User login
Paraneoplastic Pemphigus With Cicatricial Nail Involvement
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
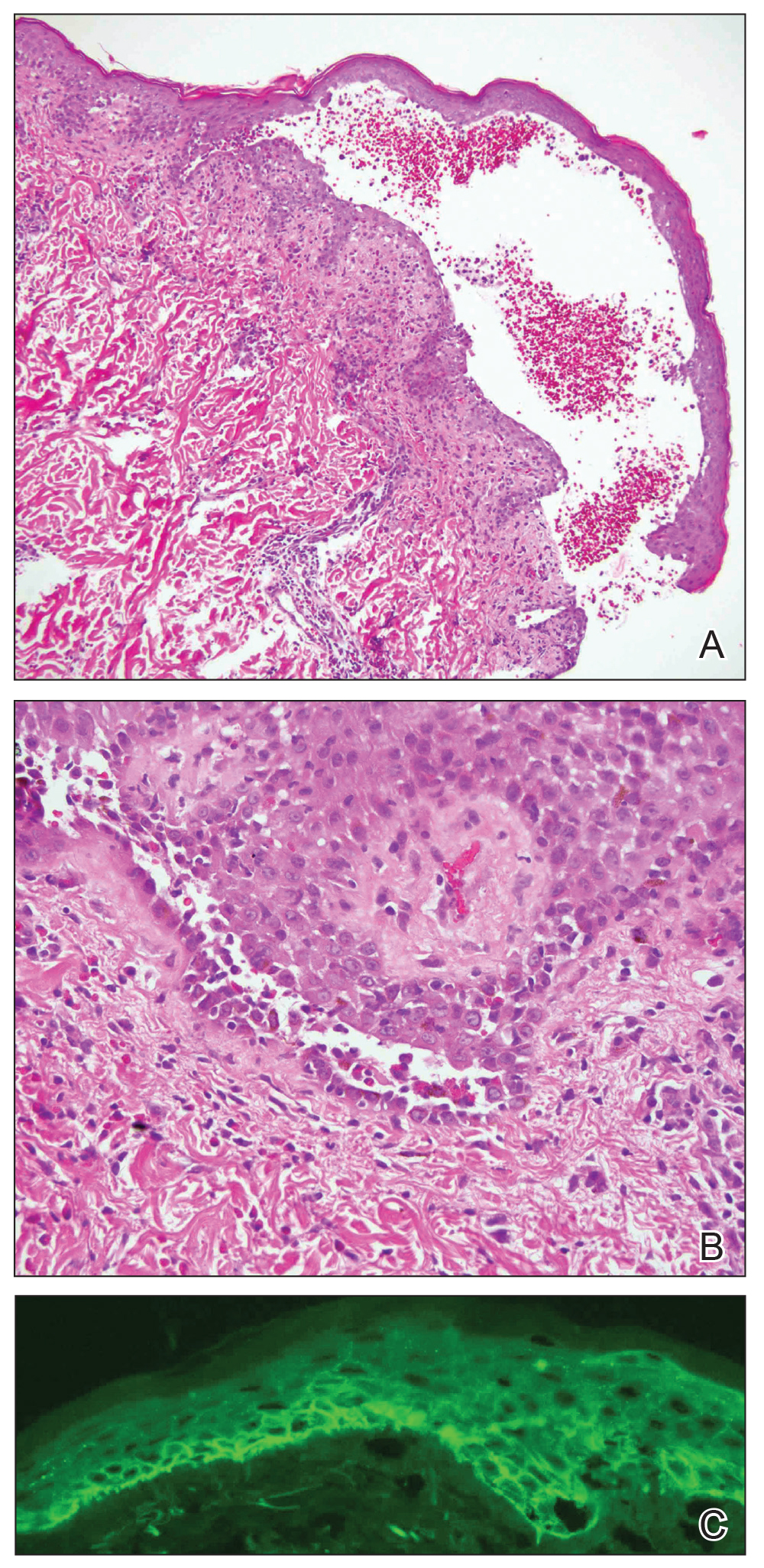
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.
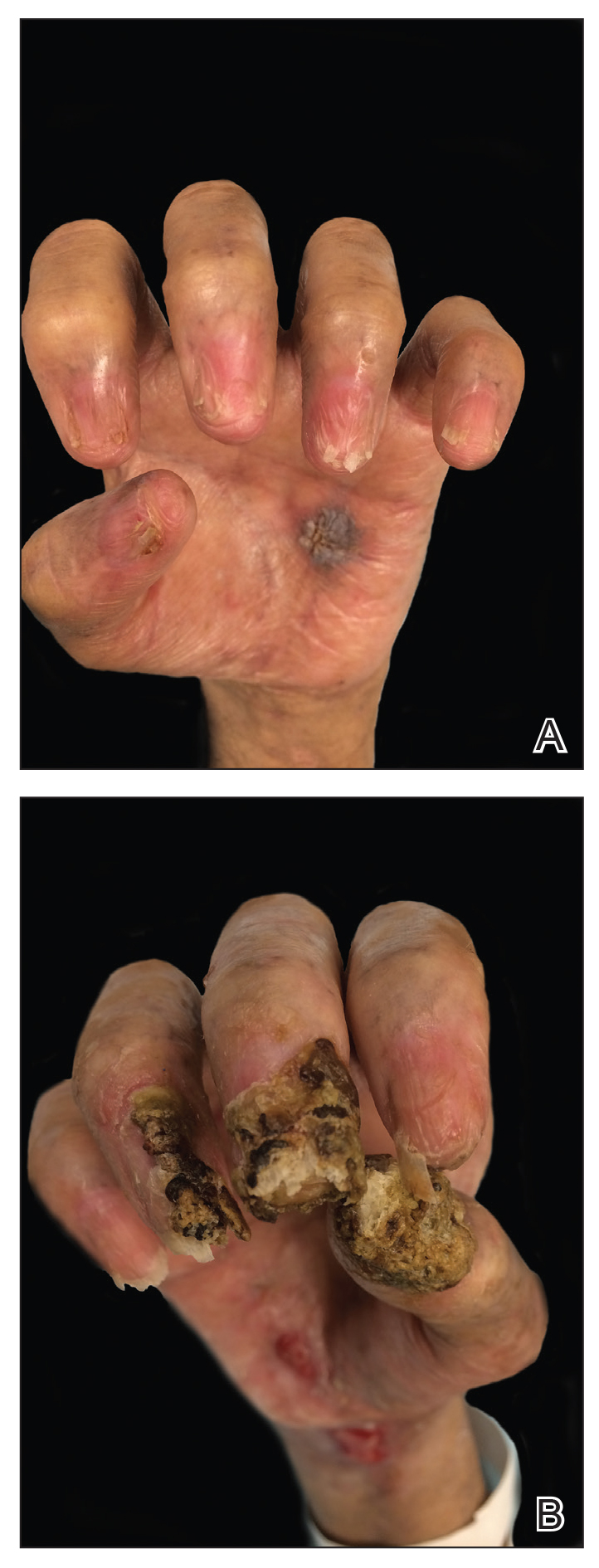
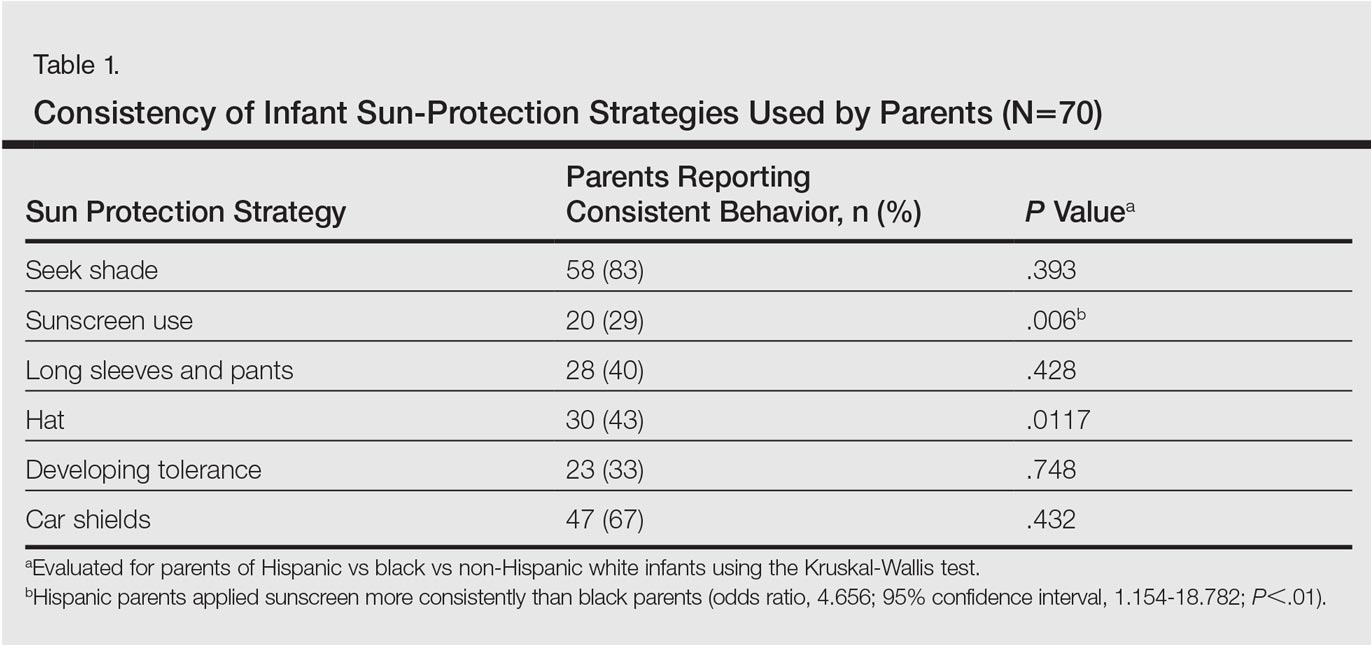
Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.
Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.
Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.
Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
To the Editor:
Paraneoplastic pemphigus (PNP), also known as paraneoplastic autoimmune multiorgan syndrome, is an autoimmune mucocutaneous blistering disease that typically occurs secondary to a lymphoproliferative disorder. Paraneoplastic pemphigus is characterized by severe erosive stomatitis, polymorphous skin lesions, and potential bronchiolitis obliterans that can mimic a wide array of conditions. The exact pathogenesis is unknown but is thought to be due to a combination of humoral and cell-mediated immunity. The condition usually confers a poor prognosis, with morbidity from 38% to upwards of 90%.1
A 47-year-old man developed prominent pink to dusky, ill-defined, targetoid, coalescing papules over the back; violaceous macules over the palms and soles; and numerous crusted oral erosions while hospitalized for an infection. He had a history of stage IVB follicular lymphoma (double-hit type immunoglobulin heavy chain/BCL2 fusion and rearrangement of BCL6) complicated by extensive erosive skin lesions and multiple lines of infections. The clinical differential diagnosis included Stevens-Johnson syndrome vs erythema multiforme (EM) major secondary to administration of oxacillin vs PNP. Herpes simplex virus polymerase chain reaction and Mycoplasma titers were negative. Skin biopsies from the back and right abdomen revealed severe lichenoid interface dermatitis (IFD) with numerous dyskeratotic cells mimicking EM and eosinophils; however, direct immunofluorescence of the abdomen biopsy revealed an apparent suprabasal acantholysis with intercellular C3 in the lower half of the epidermis. Histologically, PNP was favored, but indirect immunofluorescence with monkey esophagus IgG was negative.
The skin lesions progressed, and an additional skin biopsy from the left arm performed 1 month later revealed similar histologic features with intercellular IgG and C3 in the lower half of the epidermis with weak basement membrane C3 (Figure 1). Serology also confirmed elevated serum antidesmoglein 1 and 3 antibodies. Thus, in the clinical setting of an erosive mucositis with EM-like and pemphigoidlike eruptions associated with B-cell lymphoma, the patient was diagnosed with PNP.
