User login
Cutaneous Leishmaniasis Successfully Treated With Miltefosine
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
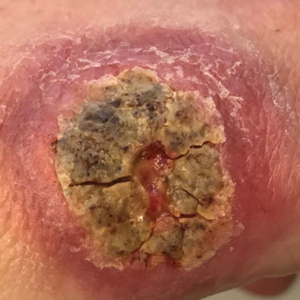
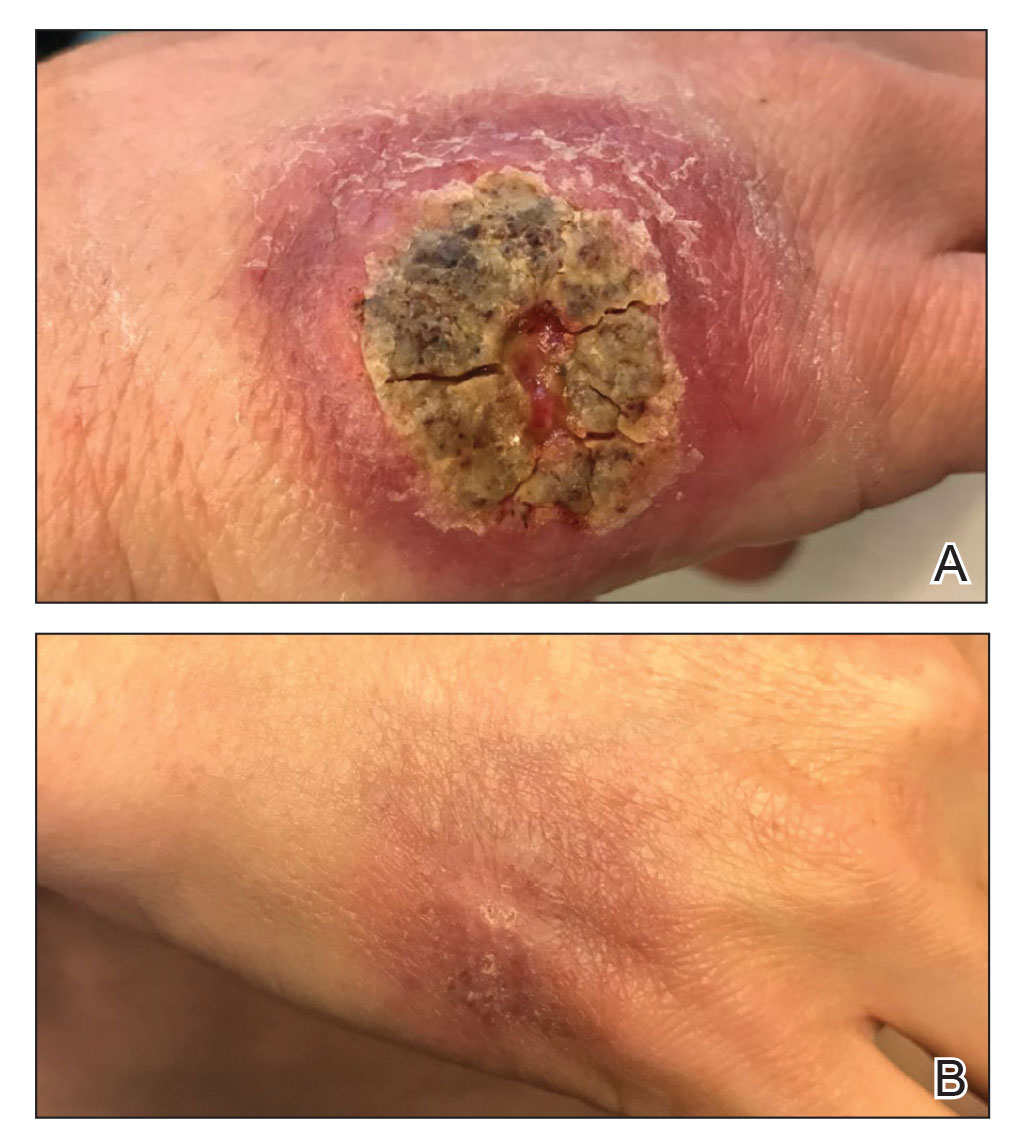
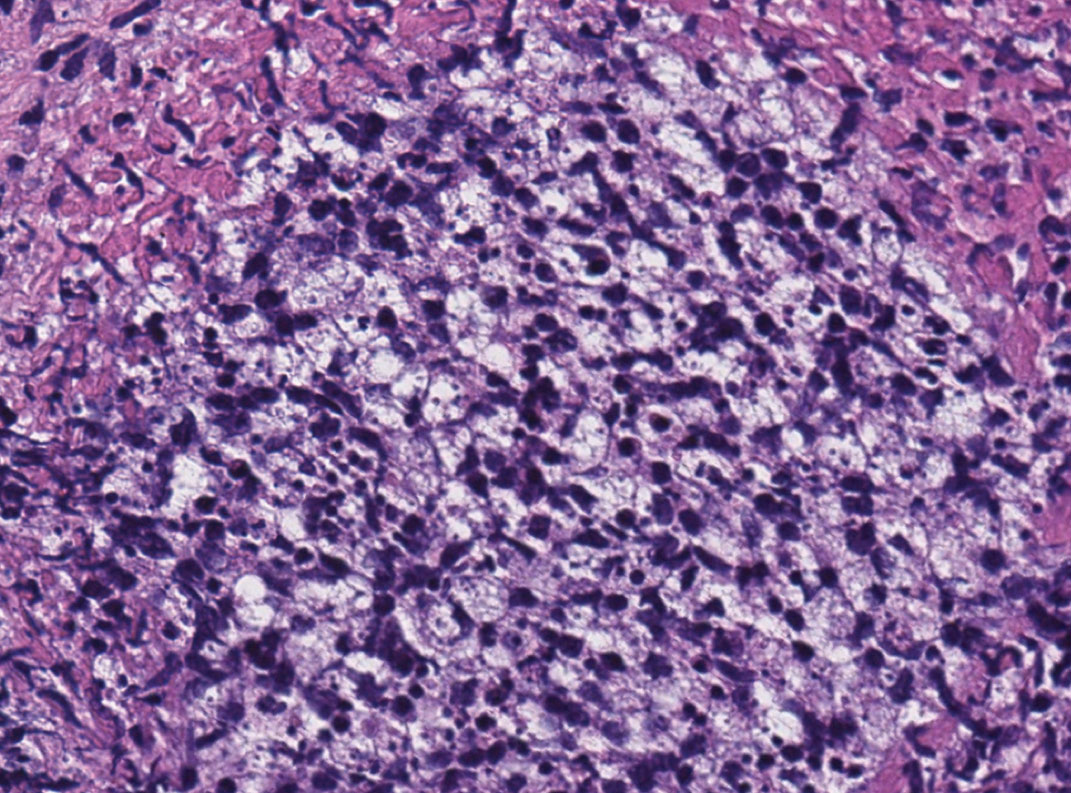
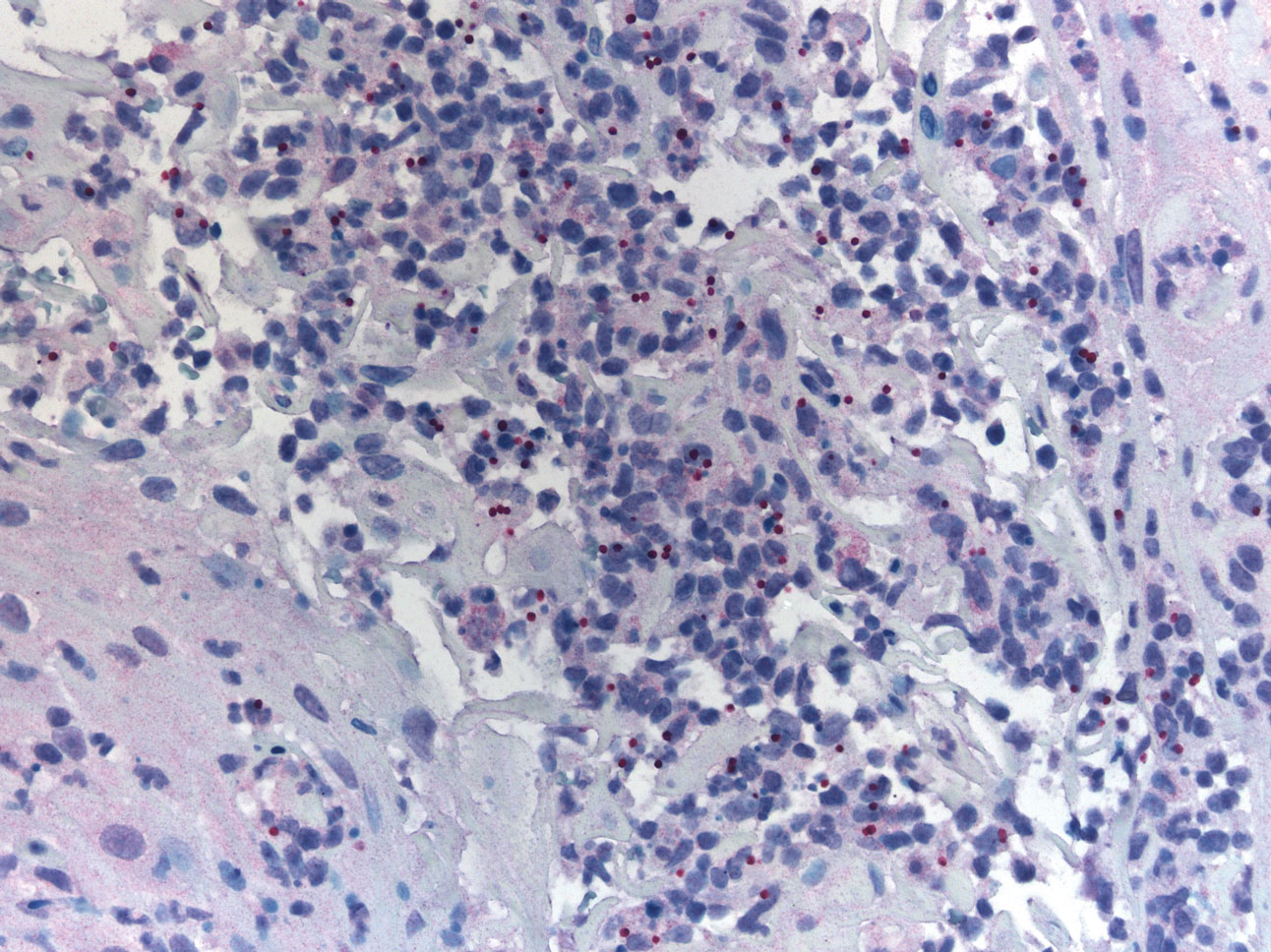
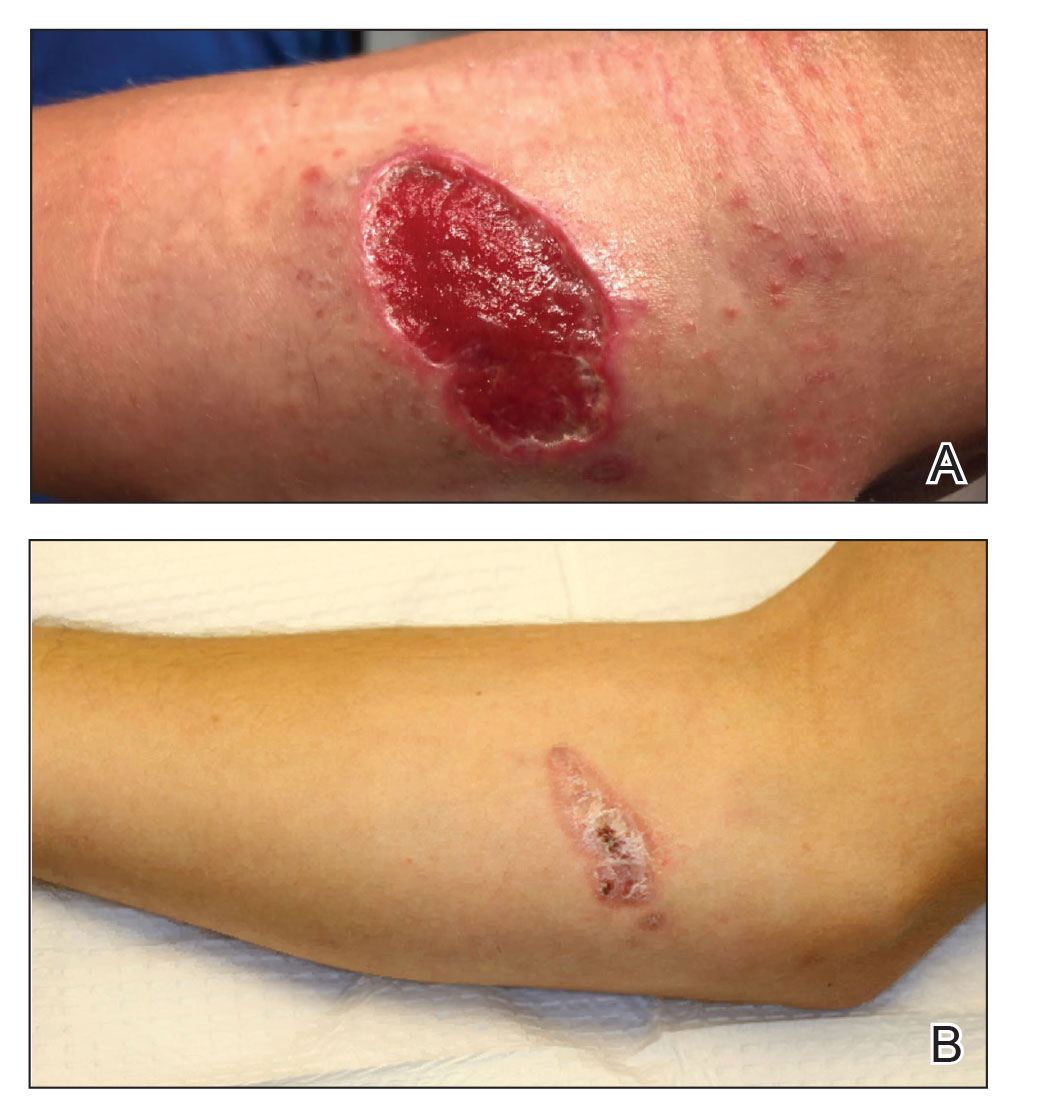
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.
Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.
Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Practice Points
