User login
Case
A 69-year-old female with metastatic ovarian cancer and chronic pain syndrome presented to the hospital with seven days of progressively worsening abdominal pain. The pain had been similar to her chronic cancer pain but more severe. She has acute renal failure secondary to volume depletion from poor intake. A CT scan of the abdomen and pelvis reveal progression of her cancer with acute pathology. What is the best method of treating this patient’s pain?
Overview
Pain is pandemic. It is the most common reason patients seek healthcare.1 Almost one-third of Americans will experience severe chronic pain at some point in their lives. Every year, approximately 25 million Americans experience acute pain and 50 million experience chronic pain. Only one in four patients with pain receives appropriate therapy and control of their pain.
Pain is the most common symptom experienced by hospitalized adults.2 Acute or chronic pain can be particularly challenging to treat because these patients are frequently opioid dependent and have many psychosocial factors. No one method of pain control is superior to another. However, one method to gain rapid control of an acute pain crisis in a patient with chronic pain is to use patient-controlled analgesia (PCA).
Review of the Data
The first commercially available PCA pumps became available in 1976.3 They were created after studies in the 1960s demonstrated that small doses of opioids given intravenously provided more effective pain relief than conventional intramuscular injections.
The majority of studies on PCAs are in the postoperative patient, with cancer pain being next most commonly studied. PCAs utilize microprocessor-controlled infusion pumps that deliver a preprogrammed dose of opioid when the patient pushes the demand button. They allow programming of dose (demand dose), time between doses (lockout interval), background infusion rate (basal rate), and nurse-initiated dose (bolus dose).
The PCA paradigm is based on the opioid pharmacologic concept of minimum effective analgesic concentration (MEAC).4,5 The MEAC is the smallest serum opioid concentration at which pain is relieved. The dose-response curve to opioids is sigmoidal such that minimal analgesia is achieved until the MEAC is reached, after which minute increases in opioid concentrations produce analgesia, until further increases produce no significant increased analgesic effect.
PCAs allow individualized dosing and titration to achieve the MEAC, with small incremental doses administered whenever the serum concentration falls below the MEAC. A major goal of PCA technology is to regulate drug delivery to rapidly achieve and maintain the MEAC.
Advantages of PCAs
- More individual dosing and titration of pain medications to account for inter-individual and intra-individual variability in the response to opioids;
- Negative feedback control system, an added safety measure to avoid respiratory depression. As patients become too sedated from opioids, they are no longer able to push the button to receive further opioids;
- Higher patient satisfaction with pain control, a major determinant being personal control over the delivery of pain relief;6-8 and
- Greater analgesic efficacy vs. conventional analgesia.
Disadvantages of PCAs
Select patient populations: Not all patients are able to understand and retain the required instructions necessary to safely or effectively use self-administered opioids (e.g., cognitively impaired patients).
Potential for opioid dosing errors: These are related to equipment factors, medical personnel prescribing or programming errors.
Increased cost: PCAs have been shown to be more expensive in comparison with intramuscular (IM) injections, the prior standard of care.9-10
PCA Prescribing
The parameters programmed into the PCA machine include the basal rate, demand (or incremental) dose, lockout interval, nurse-initiated bolus dose, and choice of opioid.
Basal rate: The continuous infusion of opioid set at an hourly rate. Most studies that compare PCA use with and without basal rates (in postoperative patients) do not show improved pain relief or sleep with basal rates.11 Basal rates have been associated with increased risk of sedation and respiratory depression.12
The routine use of basal rates is not recommended initially, unless a patient is opioid-tolerant (i.e., on chronic opioid therapy). For patients on chronic opioids, their 24-hour total opioid requirement is converted by equianalgesic dosing to the basal rate. Steady state is not achieved for eight to 12 hours of continuous infusion; therefore, it is not recommended to change the basal rate more frequently than every eight hours.13
Demand dose: The dose patients provide themselves by pushing the button. Studies on opioid-naïve patients using morphine PCAs have shown that 1 mg IV morphine was the optimal starting dose, based on good pain relief without respiratory depression. Lower doses, such as 0.5 mg IV morphine, are generally used in the elderly as opioid requirements are known to decrease with patient age.14
For patients with a basal rate, the demand dose is often set at 50% to 100% of the basal rate. The demand dose is the parameter that should be titrated up for acute pain control. World Health Organization guidelines recommend increasing the dose by 25% to 50% for mild to moderate pain, and 50% to 100% for moderate to severe pain.15
Lockout interval: Minimal allowable time between demand doses. This time is based on the time to peak effect of IV opioids and can vary from five to 15 minutes. The effects of varying lockout intervals—seven to 11 minutes for morphine and five to eight minutes for fentanyl—had no effect on pain levels or side effects.16 Ten minutes is a standard lockout interval.