Despite multiple complications followed by intermittent treatments, the initial therapy with rituximab induction and subsequent cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin hydrochloride, vincristine sulfate, and prednisone) for the B-cell lymphoma was done during his hospital stay. Toward the end of his 8-week hospitalization, the patient was noted to have new lesions involving the hands, digits, and nails. The left hand showed anonychia of several fingers with prominent scarring (Figure 2A). There were large, verrucous, crusted plaques on the distal phalanges of several fingers on the right hand (Figure 2B). At that time, he was taking 20 mg daily of prednisone (for 10 months) and had completed his 6th cycle of R-CHOP, which resulted in improvement of the skin lesions. Oral steroids were tapered, and he was maintained on rituximab infusions every 8 weeks but has since been lost to follow-up.
Paraneoplastic lymphoma is a rare condition that affects 0.012% of non-Hodgkin lymphoma and chronic lymphocytic leukemia patients.2 Reports of PNP involving the nails are even more rare, with 3 reports in the setting of underlying Castleman disease3-5 and 2 reports in patients with underlying non-Hodgkin6 and follicular1 lymphoma. These studies describe variable nail findings ranging from periungual erosions and edema, formation of dorsal pterygium, onycholysis with longitudinal furrowing, and destruction of the nail plate leading to onychomadesis and/or anonychia. These nail changes typically are seen in lichen planus or in bullous diseases affecting the basement membrane (eg, bullous pemphigoid, acquired epidermolysis bullosa) but not known in pemphigus, which is characterized by nonscarring nail changes.7
Although antidesmoglein 3 antibody was shown to be a pathologic driver in PNP, there is a weak correlation between antibody profiles and clinical presentation.8 In one case of PNP, antidesmoglein 3 antibody was negative, suggesting that lichenoid IFD may cause the phenotypic findings in PNP.9 Thus, the development of nail scarring in PNP may be explained by the presence of lichenoid IFD that is characteristic of PNP. However, the variation in antibody profile in PNP likely is a consequence of epitope spreading.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
- Miest RY, Wetter DA, Drage LA, et al. A mucocutaneous eruption. Int J Dermatol. 2014;53:1425-1427.
- Anhalt GJ, Mimouni D. Paraneoplastic pemphigus. In: LA G, Katz SI, Gilchrest, eds. Fitzpatrick’s Dermatology in General Medicine 8th Edition. Vol 1. New York, NY: McGraw Hill; 2012:600.
- Chorzelski T, Hashimoto T, Maciejewska B, et al. Paraneoplastic pemphigus associated with Castleman tumor, myasthenia gravis and bronchiolitis obliterans. J Am Acad Dermatol. 1999;41:393-400.
- Lemon MA, Weston WL, Huff JC. Childhood paraneoplastic pemphigus associated with Castleman’s tumour. Br J Dermatol. 1997;136:115-117.
