User login
Case
The orthopedic service asks you to evaluate a 76-year-old woman with a hip fracture. She has diabetes, hypertension, and hyperlipidemia but no known coronary artery disease (CAD). She says she can carry a bag of groceries up one flight of stairs without chest symptoms.
Her physical exam is significant only for a shortened, internally rotated right hip. Her blood pressure is 160/88 mm/hg, her pulse is 75 beats per minute, and her respiratory rate is 16 breaths a minute with an oxygen saturation of 95% on one liter. Her creatinine is 1.2 mg/dL, and her fasting glucose is 106 mg/dL. An electrocardiogram reveals normal sinus rhythm without evidence of prior myocardial infarction (MI).
Her medications are lisinopril, atorvastatin, aspirin, fluoxetine, and diazepam. She is scheduled for the operating room tomorrow. What is the best strategy to evaluate and minimize her perioperative cardiac risk, and does it include a beta-blocker?
Overview
There are many ways to identify patients at risk for perioperative cardiac complications—but few simple, safe, evidence-based means of mitigating risk.1
Over the past 10 years, the general approach has been that preoperative revascularization is beneficial in a limited number of clinical scenarios. Further, beta-blockers reduce risk in nearly all other high- and intermediate-risk patients. Unfortunately, routine perioperative administration of beta-blockers to intermediate-risk patients is not supported by trial evidence and may expose these patients to increased risk of adverse outcomes—including death and stroke.
Review of the Data
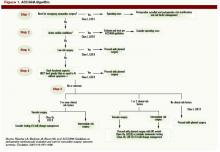
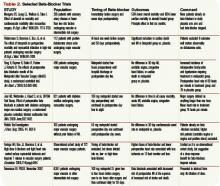
Intermediate-risk patients: Inter-mediate risk patients have recently been redefined as patients with a Revised Cardiac Risk Index (RCRI) score of two or one (See Table 1, p. 27).2,3 Older guidelines suggested noninvasive testing for such patients if they had poor functional capacity (less than four metabolic equivalents [METS]) and were undergoing intermediate-risk surgery, including orthopedic, peritoneal, and thoracic procedures.
Unfortunately, this situation is common, leading to frequent testing and unclear benefit to patients. Omission of a noninvasive evaluation in intermediate-risk orthopedic surgery patients is not associated with an increase in perioperative cardiac events.4 Most events occur in patients who did not meet criteria for preoperative testing.
The 2007 ACC/AHA Guidelines for Perioperative Evaluation and Care address this by recommending noninvasive testing only “if it will change management.” But they offer little guidance in unclear clinical situations, such as the urgent hip-fracture repair needed by our patient.
Preoperative revascularization: While it makes intuitive sense that preoperative revascularization of high-risk patients would decrease their risk of perioperative cardiac complications, evidence countering this idea is nearly definitive. In a study by McFalls, revascularization prior to major vascular surgery did not decrease the risk of perioperative MI or 30-day mortality; however, it delayed the surgical procedure, even in patients with high-risk noninvasive test results.5,6 It is generally accepted that if these high-risk patients can safely undergo major vascular surgery without revascularization, a lower-risk patient such as ours can do so at even lower risk.
In these trials, revascularization occurred in addition to medical management of coronary disease, including aspirin, statin, and—particularly in the study by Poldermans,6 where beta-blockers were started and titrated well before surgery—beta-blocker therapy.
Patients with active cardiac symptoms or signs or uncharacterized anginal symptoms should have elective surgery delayed. However, delay is rarely an option for the hospitalist, who is typically asked to address a patient’s risk shortly before urgent or emergent surgery. These difficult situations require one to weigh the cardiac risk of surgery in a patient who is not optimized versus the risk of delaying surgery to address the more urgent cardiac situation.
