveillance apps assessed drug effectiveness by monitoring clinical appearance and symptoms. The lowest ranked self-surveillance app in the top 1500 ranked Medical apps in our search monitored cancer symptoms not specific to dermatology. Although this app had a low ranking (1380/1500), it received a high number of reviews and was well rated at 4.8 out of 5 stars; therefore, it seemed more helpful than the other higher-ranked apps targeting patients, which had higher rankings but minimal to no reviews or ratings. A comparison of the ease-of-use features of all the ranked patient-targeted self-surveillance apps in the Medical category is provided in Table 4.
Physician Apps
After examining the results of apps targeting physicians, we realized that the data may be accurate but may not be as representative of all currently practicing dermatology providers. Given the increased usage of apps among younger age groups,11 our data may be skewed toward medical students and residents, supported by the fact that the top-ranked physician app in our study was an education app and the majority were reference apps. Future studies are needed to reexamine app ranking as this age group transitions from entry-level health care providers in the next 5 to 10 years. These findings also suggest less frequent app use among more veteran health care providers within our specific search parameters. Therefore, we decided to do subsequent searches for available billing/coding and EMR apps, which were many, but as mentioned above, none were specific to dermatology.
General Dermatology References
Most of the dermatology reference apps were formatted as e-books; however, other apps such as the Amazon Kindle app (categorized under Books) providing access to multiple e-books within one app were not included. Some apps included study aid features (eg, flash cards, quizzes), and topics spanned both dermatology and dermatopathology. Apps provide a unique way for on-the-go studying for dermatologists in training, and if the usage continues to grow, there may be a need for increased formal integration in dermatology education in the future.
Journals
Journal apps were not among those listed in the top-ranked apps we evaluated, which we suspect may be because journals were categorized differently from one journal to the next; for example, the Journal of the American Academy of Dermatology was ranked 1168 in the Magazines and Newspapers category. On the other hand, Dermatology World was ranked 1363 in the Reference category. An article’s citation affects the publishing journal’s impact factor, which is one of the most important variables in measuring a journal’s influence. In the future, there may be other variables that could aid in understanding journal impact as it relates to the journal’s accessibility.
Limitations
Our study did not look at Android apps. The top chart apps in the Android and Apple App Stores use undisclosed algorithms likely involving different characteristics such as number of downloads, frequency of updates, number of reviews, ratings, and more. Thus, the rankings across these different markets would not be comparable. Although our choice of keywords stemmed from the majority of prior studies looking at dermatology apps, our search was limited due to the use of these specific keywords. To avoid skewing data by cross-comparison of noncomparable categories, we could not compare apps in the Medical category versus those in other categories.
CONCLUSION
There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps. As app usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in our education, and as such, it will become more critical to develop formal scientific standards. Given these future trends, we may need to increase our current literature and understanding of apps in dermatology with regard to their impact on both patients and health care providers.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
- Poushter J, Bishop C, Chwe H. Social media use continues to rise in developing countries but plateaus across developed ones. Pew Research Center website. http://www.pewglobal.org/2018/06/19/social-media-use-continues-to-rise-in-developing-countries-but-plateaus-across-developed-ones/#table. Published June 19, 2018. Accessed August 28, 2018.
- Flaten HK, St Claire C, Schlager E, et al. Growth of mobile applications in dermatology—2017 update. Dermatol Online J. 2018;24. pii:13030/qt3hs7n9z6.
- App Annie website. https://www.appannie.com/top/. Accessed August 28, 2018.
- Number of iPhone users in the United States from 2012 to 2016 (in millions). Statista website. https://www.statista.com/statistics/232790/forecast-of-apple-users-in-the-us/. Accessed August 28, 2018.
- Burkhart C. Medical mobile apps and dermatology. Cutis. 2012;90:278-281.
- Wolf JA, Moreau JF, Patton TJ, et al. Prevalence and impact of health-related internet and smartphone use among dermatology patients. Cutis. 2015;95:323-328.
- Masud A, Shafi S, Rao BK. Mobile medical apps for patient education: a graded review of available dermatology apps. Cutis. 2018;101:141-144.
- Walocko FM, Tejasvi T. Teledermatology applications in skin cancer diagnosis. Dermatol Clin. 2017;35:559-563.
- Krupinski E, Burdick A, Pak H, et al. American Telemedicine Association’s practice guidelines for teledermatology. Telemed J E Health. 2008;14:289-302.
- Ho B, Lee M, Armstrong AW. Evaluation criteria for mobile teledermatology applications and comparison of major mobile teledermatology applications. Telemed J E Health. 2013;19:678-682.
- Number of mobile app hours per smartphone and tablet app user in the United States in June 2016, by age group. Statista website. https://www.statista.com/statistics/323522/us-user-mobile-app-engagement-age/. Accessed September 18, 2018.
Practice Points
- As mobile application (app) usage increases among dermatology providers, whose demographic is shifting younger and younger, apps may become more incorporated in dermatology education. As such, it will become more critical to develop formal scientific standards.
- The most desired dermatology apps for patients were apps that allowed them to be proactive with their health.
- There seems to be a disconnect between the apps that are popular among patients and the scientific validity of the apps.