- Avoiding phlebotomine sand fly vector bites is the most effective way to prevent leishmaniasis.
- Prompt diagnosis and treatment of cutaneous leishmaniasis caused by Leishmania species that have potential for mucocutaneous spread are key to limiting morbidity and mortality.
- Partnering with the Centers for Disease Control and Prevention is critical for timely diagnosis.
- Miltefosine should be considered as a first-line agent for cutaneous leishmaniasis given its efficacy, tolerability, and ease of administration.
APPlying Knowledge: Evidence for and Regulation of Mobile Apps for Dermatologists
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
Since the first mobile application (app) was developed in the 1990s, apps have become increasingly integrated into medical practice and training. More than 5.5 million apps were downloadable in 2019,1 of which more than 300,000 were health related.2 In the United States, more than 80% of physicians reported using smartphones for professional purposes in 2016.3 As the complexity of apps and their purpose of use has evolved, regulatory bodies have not adapted adequately to monitor apps that have broad-reaching consequences in medicine.
We review the primary literature on PubMed behind health-related apps that impact dermatologists as well as the government regulation of these apps, with a focus on the 3 most prevalent dermatology-related apps used by dermatology residents in the United States: VisualDx, UpToDate, and Mohs Surgery Appropriate Use Criteria. This prevalence is according to a survey emailed to all dermatology residents in the United States by the American Academy of Dermatology (AAD) in 2019 (unpublished data).