Bolus dose: The nurse-initiated dose that may be given initially to achieve pain control and later to counteract incidental pain (e.g., that caused by physical therapy, dressing changes, or radiology tests). A recommended dose is equivalent to the basal rate or twice the demand dose.
Choice of opioid: Morphine is the standard opioid because of its familiarity, cost, and years of study. Although inter-individual variability exists, there are no major differences in side effects among the different opioids. Renal and hepatic insufficiency can increase the effects of opioids. Morphine is especially troublesome in renal failure because it has an active metabolite—morphine-6-glucuronide—that can accumulate and increase the risk of sedation and respiratory depression.
Other Concerns
PCA complications: The most well-studied adverse effects of PCAs are nausea and respiratory depression. There is no difference between PCAs and conventional analgesia in rates of nausea or respiratory depression.17
Nausea is the most common side effect in postoperative patients on PCAs. Patients rapidly develop tolerance to nausea over a period of days. However, many clinicians are concerned about respiratory depression and the risk of death. The overall incidence of respiratory depression with PCAs is less than 1% (range from 0.1 to 0.8%), similar to conventional analgesia. However, the incidence is significantly higher when basal rates are used, rising to 1.1 to 3.9%. Other factors predisposing a patient to increased risk of respiratory depression are older age, obstructive sleep apnea, hypovolemia, renal failure, and the concurrent use of other sedating medications.18
Medication errors are also common. The overall incidence of medication mishaps with PCAs is 1.2%.19 More than 50% of these occur because of operator-related errors (e.g., improper loading, programming errors, and documentation errors). Equipment malfunction is the next most common error.
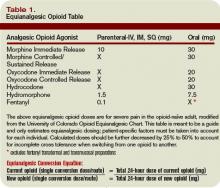
Opioid equianalgesic dosing conversions: The equianalgesic dose ratio is the ratio of the dose of two opioids required to produce the same analgesic effect. (See Table 1, right.) For example, IV morphine is three times as potent as oral morphine, with an equianalgesic dose ratio of 1:3. Equianalgesic dose tables vary somewhat in their values, which have been largely determined by single-dose administration studies.20 The generalizability of these tables to chronic opioid administration is not well studied.
Incomplete cross tolerance: When switching from one opioid to another, lower doses can be used to control pain.21, 22 Tolerance to one opioid does not completely transfer to the new opioid. Starting at half to two-thirds of the new opioid dose is generally recommended to avoid opioid-specific tolerance and inter-individual variability.23,24
Back to the Case
Opioids are the mainstay of pharmacological management of moderate-to-severe cancer pain. Evaluation of the patient reveals that her acute increase in pain is likely due to progression of her cancer. She had been taking morphine (sustained-release, 90 mg oral) twice daily for her pain and had been using approximately five doses per day of immediate-release oral morphine 20 mg for breakthrough pain. This is equivalent to a total 24-hour opioid requirement of 280 mg oral morphine.
She should be started on a PCA for rapid pain control and titration. Hydromorphone (Dilaudid) is a better PCA choice than morphine because she has acute renal failure. The equianalgesic dose ratio of oral morphine to IV hydromorphone is approximately 30:1.5. The total 24-hour opioid dose of 280 mg oral morphine is equivalent to 14 mg IV hydromorphone ([280mg morphine per day ÷ 30] x 1.5 = 14).
After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 8.4 mg IV hydromorphone (14 mg x 0.6 = 8.4 mg). This is approximately equivalent to 0.4 mg IV hydromorphone/hour (8.4 mg ÷ 24 hours), which is her initial basal rate. The demand dose should be set at 0.2 mg (50% the basal rate) with a lockout interval of 10 minutes.