- Tey HL, Tang MB. A case of paraneoplastic pemphigus associated with Castleman’s disease presenting as erosive lichen planus. Clin Exp Dermatol. 2009;34:e754-e756.
- Liang JJ, Cordes SF, Witzig TE. More than skin-deep. Cleve Clin J Med. 2013;80:632-633.
- Tosti A, Andre M, Murrell DF. Nail involvement in autoimmune bullous disorders. Dermatol Clin. 2011;29:511-513, xi.
- Ohyama M, Amagai M, Hashimoto T, et al. Clinical phenotype and anti-desmoglein autoantibody profile in paraneoplastic pemphigus. J Am Acad Dermatol. 2001;44:593-598.
- Kanwar AJ, Vinay K, Varma S, et al. Anti-desmoglein antibody-negative paraneoplastic pemphigus successfully treated with rituximab. Int J Dermatol. 2015;54:576-579.
Practice Points
- Paraneoplastic pemphigus (PNP) is a rare blistering skin eruption commonly associated with an underlying malignancy.
- Paraneoplastic pemphigus generally presents with erosive stomatitis with involvement of the vermillion lip but also can involve the skin and nails.
- Nail involvement can lead to scarring of the nails and can mimic lichen planus, bullous pemphigoid, or epidermolysis bullosa of the nails. These nail changes likely are due to the pronounced lichenoid interphase dermatitis seen in PNP.