Timing of perioperative percutaneous intervention: For patients with coronary artery disease (CAD) or coronary lesions, the interval between percutaneous revascularization (via stent or percutaneous transluminal coronary angioplasty [PTCA]) and surgery affects rates of postoperative cardiac events.7
The recommended interval between stent placement and noncardiac surgery for patients receiving bare-metal and drug-eluting stents is six weeks and one year, respectively.8 Surgery within two weeks of stent placement can carry mortality rates as high as 40%, and this risk appears to decrease out to one year.9,10 If a new stent is in place, any potential benefit appears to be offset by the increased risk of in-stent thrombosis with subsequent MI and possible death. PTCA may not be a safe alternative, although some recommend using PTCA if the patient has unstable cardiac symptoms and needs urgent/emergent surgery.11
Perioperative discontinuation of dual antiplatelet agents (e.g., clopidogrel and aspirin) is common and appears to increase thrombosis risk. This presents a challenge when patients with recent stent placement present for urgent surgery. Minimizing the interruption of dual antiplatelet therapy is the most important intervention a hospitalist can perform. Interruption is associated with increased risk of stent thrombosis, MI, and death. If clopidogrel must be discontinued in the perioperative period, continuation of aspirin is recommended and intravenous glycoprotein 2b/3a inhibitors can be considered.12
Perioperative beta-blocker: Studies on the outcomes of perioperative beta blockade strongly suggested benefits initially. But a number of randomized trials in the past three years have not shown a positive effect.
In a landmark study published in 1996, Mangano showed that initiation of beta blockade just prior to surgery reduced perioperative MI and cardiac death in a mixed surgical population.13 Similar findings were seen with initiation of beta-blocker one month prior to vascular surgery.14 Additionally, higher doses of beta-blocker and lower heart rates in the perioperative period seem to be associated with decreased troponin release.15 Finally, perioperative beta blockade was associated with decreased mortality in high-risk patients (RCRI of three or greater), but higher mortality in lower-risk patients (e.g., RCRI of zero or one).16
More recent data reveal less benefit for perioperative beta blockade. Yang, et al., suggested that initiation of beta-blockers just prior to surgery did not decrease postoperative cardiac complications in vascular surgery patients.17 Similar results were found in a cohort of diabetic patients undergoing major surgery.18 A subsequent meta-analysis concluded that, in the aggregate, perioperative beta blockade was neither beneficial nor harmful.19
Further data have shown increased mortality with perioperative beta blockade in low-risk patients. Most recently, an abstract from the largest randomized controlled trial to date, the POISE study, suggested that preoperative beta blockade decreased MI and cardiac death, but increased the risk of stroke and produced higher overall mortality.20
It is challenging to reconcile this newer evidence with the previous data. While it seems intuitive that blunting the catecholamine response would minimize cardiac workload and therefore decrease perioperative infarcts, surgical patients are also at risk for poor pain control, sepsis, hypovolemia, and venous thromboembolism. Beta blockade can obscure their clinical manifestations, delaying diagnosis or complicating therapy. Inconsistencies among studies and published guidelines make them difficult to apply broadly, particularly with the intermediate-risk patient. Finally, perioperative beta blockade is poorly defined in terms of timing of initiation, target heart rate, and duration of postoperative use.
Until more definitive trial data are published, it seems most prudent to continue beta-blockers in patients already using them. Start them as far in advance of surgery as possible in patients with high-risk features (such as a positive stress test). After surgery, pay close attention to volume status, pain, signs of sepsis, or other noncardiac complications.
Back to the Case
As per the 2007 ACC/AHA guidelines, this patient with one clinical risk factor (diabetes) and good functional capacity can proceed to the operating room without further intervention. While it is likely a patient with diabetes and hyperlipidemia has some degree of CAD, including possible vulnerable plaques, the best medical evidence offers little to decrease her operative cardiac risk. Perioperative beta blockade is not indicated at her level of risk (RCRI of one) given the inconsistent benefits and possible harm to patients like this seen in trials to date.
If she were limited in terms of functional capacity (i.e., less than four METS), the 2007 ACC/AHA algorithm suggests preoperative noninvasive testing “if it would change management.”
How might a positive stress test change management in this case? Revascularization with stenting in close proximity to noncardiac surgery is not safe, and there appears to be no benefit to preoperative revascularization before high-risk vascular surgery. However, ischemia on preoperative testing is an indication for a beta-blocker. A brief delay in her surgery to allow dose titration and use of telemetry monitoring after surgery would increase the safety of beta-blockers after surgery. How long to continue beta-blockers is an open question, but at least 30 days would seem adequate, tapering rather than abruptly discontinuing the dose. TH
Dr. Carter is an assistant professor of medicine at the University of Colorado Denver in the Section of Hospital Medicine, where he directs the Medicine Consult Service. Dr. Auerbach is an associate professor of medicine in residence, associate director of the general medicine research fellowship, director of quality improvement for the UCSF Department of Medicine, and director of the surgical care Improvement program at UCSF. His research interests include perioperative medicine and quality improvement.