Over a period of several days, the patient’s pain was controlled and her opioid requirements stabilized. She was on a basal rate of 1.4 mg/hour and a demand dose of 1 mg with a 10-minute lockout. Her total 24-hour opioid requirement was 44 mg of IV hydromorphone. As her renal function improved but did not completely normalize, oxycodone was chosen over morphine when converting her back to oral pain medications (less active renal metabolites). The equianalgesic dose ratio of oral oxycodone to IV hydromorphone is approximately 20:1.5. Her total 24-hour opioid dose of 44 mg IV hydromorphone is equivalent to 587 mg oral oxycodone (44 ÷ 1.5) x 20. After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 352 mg oral oxycodone or 180 mg of sustained-release oxycodone twice daily (352 mg ÷ 2 ≈ 180 mg). For breakthrough pain she should receive 40 mg of immediate-release oxycodone every hour as needed (10% to 15% of the 24-hour opioid requirement). TH
Dr. Youngwerth is a hospitalist and instructor of medicine, University of Colorado at Denver, assistant director, Palliative Care Consult Service, associate director, Colorado Palliative Medicine Fellowship Program, and medical director, Hospice of Saint John.
References
- American Pain Society. Pain: Current understanding of assessment, management, and treatments. National Pharmaceutical Council 2006;1-79.
- Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166:1033-1039.
- Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44-S61.
- Etches RC. Patient-controlled analgesia. Surg Clinics N Amer. 1999;79:297-312.
- Nolan MF and Wilson M-C B. Patient-controlled analgesia: A method for the controlled self-administration of opioid pain medications. Phys Ther. 1995;75:374-379.
- Ballantyne JC, Carr DB, Chalmers TC, Dear KBG, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182-193.
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:1-10.
- Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202-209.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
- Manon C, Rittenhouse BE, Perreault S, et al. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Amer Soc Anesth Inc. 1998;89:1377-1388.
- Krenn H, Oczenski W, Jellinek H, Krumpl-Ströher M, Schweitzer E, Fitzgerald RD. Nalbuphine by PCA-pump for analgesia following hysterectomy: Bolus application versus continuous infusion with bolus application. Eur J Pain. 2001;5:219-226.
- Lehmann KA. Recent developments in patient-controlled analgesia. J Pain Symptom Manage. 2005;29:S72-S89.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. 2003:1-73.
- Macintyre PC, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1995;64:357-364.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Adult cancer pain. Version 2.2005:1-30.
- Ginsberg B, Gil KM, Muir M, Sullivan F, Williams DA, Glass PSA. The influence of lockout intervals and drug selection on patient-controlled analgesia following gynecological surgery. Pain. 1995;62:95-100.
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. Acta Anaesthesiol Scand. 2001;45:795-804.
- Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125-132.
- Oswalt KE, Shrewsbury P, Stanton-Hicks M. The incidence of medication mishaps in 3,299 PCA patients. Pain. 1990;S5;S152.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose rations for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-687.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
- Mercandante S. Opioid rotation for cancer pain. Cancer. 1999;86:1856-1866.
- Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharm Sciences. 2001;22:67-70.
Case
A 69-year-old female with metastatic ovarian cancer and chronic pain syndrome presented to the hospital with seven days of progressively worsening abdominal pain. The pain had been similar to her chronic cancer pain but more severe. She has acute renal failure secondary to volume depletion from poor intake. A CT scan of the abdomen and pelvis reveal progression of her cancer with acute pathology. What is the best method of treating this patient’s pain?
Overview
Pain is pandemic. It is the most common reason patients seek healthcare.1 Almost one-third of Americans will experience severe chronic pain at some point in their lives. Every year, approximately 25 million Americans experience acute pain and 50 million experience chronic pain. Only one in four patients with pain receives appropriate therapy and control of their pain.
Pain is the most common symptom experienced by hospitalized adults.2 Acute or chronic pain can be particularly challenging to treat because these patients are frequently opioid dependent and have many psychosocial factors. No one method of pain control is superior to another. However, one method to gain rapid control of an acute pain crisis in a patient with chronic pain is to use patient-controlled analgesia (PCA).
Review of the Data
The first commercially available PCA pumps became available in 1976.3 They were created after studies in the 1960s demonstrated that small doses of opioids given intravenously provided more effective pain relief than conventional intramuscular injections.
The majority of studies on PCAs are in the postoperative patient, with cancer pain being next most commonly studied. PCAs utilize microprocessor-controlled infusion pumps that deliver a preprogrammed dose of opioid when the patient pushes the demand button. They allow programming of dose (demand dose), time between doses (lockout interval), background infusion rate (basal rate), and nurse-initiated dose (bolus dose).
The PCA paradigm is based on the opioid pharmacologic concept of minimum effective analgesic concentration (MEAC).4,5 The MEAC is the smallest serum opioid concentration at which pain is relieved. The dose-response curve to opioids is sigmoidal such that minimal analgesia is achieved until the MEAC is reached, after which minute increases in opioid concentrations produce analgesia, until further increases produce no significant increased analgesic effect.