Cutaneous Leishmaniasis Successfully Treated With Miltefosine
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
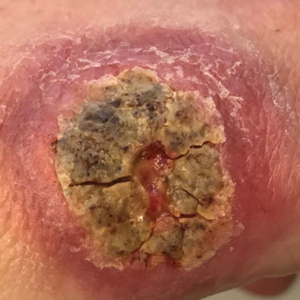
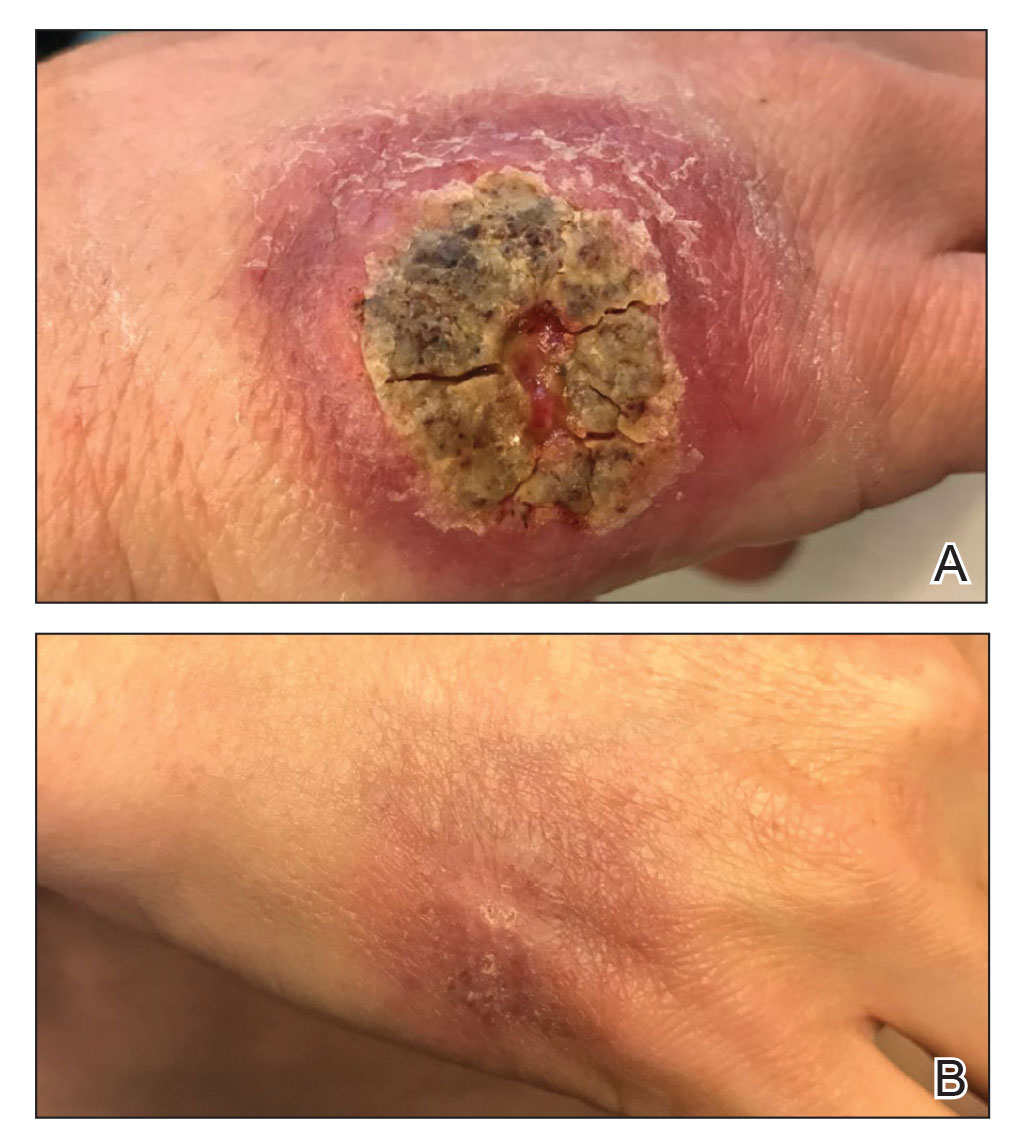
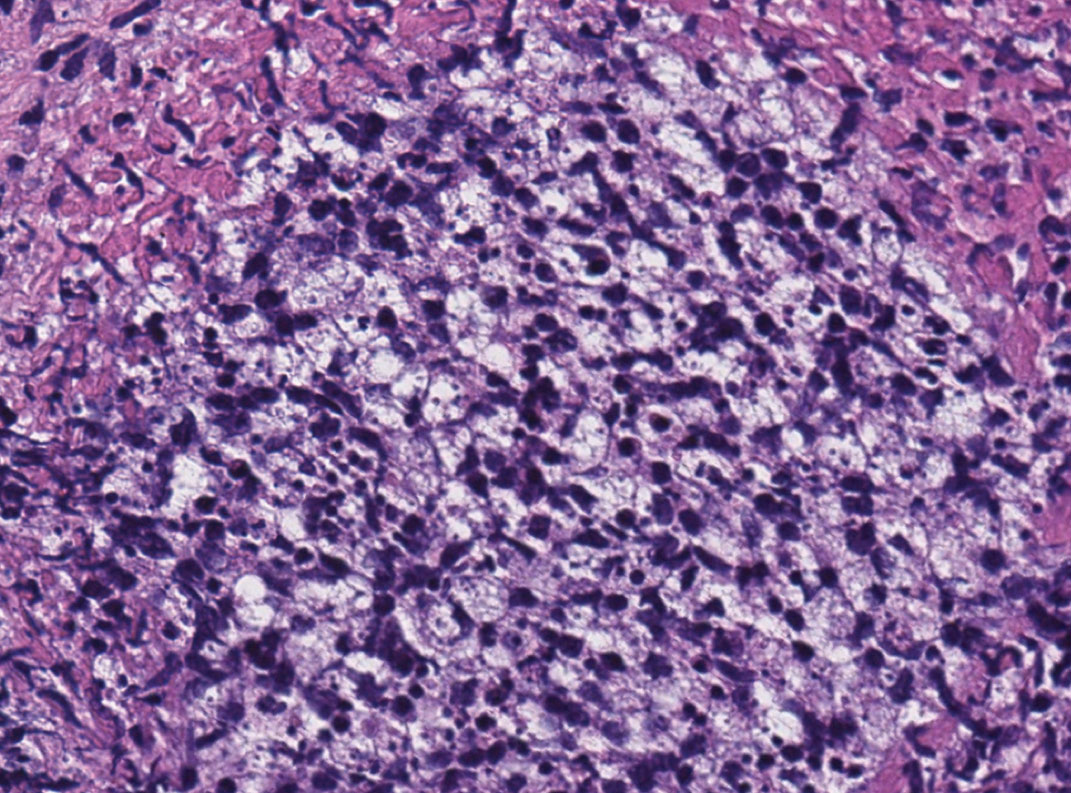
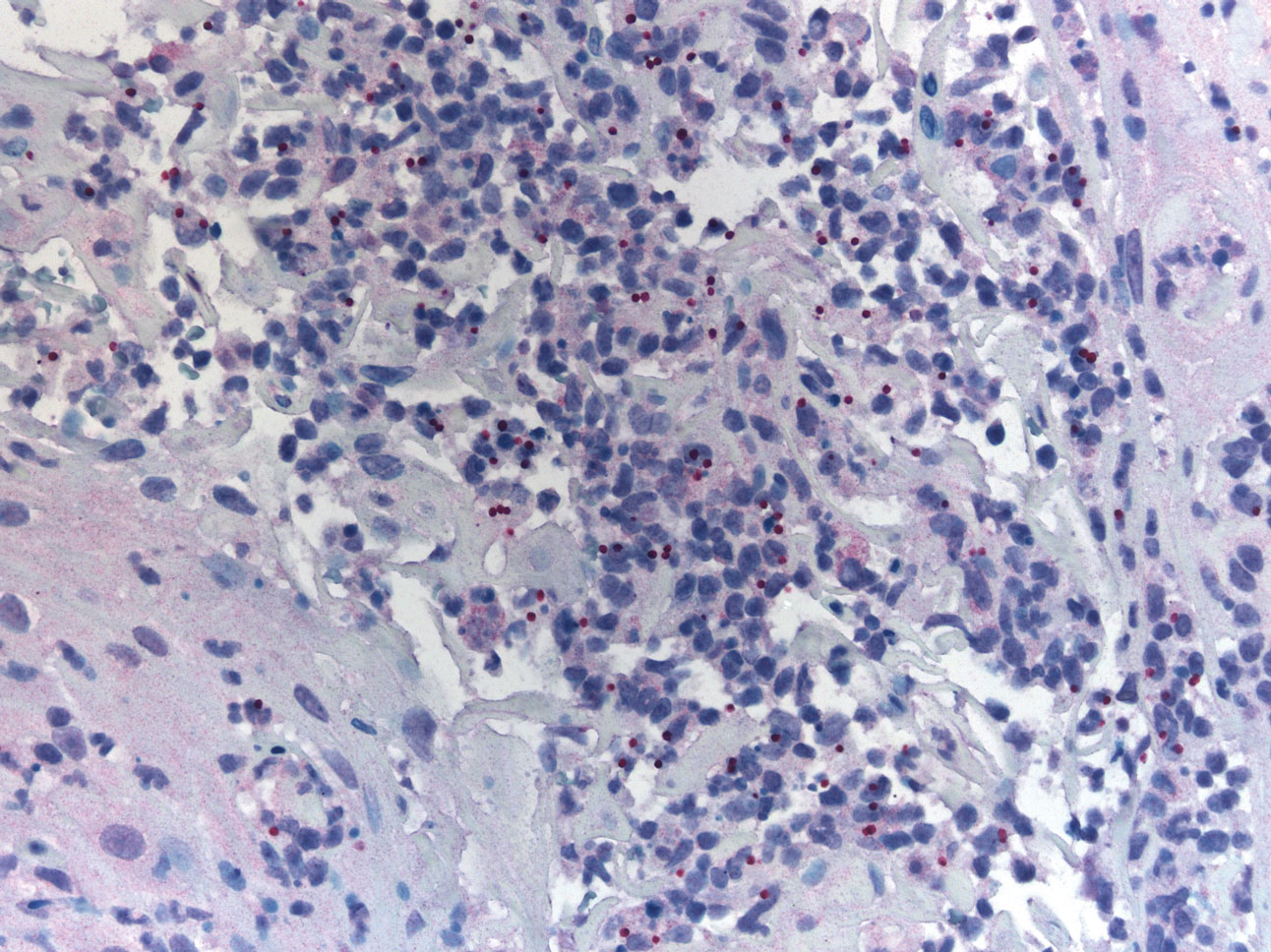
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.
Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.
Treatment
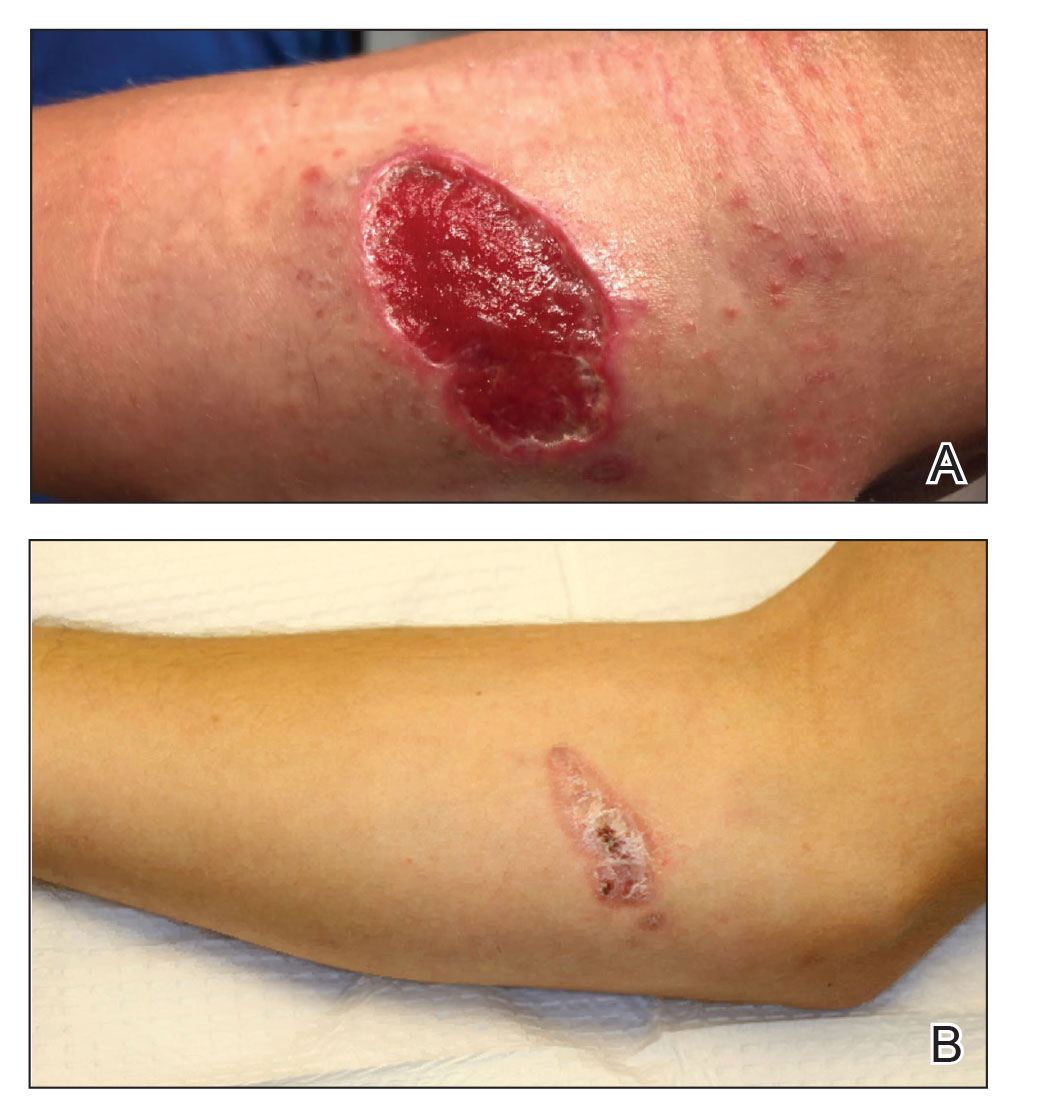
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
Leishmaniasis is a neglected parasitic disease with an estimated annual incidence of 1.3 million cases, the majority of which manifest as cutaneous leishmaniasis.1 The cutaneous and mucosal forms demonstrate substantial global burden with morbidity and socioeconomic repercussions, while the visceral form is responsible for up to 30,000 deaths annually.2 Despite increasing prevalence in the United States, awareness and diagnosis remain relatively low.3 We describe 2 cases of cutaneous leishmaniasis in New England, United States, in travelers returning from Central America, both successfully treated with miltefosine. We also review prevention, diagnosis, and treatment options.