VisualDx
VisualDx, which aims to improve diagnostic accuracy and patient safety, contains peer-reviewed data and more than 32,000 images of dermatologic conditions. The editorial board includes more than 50 physicians. It provides opportunities for continuing medical education credit, is used in more than 2300 medical settings, and costs $399.99 annually for a subscription with partial features. Prior to the launch of the app in 2010, some health science professionals noted that the website version lacked references to primary sources.4 The same issue carried over to the app, which has evolved to offer artificial intelligence (AI) analysis of photographed skin lesions. However, there are no peer-reviewed publications showing positive impact of the app on diagnostic skills among dermatology residents or on patient outcomes.
UpToDate
UpToDate is a web-based database created in the early 1990s. A corresponding app was created around 2010. Both internal and independent research has demonstrated improved outcomes, and the app is advertised as the only clinical decision support resource associated with improved outcomes, as shown in more than 80 publications.5 UpToDate covers more than 11,800 medical topics and contains more than 35,000 graphics. It cites primary sources and uses a published system for grading recommendation strength and evidence quality. The data are processed and produced by a team of more than 7100 physicians as authors, editors, and reviewers. The platform grants continuing medical education credit and is used by more than 1.9 million clinicians in more than 190 countries. A 1-year subscription for an individual US-based physician costs $559. An observational study assessed UpToDate articles for potential conflicts of interest between authors and their recommendations. Of the 6 articles that met inclusion criteria of discussing management of medical conditions that have controversial or mostly brand-name treatment options, all had conflicts of interest, such as naming drugs from companies with which the authors and/or editors had financial relationships.6
Mohs Surgery Appropriate Use Criteria
The Mohs Surgery Appropriate Use Criteria app is a free clinical decision-making tool based on a consensus statement published in 2012 by the AAD, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and American Society for Mohs Surgery.7 It helps guide management of more than 200 dermatologic scenarios. Critique has been made that the criteria are partly based on expert opinion and data largely from the United States and has not been revised to incorporate newer data.8 There are no publications regarding the app itself.
Regulation of Health-Related Apps
Health-related apps that are designed for utilization by health care providers can be a valuable tool. However, given their prevalence, cost, and potential impact on patient lives, these apps should be well regulated and researched. The general paucity of peer-reviewed literature demonstrating the utility, safety, quality, and accuracy of health-related apps commonly used by providers is a reflection of insufficient mobile health regulation in the United States.
There are 3 primary government agencies responsible for regulating mobile medical apps: the US Food and Drug Administration (FDA), Federal Trade Commission, and Office for Civil Rights.9 The FDA does not regulate all medical devices. Apps intended for use in the diagnosis, cure, mitigation, prevention, or treatment of a disease or condition are considered to be medical devices.10 The FDA regulates those apps only if they are judged to pose more than minimal risk. Apps that are designed only to provide easy access to information related to health conditions or treatment are considered to be minimal risk but can develop into a different risk level such as by offering AI.11 Although the FDA does update its approach to medical devices, including apps and AI- and machine learning–based software, the rate and direction of update has not kept pace with the rapid evolution of apps.12 In 2019, the FDA began piloting a precertification program that grants long-term approval to organizations that develop apps instead of reviewing each app product individually.13 This decrease in premarket oversight is intended to expedite innovation with the hopeful upside of improving patient outcomes but is inconsistent, with the FDA still reviewing other types of medical devices individually.
For apps that are already in use, the Federal Trade Commission only gets involved in response to deceptive or unfair acts or practices relating to privacy, data security, and false or misleading claims about safety or performance. It may be more beneficial for consumers if those apps had a more stringent initial approval process. The Office for Civil Rights enforces the Health Insurance Portability and Accountability Act when relevant to apps.
Nongovernment agencies also are involved in app regulation. The FDA believes sharing more regulatory responsibility with private industry would promote efficiency.14 Google does not allow apps that contain false or misleading health claims,15 and Apple may scrutinize medical apps that could provide inaccurate data or be used for diagnosing or treating patients.16 Xcertia, a nonprofit organization founded by the American Medical Association and others, develops standards for the security, privacy, content, and operability of health-related apps, but those standards have not been adopted by other parties. Ultimately, nongovernment agencies are not responsible for public health and do not boast the government’s ability to enforce rules or ensure public safety.