PCAs allow individualized dosing and titration to achieve the MEAC, with small incremental doses administered whenever the serum concentration falls below the MEAC. A major goal of PCA technology is to regulate drug delivery to rapidly achieve and maintain the MEAC.
Advantages of PCAs
- More individual dosing and titration of pain medications to account for inter-individual and intra-individual variability in the response to opioids;
- Negative feedback control system, an added safety measure to avoid respiratory depression. As patients become too sedated from opioids, they are no longer able to push the button to receive further opioids;
- Higher patient satisfaction with pain control, a major determinant being personal control over the delivery of pain relief;6-8 and
- Greater analgesic efficacy vs. conventional analgesia.
Disadvantages of PCAs
Select patient populations: Not all patients are able to understand and retain the required instructions necessary to safely or effectively use self-administered opioids (e.g., cognitively impaired patients).
Potential for opioid dosing errors: These are related to equipment factors, medical personnel prescribing or programming errors.
Increased cost: PCAs have been shown to be more expensive in comparison with intramuscular (IM) injections, the prior standard of care.9-10
PCA Prescribing
The parameters programmed into the PCA machine include the basal rate, demand (or incremental) dose, lockout interval, nurse-initiated bolus dose, and choice of opioid.
Basal rate: The continuous infusion of opioid set at an hourly rate. Most studies that compare PCA use with and without basal rates (in postoperative patients) do not show improved pain relief or sleep with basal rates.11 Basal rates have been associated with increased risk of sedation and respiratory depression.12
The routine use of basal rates is not recommended initially, unless a patient is opioid-tolerant (i.e., on chronic opioid therapy). For patients on chronic opioids, their 24-hour total opioid requirement is converted by equianalgesic dosing to the basal rate. Steady state is not achieved for eight to 12 hours of continuous infusion; therefore, it is not recommended to change the basal rate more frequently than every eight hours.13
Demand dose: The dose patients provide themselves by pushing the button. Studies on opioid-naïve patients using morphine PCAs have shown that 1 mg IV morphine was the optimal starting dose, based on good pain relief without respiratory depression. Lower doses, such as 0.5 mg IV morphine, are generally used in the elderly as opioid requirements are known to decrease with patient age.14
For patients with a basal rate, the demand dose is often set at 50% to 100% of the basal rate. The demand dose is the parameter that should be titrated up for acute pain control. World Health Organization guidelines recommend increasing the dose by 25% to 50% for mild to moderate pain, and 50% to 100% for moderate to severe pain.15
Lockout interval: Minimal allowable time between demand doses. This time is based on the time to peak effect of IV opioids and can vary from five to 15 minutes. The effects of varying lockout intervals—seven to 11 minutes for morphine and five to eight minutes for fentanyl—had no effect on pain levels or side effects.16 Ten minutes is a standard lockout interval.
Bolus dose: The nurse-initiated dose that may be given initially to achieve pain control and later to counteract incidental pain (e.g., that caused by physical therapy, dressing changes, or radiology tests). A recommended dose is equivalent to the basal rate or twice the demand dose.
Choice of opioid: Morphine is the standard opioid because of its familiarity, cost, and years of study. Although inter-individual variability exists, there are no major differences in side effects among the different opioids. Renal and hepatic insufficiency can increase the effects of opioids. Morphine is especially troublesome in renal failure because it has an active metabolite—morphine-6-glucuronide—that can accumulate and increase the risk of sedation and respiratory depression.
Other Concerns
PCA complications: The most well-studied adverse effects of PCAs are nausea and respiratory depression. There is no difference between PCAs and conventional analgesia in rates of nausea or respiratory depression.17
Nausea is the most common side effect in postoperative patients on PCAs. Patients rapidly develop tolerance to nausea over a period of days. However, many clinicians are concerned about respiratory depression and the risk of death. The overall incidence of respiratory depression with PCAs is less than 1% (range from 0.1 to 0.8%), similar to conventional analgesia. However, the incidence is significantly higher when basal rates are used, rising to 1.1 to 3.9%. Other factors predisposing a patient to increased risk of respiratory depression are older age, obstructive sleep apnea, hypovolemia, renal failure, and the concurrent use of other sedating medications.18
Medication errors are also common. The overall incidence of medication mishaps with PCAs is 1.2%.19 More than 50% of these occur because of operator-related errors (e.g., improper loading, programming errors, and documentation errors). Equipment malfunction is the next most common error.