Case Reports
Patient 1
A 47-year-old woman presented with an enlarging, 2-cm, erythematous, ulcerated nodule on the right dorsal hand of 2 weeks’ duration with accompanying right epitrochlear lymphadenopathy (Figure 1A). She noticed the lesion 10 weeks after returning from Panama, where she had been photographing the jungle. Prior to the initial presentation to dermatology, salicylic acid wart remover, intramuscular ceftriaxone, and oral trimethoprim had failed to alleviate the lesion. Her laboratory results were notable for an elevated C-reactive protein level of 5.4 mg/L (reference range, ≤4.9 mg/L). A punch biopsy demonstrated pseudoepitheliomatous hyperplasia with diffuse dermal lymphohistiocytic inflammation and small intracytoplasmic structures within histiocytes consistent with leishmaniasis (Figure 2). Immunohistochemistry was consistent with leishmaniasis (Figure 3), and polymerase chain reaction performed by the Centers for Disease Control and Prevention (CDC) identified the pathogen as Leishmania braziliensis.



Patient 2
An 18-year-old man presented with an enlarging, well-delineated, tender ulcer of 6 weeks’ duration measuring 2.5×2 cm with an erythematous and edematous border on the right medial forearm with associated epitrochlear lymphadenopathy (Figure 4). Nine weeks prior to initial presentation, he had returned from a 3-month outdoor adventure trip to the Florida Keys, Costa Rica, and Panama. He had used bug repellent intermittently, slept under a bug net, and did not recall any trauma or bite at the ulcer site. Biopsy and tissue culture were obtained, and histopathology demonstrated an ulcer with a dense dermal lymphogranulomatous infiltrate and intracytoplasmic organisms consistent with leishmaniasis. Polymerase chain reaction by the CDC identified the pathogen as Leishmania panamensis.

Treatment
Both patients were prescribed oral miltefosine 50 mg twice daily for 28 days. Patient 1 initiated treatment 1 month after lesion onset, and patient 2 initiated treatment 2.5 months after initial presentation. Both patients had noticeable clinical improvement within 21 days of starting treatment, with lesions diminishing in size and lymphadenopathy resolving. Within 2 months of treatment, patient 1’s ulcer completely resolved with only postinflammatory hyperpigmentation (Figure 1B), while patient 2’s ulcer was noticeably smaller and shallower compared with its peak size of 4.2×2.4 cm (Figure 4B). Miltefosine was well tolerated by both patients; emesis resolved with ondansetron in patient 1 and spontaneously in patient 2, who had asymptomatic temporary hyperkalemia of 5.2 mmol/L (reference range, 3.5–5.0 mmol/L).
Comment
Epidemiology and Prevention
Risk factors for leishmaniasis include weak immunity, poverty, poor housing, poor sanitation, malnutrition, urbanization, climate change, and human migration.4 Our patients were most directly affected by travel to locations where leishmaniasis is endemic. Despite an increasing prevalence of endemic leishmaniasis and new animal hosts in the southern United States, most patients diagnosed in the United States are infected abroad by Leishmania mexicana and L braziliensis, both cutaneous New World species.3 Our patients were infected by species within the New World subgenus Viannia that have potential for mucocutaneous spread.4
Because there is no chemoprophylaxis or acquired active immunity such as vaccines that can mitigate the risk for leishmaniasis, public health efforts focus on preventive measures. Although difficult to achieve, avoidance of the phlebotomine sand fly species that transmit the obligate intracellular Leishmania parasite is a most effective measure.4 Travelers entering geographic regions with higher risk for leishmaniasis should be aware of the inherent risk and determine which methods of prevention, such as N,N-diethyl-meta-toluamide (DEET) insecticides or permethrin-treated protective clothing, are most feasible. Although higher concentrations of DEET provide longer protection, the effectiveness tends to plateau at approximately 50%.5
Presentation and Prognosis
For patients who develop leishmaniasis, the disease course and prognosis depend greatly on the species and manifestation. The most common form of leishmaniasis is localized cutaneous leishmaniasis, which has an annual incidence of up to 1 million cases. It initially presents as macules, usually at the site of inoculation within several months to years of infection.6 The macules expand into papules and plaques that reach maximum size over at least 1 week4 and then progress into crusted ulcers up to 5 cm in diameter with raised edges. Although usually painless and self-limited, these lesions can take years to spontaneously heal, with the risk for atrophic scarring and altered pigmentation. Lymphatic involvement manifests as lymphadenitis or regional lymphadenopathy and is common with lesions caused by the subgenus Viannia.6
Leishmania braziliensis and L panamensis, the species that infected our patients, can uniquely cause cutaneous leishmaniasis that metastasizes into mucocutaneous leishmaniasis, which always affects the nasal mucosa. Risk factors for transformation include a primary lesion site above the waist, multiple or large primary lesions, and delayed healing of primary cutaneous leishmaniasis. Mucocutaneous leishmaniasis can result in notable morbidity and even mortality from invasion and destruction of nasal and oropharyngeal mucosa, as well as intercurrent pneumonia, especially if treatment is insufficient or delayed.4
Diagnosis
Prompt treatment relies on accurate and timely diagnosis, which is complicated by the relative unfamiliarity with leishmaniasis in the United States. The differential diagnosis for cutaneous leishmaniasis is broad, including deep fungal infection, Mycobacterium infection, cutaneous granulomatous conditions, nonmelanoma cutaneous neoplasms, and trauma. Taking a thorough patient history, including potential exposures and travels; having high clinical suspicion; and being aware of classic presentation allows for identification of leishmaniasis and subsequent stratification by manifestation.7
Diagnosis is made by detecting Leishmania organisms or DNA using light microscopy and staining to visualize the kinetoplast in an amastigote, molecular methods, or specialized culturing.7 The CDC is a valuable diagnostic partner for confirmation and speciation. Specific instructions for specimen collection and transportation can be found by contacting the CDC or reading their guide.8 To provide prompt care and reassurance to patients, it is important to be aware of the coordination effort that may be needed to send samples, receive results, and otherwise correspond with a separate institution.