References
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. Circulation 2002;105:1257-1267.
- Fleischer LA, Beckman JA, Brown KA, et al. ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for noncardiac surgery: executive summary. Circulation. 2007;116:1971-1996.
- Salerno SM, Carlson DW, Soh EK, et al. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763-1769.
- Wilson, SH, Fasseas P, Orford JL, et al. Clinical outcomes of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
- Grines, CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007; 115:813-818.
- Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. Am J Coll Cardiol. 2000;35(5):1288-1294.
- Schouten O, Bax JJ, Damen J, et al. Coronary stent placement immediately before non cardiac surgery: a potential risk? Anesthesiology 106(5);2007:1067.
- Leibowitz D, Cohen M, Planer D, et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
- Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006;113:1361-1376.
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335(23):1713-1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341(24):1789-1794.
- Feringa HH, Bax JJ, Boersma E, et al. High dose b-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. 2006;114(supp):I344.
- Lindenauer PK Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative ß blockade in patients with diabetes undergoing major non-cardiac surgery: randomized placebo controlled, blinded multicentre trial. BMJ. 2006 June;332:1482.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative ß blockers in noncardiac surgery? Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;331:313.
- Devereaux PJ. POISE Abstract. American Heart Association Annual Scientific Session, Orlando, Fla., November 2007.
Case
The orthopedic service asks you to evaluate a 76-year-old woman with a hip fracture. She has diabetes, hypertension, and hyperlipidemia but no known coronary artery disease (CAD). She says she can carry a bag of groceries up one flight of stairs without chest symptoms.
Her physical exam is significant only for a shortened, internally rotated right hip. Her blood pressure is 160/88 mm/hg, her pulse is 75 beats per minute, and her respiratory rate is 16 breaths a minute with an oxygen saturation of 95% on one liter. Her creatinine is 1.2 mg/dL, and her fasting glucose is 106 mg/dL. An electrocardiogram reveals normal sinus rhythm without evidence of prior myocardial infarction (MI).
Her medications are lisinopril, atorvastatin, aspirin, fluoxetine, and diazepam. She is scheduled for the operating room tomorrow. What is the best strategy to evaluate and minimize her perioperative cardiac risk, and does it include a beta-blocker?
Overview
There are many ways to identify patients at risk for perioperative cardiac complications—but few simple, safe, evidence-based means of mitigating risk.1
Over the past 10 years, the general approach has been that preoperative revascularization is beneficial in a limited number of clinical scenarios. Further, beta-blockers reduce risk in nearly all other high- and intermediate-risk patients. Unfortunately, routine perioperative administration of beta-blockers to intermediate-risk patients is not supported by trial evidence and may expose these patients to increased risk of adverse outcomes—including death and stroke.
Review of the Data
Intermediate-risk patients: Inter-mediate risk patients have recently been redefined as patients with a Revised Cardiac Risk Index (RCRI) score of two or one (See Table 1, p. 27).2,3 Older guidelines suggested noninvasive testing for such patients if they had poor functional capacity (less than four metabolic equivalents [METS]) and were undergoing intermediate-risk surgery, including orthopedic, peritoneal, and thoracic procedures.
Unfortunately, this situation is common, leading to frequent testing and unclear benefit to patients. Omission of a noninvasive evaluation in intermediate-risk orthopedic surgery patients is not associated with an increase in perioperative cardiac events.4 Most events occur in patients who did not meet criteria for preoperative testing.
The 2007 ACC/AHA Guidelines for Perioperative Evaluation and Care address this by recommending noninvasive testing only “if it will change management.” But they offer little guidance in unclear clinical situations, such as the urgent hip-fracture repair needed by our patient.