Opioid equianalgesic dosing conversions: The equianalgesic dose ratio is the ratio of the dose of two opioids required to produce the same analgesic effect. (See Table 1, right.) For example, IV morphine is three times as potent as oral morphine, with an equianalgesic dose ratio of 1:3. Equianalgesic dose tables vary somewhat in their values, which have been largely determined by single-dose administration studies.20 The generalizability of these tables to chronic opioid administration is not well studied.
Incomplete cross tolerance: When switching from one opioid to another, lower doses can be used to control pain.21, 22 Tolerance to one opioid does not completely transfer to the new opioid. Starting at half to two-thirds of the new opioid dose is generally recommended to avoid opioid-specific tolerance and inter-individual variability.23,24
Back to the Case
Opioids are the mainstay of pharmacological management of moderate-to-severe cancer pain. Evaluation of the patient reveals that her acute increase in pain is likely due to progression of her cancer. She had been taking morphine (sustained-release, 90 mg oral) twice daily for her pain and had been using approximately five doses per day of immediate-release oral morphine 20 mg for breakthrough pain. This is equivalent to a total 24-hour opioid requirement of 280 mg oral morphine.
She should be started on a PCA for rapid pain control and titration. Hydromorphone (Dilaudid) is a better PCA choice than morphine because she has acute renal failure. The equianalgesic dose ratio of oral morphine to IV hydromorphone is approximately 30:1.5. The total 24-hour opioid dose of 280 mg oral morphine is equivalent to 14 mg IV hydromorphone ([280mg morphine per day ÷ 30] x 1.5 = 14).
After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 8.4 mg IV hydromorphone (14 mg x 0.6 = 8.4 mg). This is approximately equivalent to 0.4 mg IV hydromorphone/hour (8.4 mg ÷ 24 hours), which is her initial basal rate. The demand dose should be set at 0.2 mg (50% the basal rate) with a lockout interval of 10 minutes.
Over a period of several days, the patient’s pain was controlled and her opioid requirements stabilized. She was on a basal rate of 1.4 mg/hour and a demand dose of 1 mg with a 10-minute lockout. Her total 24-hour opioid requirement was 44 mg of IV hydromorphone. As her renal function improved but did not completely normalize, oxycodone was chosen over morphine when converting her back to oral pain medications (less active renal metabolites). The equianalgesic dose ratio of oral oxycodone to IV hydromorphone is approximately 20:1.5. Her total 24-hour opioid dose of 44 mg IV hydromorphone is equivalent to 587 mg oral oxycodone (44 ÷ 1.5) x 20. After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 352 mg oral oxycodone or 180 mg of sustained-release oxycodone twice daily (352 mg ÷ 2 ≈ 180 mg). For breakthrough pain she should receive 40 mg of immediate-release oxycodone every hour as needed (10% to 15% of the 24-hour opioid requirement). TH
Dr. Youngwerth is a hospitalist and instructor of medicine, University of Colorado at Denver, assistant director, Palliative Care Consult Service, associate director, Colorado Palliative Medicine Fellowship Program, and medical director, Hospice of Saint John.
References
- American Pain Society. Pain: Current understanding of assessment, management, and treatments. National Pharmaceutical Council 2006;1-79.
- Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166:1033-1039.
- Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44-S61.
- Etches RC. Patient-controlled analgesia. Surg Clinics N Amer. 1999;79:297-312.
- Nolan MF and Wilson M-C B. Patient-controlled analgesia: A method for the controlled self-administration of opioid pain medications. Phys Ther. 1995;75:374-379.
- Ballantyne JC, Carr DB, Chalmers TC, Dear KBG, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182-193.
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:1-10.
- Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202-209.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
- Manon C, Rittenhouse BE, Perreault S, et al. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Amer Soc Anesth Inc. 1998;89:1377-1388.
- Krenn H, Oczenski W, Jellinek H, Krumpl-Ströher M, Schweitzer E, Fitzgerald RD. Nalbuphine by PCA-pump for analgesia following hysterectomy: Bolus application versus continuous infusion with bolus application. Eur J Pain. 2001;5:219-226.
- Lehmann KA. Recent developments in patient-controlled analgesia. J Pain Symptom Manage. 2005;29:S72-S89.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. 2003:1-73.
- Macintyre PC, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1995;64:357-364.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Adult cancer pain. Version 2.2005:1-30.
- Ginsberg B, Gil KM, Muir M, Sullivan F, Williams DA, Glass PSA. The influence of lockout intervals and drug selection on patient-controlled analgesia following gynecological surgery. Pain. 1995;62:95-100.