Treatment
Treatment of cutaneous leishmaniasis is indicated to decrease the risk for mucosal dissemination and clinical reactivation of lesions, accelerate healing of lesions, decrease local morbidity caused by large or persistent lesions, and decrease the reservoir of infection in places where infected humans serve as reservoir hosts. Oral treatments include ketoconazole, itraconazole, and fluconazole, recommended at doses ranging from 200 to 600 mg daily for at least 28 days. For severe, refractory, or visceral leishmaniasis, parenteral choices include
Miltefosine is becoming a more common treatment of leishmaniasis because of its oral route, tolerability in nonpregnant patients, and commercial availability. It was approved by the US Food and Drug Administration in 2014 for cutaneous leishmaniasis due to L braziliensis, L panamensis, and Leishmania guyanensis; mucosal leishmaniasis due to L braziliensis; and visceral leishmaniasis due to Leishmania donovani in patients at least 12 years of age. For cutaneous leishmaniasis, the standard dosage of 50 mg twice daily (for patients weighing 30–44 kg) or 3 times daily (for patients weighing 45 kg or more) for 28 consecutive days has cure rates of 48% to 85% by 6 months after therapy ends. Cure is defined as epithelialization of lesions, no enlargement greater than 50% in lesions, no appearance of new lesions, and/or negative parasitology. The antileishmanial mechanism of action is unknown and likely involves interaction with lipids, inhibition of cytochrome c oxidase, and apoptosislike cell death. Miltefosine is contraindicated in pregnancy. The most common adverse reactions in patients include nausea (35.9%–41.7%), motion sickness (29.2%), headache (28.1%), and emesis (4.5%–27.5%). With the exception of headache, these adverse reactions can decrease with administration of food, fluids, and antiemetics. Potentially more serious but rarer adverse reactions include elevated serum creatinine (5%–25%) and transaminases (5%). Although our patients had mild hyperkalemia, it is not an established adverse reaction. However, renal injury has been reported.10
Conclusion
Cutaneous leishmaniasis is increasing in prevalence in the United States due to increased foreign travel. Providers should be familiar with the cutaneous presentation of leishmaniasis, even in areas of low prevalence, to limit the risk for mucocutaneous dissemination from infection with the subgenus Viannia. Prompt treatment is vital to ensuring the best prognosis, and first-line treatment with miltefosine should be strongly considered given its efficacy and tolerability.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
- Babuadze G, Alvar J, Argaw D, et al. Epidemiology of visceral leishmaniasis in Georgia. PLoS Negl Trop Dis. 2014;8:e2725.
- Leishmaniasis. World Health Organization website. https://www.afro.who.int/health-topics/Leishmaniasis. Accessed September 15, 2020.
- McIlwee BE, Weis SE, Hosler GA. Incidence of endemic human cutaneous leishmaniasis in the United States. JAMA Dermatol. 2018;154:1032-1039.
- Leishmaniasis. World Health Organization website. https://www.who.int/news-room/fact-sheets/detail/leishmaniasis. Update March 2, 2020. Accessed September 15, 2020.
- Centers for Disease Control and Prevention. Guidelines for DEET insect repellent use. https://www.cdc.gov/malaria/toolkit/DEET.pdf. Accessed September 20, 2020.
- Buescher MD, Rutledge LC, Wirtz RA, et al. The dose-persistence relationship of DEET against Aedes aegypti. Mosq News. 1983;43:364-366.
- Aronson N, Herwaldt BL, Libman M, et al. Diagnosis and treatment of leishmaniasis: clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Clin Infect Dis. 2016;63:e202-e264.
- US Department of Health and Human Services. Practical guide for specimen collection and reference diagnosis of leishmaniasis. Centers for Disease Control and Prevention website. https://www.cdc.gov/parasites/leishmaniasis/resources/pdf/cdc_diagnosis_guide_leishmaniasis_2016.pdf. Accessed September 15, 2020.
- Visceral leishmaniasis. Drugs for Neglected Diseases Initiative website. https://www.dndi.org/diseases-projects/leishmaniasis/. Accessed September 15, 2020.
- Impavido Medication Guide. Food and Drug Administration Web site. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/204684s000lbl.pdf. Revised March 2014. Accessed May 18, 2020.
Practice Points
- Avoiding phlebotomine sand fly vector bites is the most effective way to prevent leishmaniasis.
- Prompt diagnosis and treatment of cutaneous leishmaniasis caused by Leishmania species that have potential for mucocutaneous spread are key to limiting morbidity and mortality.
- Partnering with the Centers for Disease Control and Prevention is critical for timely diagnosis.
- Miltefosine should be considered as a first-line agent for cutaneous leishmaniasis given its efficacy, tolerability, and ease of administration.
Sun Protection for Infants: Parent Behaviors and Beliefs in Miami, Florida
Sun exposure and sunburns sustained during childhood are linked to an increased risk for development of skin cancers in adulthood. In infants, the skin is particularly vulnerable and is considered to be at increased risk for UV radiation damage,1 even as early as the first 6 months of life.2 Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 To effectively teach parents proper sun-safe practices, it is essential to understand their existing perceptions and behaviors. This study sought to examine differences in infant sun-safety practices during the first 6 months of life among black, Hispanic, and non-Hispanic white (NHW) parents in Miami, Florida.
Methods
Parents presenting to the University of Miami general pediatrics clinic from February 2015 through April 2015 with a child younger than 5 years were administered a 15-item questionnaire that included items on demographics, sun-safety strategies, sunburns and tanning, beliefs and limitations regarding sunscreen, and primary information source regarding sun safety (eg, physician, Internet, media, instincts). Parents were approached by the investigators consecutively for participation in scheduled blocks, with the exception of those who were otherwise engaged in appointment-related tasks (eg, paperwork). The study was approved by the University of Miami Miller School of Medicine institutional review board. The primary objective of this study was to determine the sun protection behaviors that black and Hispanic parents in Miami, Florida, employ in infants younger than 6 months. Secondary objectives included determining if this patient population is at risk for infant sunburns and tanning, beliefs among parents regarding sunscreen's efficacy in the prevention of skin cancers, and limitations of sunscreen use.
All data were analyzed using SAS software version 9.3. Wilcoxon signed rank test, Kruskal-Wallis test, Fisher exact test, and proportional-odds cumulative logit model were used to compare nonparametric data. Parents reporting on the full first 6 months of life (ie, the child was older than 6 months at the time of study completion) were included for analysis of sun-safety strategies. All survey respondents were included for analysis of secondary objectives. Responses from parents of infants of mixed racial and ethnic backgrounds were excluded from applicable subgroup analyses.
Results
Ninety-eight parents were approached for participation in the study; 97 consented to participate and 95 completed the survey. Seventy parents had children who were at least 6 months of age and were included for analysis of the primary objectives (ie, sun-protection strategies in the first 6 months of life). The cohort included 49 Hispanic parents, 26 black parents, and 9 NHW parents; 5 parents indicated their child was of mixed racial and ethnic background. Six respondents indicated another minority group (eg, Native American, Pacific Islander). Eighty-three respondents were mothers, 72 were educated beyond high school, and 14 were Spanish-speaking only. Four reported a known family history of skin cancer.