Final Thoughts
The AAD survey of US dermatology residents found that the top consideration when choosing apps was up-to-date and accurate information; however, the 3 most prevalent apps among those same respondents did not need government approval and are not required to contain up-to-date data or to improve clinical outcomes, similar to most other health-related apps. This discrepancy is concerning considering the increasing utilization of apps for physician education and health care delivery and the increasing complexity of those apps. In light of these results, the potential decrease in federal premarket regulation suggested by the FDA’s precertification program seems inappropriate. It is important for the government to take responsibility for regulating health-related apps and to find a balance between too much regulation delaying innovation and too little regulation hurting physician training and patient care. It also is important for providers to be aware of the evidence and oversight behind the technologies they use for professional purposes.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
- Clement J. Number of apps available in leading app stores as of 1st quarter 2020. Statista website. https://www.statista.com/statistics/276623/number-of-apps-available-in-leading-app-stores/. Published May 4, 2020. Accessed July 23, 2020.
- mHealth App Economics 2017/2018. Current Status and Future Trends in Mobile Health. Berlin, Germany: Research 2 Guidance; 2018.
- Healthcare Client Services. Professional usage of smartphones by doctors. Kantar website. https://www.kantarmedia.com/us/thinking-and-resources/blog/professional-usage-of-smartphones-by-doctors-2016. Published November 16, 2016. Accessed July 23, 2020.
- Skhal KJ, Koffel J. VisualDx. J Med Libr Assoc. 2007;95:470-471.
- UpToDate is the only clinical decision support resource associated with improved outcomes. UpToDate website. https://www.uptodate.com/home/research. Accessed July 29, 2020.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Amber KT, Dhiman G, Goodman KW. Conflict of interest in online point-of-care clinical support websites. J Med Ethics. 2014;40:578-580.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs micrographic surgery appropriate use criteria. J Am Acad Dermatol. 2020;82:E55.
- Mobile health apps interactive tool. Federal Trade Commission website. https://www.ftc.gov/tips-advice/business-center/guidance/mobile-health-apps-interactive-tool. Published April 2016. Accessed May 23, 2020.
- Federal Food, Drug, and Cosmetic Act, 21 USC §321 (2018).
- US Food and Drug Administration. Examples of software functions for which the FDA will exercise enforcement discretion. https://www.fda.gov/medical-devices/device-software-functions-including-mobile-medical-applications/examples-software-functions-which-fda-will-exercise-enforcement-discretion. Updated September 26, 2019. Accessed July 29, 2020.
- US Food and Drug Administration. Proposed regulatory framework for modifications to artificial intelligence/machine learning (AI/ML)‐based software as a medical device (SaMD). https://www.fda.gov/downloads/MedicalDevices/DigitalHealth/SoftwareasaMedicalDevice/UCM635052.pdf. Accessed July 23, 2020.
- US Food and Drug Administration. Digital health software precertification (pre-cert) program. https://www.fda.gov/medical-devices/digital-health/digital-health-software-precertification-pre-cert-program. Updated July 18, 2019. Accessed July 23, 2020.
- Gottlieb S. Fostering medical innovation: a plan for digital health devices. US Food and Drug Administration website. https://www.fda.gov/news-events/fda-voices/fostering-medical-innovation-plan-digital-health-devices. Published June 15, 2017. Accessed July 23, 2020.
- Restricted content: unapproved substances. Google Play website. https://play.google.com/about/restricted-content/unapproved-substances. Accessed July 23, 2020.
- App store review guidelines. Apple Developer website. https://developer.apple.com/app-store/review/guidelines. Updated March 4, 2020. Accessed July 23, 2020.
Practice Points
- Physicians who are selecting an app for self-education or patient care should take into consideration the strength of the evidence supporting the app as well as the rigor of any approval process the app had to undergo.
- Only a minority of health-related apps are regulated by the government. This regulation has not kept up with the evolution of app software and may become more indirect.