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. Acta Anaesthesiol Scand. 2001;45:795-804.
- Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125-132.
- Oswalt KE, Shrewsbury P, Stanton-Hicks M. The incidence of medication mishaps in 3,299 PCA patients. Pain. 1990;S5;S152.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose rations for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-687.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
- Mercandante S. Opioid rotation for cancer pain. Cancer. 1999;86:1856-1866.
- Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharm Sciences. 2001;22:67-70.
Case
A 69-year-old female with metastatic ovarian cancer and chronic pain syndrome presented to the hospital with seven days of progressively worsening abdominal pain. The pain had been similar to her chronic cancer pain but more severe. She has acute renal failure secondary to volume depletion from poor intake. A CT scan of the abdomen and pelvis reveal progression of her cancer with acute pathology. What is the best method of treating this patient’s pain?
Overview
Pain is pandemic. It is the most common reason patients seek healthcare.1 Almost one-third of Americans will experience severe chronic pain at some point in their lives. Every year, approximately 25 million Americans experience acute pain and 50 million experience chronic pain. Only one in four patients with pain receives appropriate therapy and control of their pain.
Pain is the most common symptom experienced by hospitalized adults.2 Acute or chronic pain can be particularly challenging to treat because these patients are frequently opioid dependent and have many psychosocial factors. No one method of pain control is superior to another. However, one method to gain rapid control of an acute pain crisis in a patient with chronic pain is to use patient-controlled analgesia (PCA).
Review of the Data
The first commercially available PCA pumps became available in 1976.3 They were created after studies in the 1960s demonstrated that small doses of opioids given intravenously provided more effective pain relief than conventional intramuscular injections.
The majority of studies on PCAs are in the postoperative patient, with cancer pain being next most commonly studied. PCAs utilize microprocessor-controlled infusion pumps that deliver a preprogrammed dose of opioid when the patient pushes the demand button. They allow programming of dose (demand dose), time between doses (lockout interval), background infusion rate (basal rate), and nurse-initiated dose (bolus dose).
The PCA paradigm is based on the opioid pharmacologic concept of minimum effective analgesic concentration (MEAC).4,5 The MEAC is the smallest serum opioid concentration at which pain is relieved. The dose-response curve to opioids is sigmoidal such that minimal analgesia is achieved until the MEAC is reached, after which minute increases in opioid concentrations produce analgesia, until further increases produce no significant increased analgesic effect.
PCAs allow individualized dosing and titration to achieve the MEAC, with small incremental doses administered whenever the serum concentration falls below the MEAC. A major goal of PCA technology is to regulate drug delivery to rapidly achieve and maintain the MEAC.
Advantages of PCAs
- More individual dosing and titration of pain medications to account for inter-individual and intra-individual variability in the response to opioids;
- Negative feedback control system, an added safety measure to avoid respiratory depression. As patients become too sedated from opioids, they are no longer able to push the button to receive further opioids;
- Higher patient satisfaction with pain control, a major determinant being personal control over the delivery of pain relief;6-8 and
- Greater analgesic efficacy vs. conventional analgesia.
Disadvantages of PCAs
Select patient populations: Not all patients are able to understand and retain the required instructions necessary to safely or effectively use self-administered opioids (e.g., cognitively impaired patients).
Potential for opioid dosing errors: These are related to equipment factors, medical personnel prescribing or programming errors.
Increased cost: PCAs have been shown to be more expensive in comparison with intramuscular (IM) injections, the prior standard of care.9-10
PCA Prescribing
The parameters programmed into the PCA machine include the basal rate, demand (or incremental) dose, lockout interval, nurse-initiated bolus dose, and choice of opioid.
Basal rate: The continuous infusion of opioid set at an hourly rate. Most studies that compare PCA use with and without basal rates (in postoperative patients) do not show improved pain relief or sleep with basal rates.11 Basal rates have been associated with increased risk of sedation and respiratory depression.12
The routine use of basal rates is not recommended initially, unless a patient is opioid-tolerant (i.e., on chronic opioid therapy). For patients on chronic opioids, their 24-hour total opioid requirement is converted by equianalgesic dosing to the basal rate. Steady state is not achieved for eight to 12 hours of continuous infusion; therefore, it is not recommended to change the basal rate more frequently than every eight hours.13
Demand dose: The dose patients provide themselves by pushing the button. Studies on opioid-naïve patients using morphine PCAs have shown that 1 mg IV morphine was the optimal starting dose, based on good pain relief without respiratory depression. Lower doses, such as 0.5 mg IV morphine, are generally used in the elderly as opioid requirements are known to decrease with patient age.14
For patients with a basal rate, the demand dose is often set at 50% to 100% of the basal rate. The demand dose is the parameter that should be titrated up for acute pain control. World Health Organization guidelines recommend increasing the dose by 25% to 50% for mild to moderate pain, and 50% to 100% for moderate to severe pain.15
Lockout interval: Minimal allowable time between demand doses. This time is based on the time to peak effect of IV opioids and can vary from five to 15 minutes. The effects of varying lockout intervals—seven to 11 minutes for morphine and five to eight minutes for fentanyl—had no effect on pain levels or side effects.16 Ten minutes is a standard lockout interval.