There were notable differences in application of sunscreen, belief in the efficacy of sunscreen, and primary source of information between parents (Tables 1 and 2). Hispanic parents reported applying sunscreen more consistently than black parents (odds ratio, 4.656; 95% confidence interval, 1.154-18.782; P<.01). Hispanic parents also were more likely than black parents to believe sunscreen is effective in the prevention of skin cancers (odds ratio, 7.499; 95% confidence interval, 1.535-36.620; P<.01). Hispanic parents were more likely to report receiving information regarding sun-safety practices for infants from their pediatrician, whereas NHW parents were more likely to follow their instincts regarding how and if infants should be exposed to the sun (P<.05). No significant differences were found in the reported primary source of information in black versus Hispanic parents or in black versus NHW parents. Three percent (3/95) of respondents reported a sunburn in the infant's first 6 months of life, and 12% (11/95) reported tanning of infants' skin from sun exposure. Tanning was associated with inconsistent shade (P<.01), inconsistent clothing coverage (P<.01), and consistently allowing infants to "develop tolerance to the sun's rays by slowly increasing sun exposure each day" (P<.05).
Comment
The survey results indicated suboptimal sun-protection practices among parents of black and Hispanic infants in Miami. Although the majority of respondents (83% [58/70]) reported keeping their infants in the shade, less than half of parents consistently covered their infants adequately with clothing and hats (40% [28/70] and 43% [30/70], respectively). More alarmingly, one-third of parents reported intentionally increasing their infant's level of sun exposure to develop his/her tolerance to the sun. A minority of parents reported sunburns (3%) and tanning (12%) within the first 6 months of life. Twenty-nine percent of parents (20/70) reported consistently applying sunscreen to their infants who were younger than 6 months despite limited safety data available for this age group.
Although our study included a limited sample size and represents a narrow geographic distribution, these results suggest that shortcomings in current practices in sun protection for black and Hispanic infants younger than 6 months may be a widespread problem. Black and Hispanic patients have a lower incidence of skin cancer, but the diagnosis often is delayed and the mortality is higher when skin cancer does occur.4 The common perception among laypeople as well as many health care providers that black and Hispanic individuals are not at risk for skin cancer may limit sun-safety counseling as well as the overall knowledge base of this patient demographic. As demonstrated by the results of this study, there is a need for counseling on sun-safe behaviors from a young age among this population.
Conclusion
This study highlights potential shortcomings in current sun-protection practices for black and Hispanic infants younger than 6 months. Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 Additional studies are needed to further define sun-safety behaviors in black and Hispanic children across the United States. Further, additional studies should focus on developing interventions that positively influence sun-safety behaviors in this patient population.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics. 2011;128:92-102.
- Benjes LS, Brooks DR, Zhang Z, et al. Changing patterns of sun protection between the first and second summers for very young children. Arch Dermatol. 2004;140:925-930.
- Oliveria SA, Saraiya M, Geller AC, et al. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131-138.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
Sun exposure and sunburns sustained during childhood are linked to an increased risk for development of skin cancers in adulthood. In infants, the skin is particularly vulnerable and is considered to be at increased risk for UV radiation damage,1 even as early as the first 6 months of life.2 Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 To effectively teach parents proper sun-safe practices, it is essential to understand their existing perceptions and behaviors. This study sought to examine differences in infant sun-safety practices during the first 6 months of life among black, Hispanic, and non-Hispanic white (NHW) parents in Miami, Florida.
Methods
Parents presenting to the University of Miami general pediatrics clinic from February 2015 through April 2015 with a child younger than 5 years were administered a 15-item questionnaire that included items on demographics, sun-safety strategies, sunburns and tanning, beliefs and limitations regarding sunscreen, and primary information source regarding sun safety (eg, physician, Internet, media, instincts). Parents were approached by the investigators consecutively for participation in scheduled blocks, with the exception of those who were otherwise engaged in appointment-related tasks (eg, paperwork). The study was approved by the University of Miami Miller School of Medicine institutional review board. The primary objective of this study was to determine the sun protection behaviors that black and Hispanic parents in Miami, Florida, employ in infants younger than 6 months. Secondary objectives included determining if this patient population is at risk for infant sunburns and tanning, beliefs among parents regarding sunscreen's efficacy in the prevention of skin cancers, and limitations of sunscreen use.
All data were analyzed using SAS software version 9.3. Wilcoxon signed rank test, Kruskal-Wallis test, Fisher exact test, and proportional-odds cumulative logit model were used to compare nonparametric data. Parents reporting on the full first 6 months of life (ie, the child was older than 6 months at the time of study completion) were included for analysis of sun-safety strategies. All survey respondents were included for analysis of secondary objectives. Responses from parents of infants of mixed racial and ethnic backgrounds were excluded from applicable subgroup analyses.
Results
Ninety-eight parents were approached for participation in the study; 97 consented to participate and 95 completed the survey. Seventy parents had children who were at least 6 months of age and were included for analysis of the primary objectives (ie, sun-protection strategies in the first 6 months of life). The cohort included 49 Hispanic parents, 26 black parents, and 9 NHW parents; 5 parents indicated their child was of mixed racial and ethnic background. Six respondents indicated another minority group (eg, Native American, Pacific Islander). Eighty-three respondents were mothers, 72 were educated beyond high school, and 14 were Spanish-speaking only. Four reported a known family history of skin cancer.
There were notable differences in application of sunscreen, belief in the efficacy of sunscreen, and primary source of information between parents (Tables 1 and 2). Hispanic parents reported applying sunscreen more consistently than black parents (odds ratio, 4.656; 95% confidence interval, 1.154-18.782; P<.01). Hispanic parents also were more likely than black parents to believe sunscreen is effective in the prevention of skin cancers (odds ratio, 7.499; 95% confidence interval, 1.535-36.620; P<.01). Hispanic parents were more likely to report receiving information regarding sun-safety practices for infants from their pediatrician, whereas NHW parents were more likely to follow their instincts regarding how and if infants should be exposed to the sun (P<.05). No significant differences were found in the reported primary source of information in black versus Hispanic parents or in black versus NHW parents. Three percent (3/95) of respondents reported a sunburn in the infant's first 6 months of life, and 12% (11/95) reported tanning of infants' skin from sun exposure. Tanning was associated with inconsistent shade (P<.01), inconsistent clothing coverage (P<.01), and consistently allowing infants to "develop tolerance to the sun's rays by slowly increasing sun exposure each day" (P<.05).
Comment
The survey results indicated suboptimal sun-protection practices among parents of black and Hispanic infants in Miami. Although the majority of respondents (83% [58/70]) reported keeping their infants in the shade, less than half of parents consistently covered their infants adequately with clothing and hats (40% [28/70] and 43% [30/70], respectively). More alarmingly, one-third of parents reported intentionally increasing their infant's level of sun exposure to develop his/her tolerance to the sun. A minority of parents reported sunburns (3%) and tanning (12%) within the first 6 months of life. Twenty-nine percent of parents (20/70) reported consistently applying sunscreen to their infants who were younger than 6 months despite limited safety data available for this age group.