Bolus dose: The nurse-initiated dose that may be given initially to achieve pain control and later to counteract incidental pain (e.g., that caused by physical therapy, dressing changes, or radiology tests). A recommended dose is equivalent to the basal rate or twice the demand dose.
Choice of opioid: Morphine is the standard opioid because of its familiarity, cost, and years of study. Although inter-individual variability exists, there are no major differences in side effects among the different opioids. Renal and hepatic insufficiency can increase the effects of opioids. Morphine is especially troublesome in renal failure because it has an active metabolite—morphine-6-glucuronide—that can accumulate and increase the risk of sedation and respiratory depression.
Other Concerns
PCA complications: The most well-studied adverse effects of PCAs are nausea and respiratory depression. There is no difference between PCAs and conventional analgesia in rates of nausea or respiratory depression.17
Nausea is the most common side effect in postoperative patients on PCAs. Patients rapidly develop tolerance to nausea over a period of days. However, many clinicians are concerned about respiratory depression and the risk of death. The overall incidence of respiratory depression with PCAs is less than 1% (range from 0.1 to 0.8%), similar to conventional analgesia. However, the incidence is significantly higher when basal rates are used, rising to 1.1 to 3.9%. Other factors predisposing a patient to increased risk of respiratory depression are older age, obstructive sleep apnea, hypovolemia, renal failure, and the concurrent use of other sedating medications.18
Medication errors are also common. The overall incidence of medication mishaps with PCAs is 1.2%.19 More than 50% of these occur because of operator-related errors (e.g., improper loading, programming errors, and documentation errors). Equipment malfunction is the next most common error.
Opioid equianalgesic dosing conversions: The equianalgesic dose ratio is the ratio of the dose of two opioids required to produce the same analgesic effect. (See Table 1, right.) For example, IV morphine is three times as potent as oral morphine, with an equianalgesic dose ratio of 1:3. Equianalgesic dose tables vary somewhat in their values, which have been largely determined by single-dose administration studies.20 The generalizability of these tables to chronic opioid administration is not well studied.
Incomplete cross tolerance: When switching from one opioid to another, lower doses can be used to control pain.21, 22 Tolerance to one opioid does not completely transfer to the new opioid. Starting at half to two-thirds of the new opioid dose is generally recommended to avoid opioid-specific tolerance and inter-individual variability.23,24
Back to the Case
Opioids are the mainstay of pharmacological management of moderate-to-severe cancer pain. Evaluation of the patient reveals that her acute increase in pain is likely due to progression of her cancer. She had been taking morphine (sustained-release, 90 mg oral) twice daily for her pain and had been using approximately five doses per day of immediate-release oral morphine 20 mg for breakthrough pain. This is equivalent to a total 24-hour opioid requirement of 280 mg oral morphine.
She should be started on a PCA for rapid pain control and titration. Hydromorphone (Dilaudid) is a better PCA choice than morphine because she has acute renal failure. The equianalgesic dose ratio of oral morphine to IV hydromorphone is approximately 30:1.5. The total 24-hour opioid dose of 280 mg oral morphine is equivalent to 14 mg IV hydromorphone ([280mg morphine per day ÷ 30] x 1.5 = 14).
After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 8.4 mg IV hydromorphone (14 mg x 0.6 = 8.4 mg). This is approximately equivalent to 0.4 mg IV hydromorphone/hour (8.4 mg ÷ 24 hours), which is her initial basal rate. The demand dose should be set at 0.2 mg (50% the basal rate) with a lockout interval of 10 minutes.