Although our study included a limited sample size and represents a narrow geographic distribution, these results suggest that shortcomings in current practices in sun protection for black and Hispanic infants younger than 6 months may be a widespread problem. Black and Hispanic patients have a lower incidence of skin cancer, but the diagnosis often is delayed and the mortality is higher when skin cancer does occur.4 The common perception among laypeople as well as many health care providers that black and Hispanic individuals are not at risk for skin cancer may limit sun-safety counseling as well as the overall knowledge base of this patient demographic. As demonstrated by the results of this study, there is a need for counseling on sun-safe behaviors from a young age among this population.
Conclusion
This study highlights potential shortcomings in current sun-protection practices for black and Hispanic infants younger than 6 months. Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 Additional studies are needed to further define sun-safety behaviors in black and Hispanic children across the United States. Further, additional studies should focus on developing interventions that positively influence sun-safety behaviors in this patient population.
Sun exposure and sunburns sustained during childhood are linked to an increased risk for development of skin cancers in adulthood. In infants, the skin is particularly vulnerable and is considered to be at increased risk for UV radiation damage,1 even as early as the first 6 months of life.2 Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 To effectively teach parents proper sun-safe practices, it is essential to understand their existing perceptions and behaviors. This study sought to examine differences in infant sun-safety practices during the first 6 months of life among black, Hispanic, and non-Hispanic white (NHW) parents in Miami, Florida.
Methods
Parents presenting to the University of Miami general pediatrics clinic from February 2015 through April 2015 with a child younger than 5 years were administered a 15-item questionnaire that included items on demographics, sun-safety strategies, sunburns and tanning, beliefs and limitations regarding sunscreen, and primary information source regarding sun safety (eg, physician, Internet, media, instincts). Parents were approached by the investigators consecutively for participation in scheduled blocks, with the exception of those who were otherwise engaged in appointment-related tasks (eg, paperwork). The study was approved by the University of Miami Miller School of Medicine institutional review board. The primary objective of this study was to determine the sun protection behaviors that black and Hispanic parents in Miami, Florida, employ in infants younger than 6 months. Secondary objectives included determining if this patient population is at risk for infant sunburns and tanning, beliefs among parents regarding sunscreen's efficacy in the prevention of skin cancers, and limitations of sunscreen use.
All data were analyzed using SAS software version 9.3. Wilcoxon signed rank test, Kruskal-Wallis test, Fisher exact test, and proportional-odds cumulative logit model were used to compare nonparametric data. Parents reporting on the full first 6 months of life (ie, the child was older than 6 months at the time of study completion) were included for analysis of sun-safety strategies. All survey respondents were included for analysis of secondary objectives. Responses from parents of infants of mixed racial and ethnic backgrounds were excluded from applicable subgroup analyses.
Results
Ninety-eight parents were approached for participation in the study; 97 consented to participate and 95 completed the survey. Seventy parents had children who were at least 6 months of age and were included for analysis of the primary objectives (ie, sun-protection strategies in the first 6 months of life). The cohort included 49 Hispanic parents, 26 black parents, and 9 NHW parents; 5 parents indicated their child was of mixed racial and ethnic background. Six respondents indicated another minority group (eg, Native American, Pacific Islander). Eighty-three respondents were mothers, 72 were educated beyond high school, and 14 were Spanish-speaking only. Four reported a known family history of skin cancer.
There were notable differences in application of sunscreen, belief in the efficacy of sunscreen, and primary source of information between parents (Tables 1 and 2). Hispanic parents reported applying sunscreen more consistently than black parents (odds ratio, 4.656; 95% confidence interval, 1.154-18.782; P<.01). Hispanic parents also were more likely than black parents to believe sunscreen is effective in the prevention of skin cancers (odds ratio, 7.499; 95% confidence interval, 1.535-36.620; P<.01). Hispanic parents were more likely to report receiving information regarding sun-safety practices for infants from their pediatrician, whereas NHW parents were more likely to follow their instincts regarding how and if infants should be exposed to the sun (P<.05). No significant differences were found in the reported primary source of information in black versus Hispanic parents or in black versus NHW parents. Three percent (3/95) of respondents reported a sunburn in the infant's first 6 months of life, and 12% (11/95) reported tanning of infants' skin from sun exposure. Tanning was associated with inconsistent shade (P<.01), inconsistent clothing coverage (P<.01), and consistently allowing infants to "develop tolerance to the sun's rays by slowly increasing sun exposure each day" (P<.05).
Comment
The survey results indicated suboptimal sun-protection practices among parents of black and Hispanic infants in Miami. Although the majority of respondents (83% [58/70]) reported keeping their infants in the shade, less than half of parents consistently covered their infants adequately with clothing and hats (40% [28/70] and 43% [30/70], respectively). More alarmingly, one-third of parents reported intentionally increasing their infant's level of sun exposure to develop his/her tolerance to the sun. A minority of parents reported sunburns (3%) and tanning (12%) within the first 6 months of life. Twenty-nine percent of parents (20/70) reported consistently applying sunscreen to their infants who were younger than 6 months despite limited safety data available for this age group.
Although our study included a limited sample size and represents a narrow geographic distribution, these results suggest that shortcomings in current practices in sun protection for black and Hispanic infants younger than 6 months may be a widespread problem. Black and Hispanic patients have a lower incidence of skin cancer, but the diagnosis often is delayed and the mortality is higher when skin cancer does occur.4 The common perception among laypeople as well as many health care providers that black and Hispanic individuals are not at risk for skin cancer may limit sun-safety counseling as well as the overall knowledge base of this patient demographic. As demonstrated by the results of this study, there is a need for counseling on sun-safe behaviors from a young age among this population.
Conclusion
This study highlights potential shortcomings in current sun-protection practices for black and Hispanic infants younger than 6 months. Sun-safe behaviors instituted from a young age may help reduce the risk for future skin cancers.3 Additional studies are needed to further define sun-safety behaviors in black and Hispanic children across the United States. Further, additional studies should focus on developing interventions that positively influence sun-safety behaviors in this patient population.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics. 2011;128:92-102.
- Benjes LS, Brooks DR, Zhang Z, et al. Changing patterns of sun protection between the first and second summers for very young children. Arch Dermatol. 2004;140:925-930.
- Oliveria SA, Saraiya M, Geller AC, et al. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131-138.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
- Paller AS, Hawk JL, Honig P, et al. New insights about infant and toddler skin: implications for sun protection. Pediatrics. 2011;128:92-102.
- Benjes LS, Brooks DR, Zhang Z, et al. Changing patterns of sun protection between the first and second summers for very young children. Arch Dermatol. 2004;140:925-930.
- Oliveria SA, Saraiya M, Geller AC, et al. Sun exposure and risk of melanoma. Arch Dis Child. 2006;91:131-138.
- Wu XC, Eide MJ, King J, et al. Racial and ethnic variations in incidence and survival of cutaneous melanoma in the United States, 1999-2006. J Am Acad Dermatol. 2011;65(5 suppl 1):S26-S37.
Practice Points
- Infants of all racial and ethnic backgrounds need protection from the sun's rays. Remember to counsel parents on the importance of sun protection.
- Instruct parents to keep infants in the shade when outdoors and to dress infants in a long-sleeved shirt, pants, and a hat. Intentional sun exposure for infants is not recommended.
- The American Academy of Dermatology currently recommends that parents begin sunscreen application when their child reaches 6 months of age. Broad-spectrum barrier sunscreens containing zinc oxide or titanium dioxide are preferred and should provide a sun protection factor of 30 or greater.