Over a period of several days, the patient’s pain was controlled and her opioid requirements stabilized. She was on a basal rate of 1.4 mg/hour and a demand dose of 1 mg with a 10-minute lockout. Her total 24-hour opioid requirement was 44 mg of IV hydromorphone. As her renal function improved but did not completely normalize, oxycodone was chosen over morphine when converting her back to oral pain medications (less active renal metabolites). The equianalgesic dose ratio of oral oxycodone to IV hydromorphone is approximately 20:1.5. Her total 24-hour opioid dose of 44 mg IV hydromorphone is equivalent to 587 mg oral oxycodone (44 ÷ 1.5) x 20. After adjusting for 60% incomplete cross tolerance, the total 24-hour opioid dose is reduced to 352 mg oral oxycodone or 180 mg of sustained-release oxycodone twice daily (352 mg ÷ 2 ≈ 180 mg). For breakthrough pain she should receive 40 mg of immediate-release oxycodone every hour as needed (10% to 15% of the 24-hour opioid requirement). TH
Dr. Youngwerth is a hospitalist and instructor of medicine, University of Colorado at Denver, assistant director, Palliative Care Consult Service, associate director, Colorado Palliative Medicine Fellowship Program, and medical director, Hospice of Saint John.
References
- American Pain Society. Pain: Current understanding of assessment, management, and treatments. National Pharmaceutical Council 2006;1-79.
- Morrison RS, Meier DE, Fischberg D, et al. Improving the management of pain in hospitalized adults. Arch Intern Med. 2006;166:1033-1039.
- Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101:S44-S61.
- Etches RC. Patient-controlled analgesia. Surg Clinics N Amer. 1999;79:297-312.
- Nolan MF and Wilson M-C B. Patient-controlled analgesia: A method for the controlled self-administration of opioid pain medications. Phys Ther. 1995;75:374-379.
- Ballantyne JC, Carr DB, Chalmers TC, Dear KBG, Angelillo IF, Mosteller F. Postoperative patient-controlled analgesia: Meta-analyses of initial randomized control trials. J Clin Anesth. 1993;5:182-193.
- Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database of Systematic Reviews. 2006;4:1-10.
- Sidebotham D, Dijkhuizen MRJ, Schug SA. The safety and utilization of patient-controlled analgesia. J Pain Symptom Manage. 1997;14:202-209.
- Macintyre PE. Safety and efficacy of patient-controlled analgesia. Br J Anaesth. 2001;87:36-46.
- Manon C, Rittenhouse BE, Perreault S, et al. Efficacy and costs of patient-controlled analgesia versus regularly administered intramuscular opioid therapy. Amer Soc Anesth Inc. 1998;89:1377-1388.
- Krenn H, Oczenski W, Jellinek H, Krumpl-Ströher M, Schweitzer E, Fitzgerald RD. Nalbuphine by PCA-pump for analgesia following hysterectomy: Bolus application versus continuous infusion with bolus application. Eur J Pain. 2001;5:219-226.
- Lehmann KA. Recent developments in patient-controlled analgesia. J Pain Symptom Manage. 2005;29:S72-S89.
- American Pain Society. Principles of analgesic use in the treatment of acute pain and cancer pain. 5th ed. 2003:1-73.
- Macintyre PC, Jarvis DA. Age is the best predictor of postoperative morphine requirements. Pain. 1995;64:357-364.
- National Comprehensive Cancer Network. Clinical practice guidelines in oncology: Adult cancer pain. Version 2.2005:1-30.
- Ginsberg B, Gil KM, Muir M, Sullivan F, Williams DA, Glass PSA. The influence of lockout intervals and drug selection on patient-controlled analgesia following gynecological surgery. Pain. 1995;62:95-100.
- Walder B, Schafer M, Henzi I, Tramer MR. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. Acta Anaesthesiol Scand. 2001;45:795-804.
- Etches RC. Respiratory depression associated with patient-controlled analgesia: a review of eight cases. Can J Anaesth. 1994;41:125-132.
- Oswalt KE, Shrewsbury P, Stanton-Hicks M. The incidence of medication mishaps in 3,299 PCA patients. Pain. 1990;S5;S152.
- Pereira J, Lawlor P, Vigano A, Dorgan M, Bruera E. Equianalgesic dose rations for opioids: A critical review and proposals for long-term dosing. J Pain Symptom Manage. 2001;22:672-687.
- Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943-1953.
- Mercandante S. Opioid rotation for cancer pain. Cancer. 1999;86:1856-1866.
- Mehta V, Langford RM. Acute pain management for opioid dependent patients. Anaesthesia. 2006;61:269-276.
- Pasternak GW. Incomplete cross tolerance and multiple mu opioid peptide receptors. Trends Pharm Sciences. 2001;22:67-70.