Preoperative revascularization: While it makes intuitive sense that preoperative revascularization of high-risk patients would decrease their risk of perioperative cardiac complications, evidence countering this idea is nearly definitive. In a study by McFalls, revascularization prior to major vascular surgery did not decrease the risk of perioperative MI or 30-day mortality; however, it delayed the surgical procedure, even in patients with high-risk noninvasive test results.5,6 It is generally accepted that if these high-risk patients can safely undergo major vascular surgery without revascularization, a lower-risk patient such as ours can do so at even lower risk.
In these trials, revascularization occurred in addition to medical management of coronary disease, including aspirin, statin, and—particularly in the study by Poldermans,6 where beta-blockers were started and titrated well before surgery—beta-blocker therapy.
Patients with active cardiac symptoms or signs or uncharacterized anginal symptoms should have elective surgery delayed. However, delay is rarely an option for the hospitalist, who is typically asked to address a patient’s risk shortly before urgent or emergent surgery. These difficult situations require one to weigh the cardiac risk of surgery in a patient who is not optimized versus the risk of delaying surgery to address the more urgent cardiac situation.
Timing of perioperative percutaneous intervention: For patients with coronary artery disease (CAD) or coronary lesions, the interval between percutaneous revascularization (via stent or percutaneous transluminal coronary angioplasty [PTCA]) and surgery affects rates of postoperative cardiac events.7
The recommended interval between stent placement and noncardiac surgery for patients receiving bare-metal and drug-eluting stents is six weeks and one year, respectively.8 Surgery within two weeks of stent placement can carry mortality rates as high as 40%, and this risk appears to decrease out to one year.9,10 If a new stent is in place, any potential benefit appears to be offset by the increased risk of in-stent thrombosis with subsequent MI and possible death. PTCA may not be a safe alternative, although some recommend using PTCA if the patient has unstable cardiac symptoms and needs urgent/emergent surgery.11
Perioperative discontinuation of dual antiplatelet agents (e.g., clopidogrel and aspirin) is common and appears to increase thrombosis risk. This presents a challenge when patients with recent stent placement present for urgent surgery. Minimizing the interruption of dual antiplatelet therapy is the most important intervention a hospitalist can perform. Interruption is associated with increased risk of stent thrombosis, MI, and death. If clopidogrel must be discontinued in the perioperative period, continuation of aspirin is recommended and intravenous glycoprotein 2b/3a inhibitors can be considered.12
Perioperative beta-blocker: Studies on the outcomes of perioperative beta blockade strongly suggested benefits initially. But a number of randomized trials in the past three years have not shown a positive effect.
In a landmark study published in 1996, Mangano showed that initiation of beta blockade just prior to surgery reduced perioperative MI and cardiac death in a mixed surgical population.13 Similar findings were seen with initiation of beta-blocker one month prior to vascular surgery.14 Additionally, higher doses of beta-blocker and lower heart rates in the perioperative period seem to be associated with decreased troponin release.15 Finally, perioperative beta blockade was associated with decreased mortality in high-risk patients (RCRI of three or greater), but higher mortality in lower-risk patients (e.g., RCRI of zero or one).16
More recent data reveal less benefit for perioperative beta blockade. Yang, et al., suggested that initiation of beta-blockers just prior to surgery did not decrease postoperative cardiac complications in vascular surgery patients.17 Similar results were found in a cohort of diabetic patients undergoing major surgery.18 A subsequent meta-analysis concluded that, in the aggregate, perioperative beta blockade was neither beneficial nor harmful.19
Further data have shown increased mortality with perioperative beta blockade in low-risk patients. Most recently, an abstract from the largest randomized controlled trial to date, the POISE study, suggested that preoperative beta blockade decreased MI and cardiac death, but increased the risk of stroke and produced higher overall mortality.20
It is challenging to reconcile this newer evidence with the previous data. While it seems intuitive that blunting the catecholamine response would minimize cardiac workload and therefore decrease perioperative infarcts, surgical patients are also at risk for poor pain control, sepsis, hypovolemia, and venous thromboembolism. Beta blockade can obscure their clinical manifestations, delaying diagnosis or complicating therapy. Inconsistencies among studies and published guidelines make them difficult to apply broadly, particularly with the intermediate-risk patient. Finally, perioperative beta blockade is poorly defined in terms of timing of initiation, target heart rate, and duration of postoperative use.
Until more definitive trial data are published, it seems most prudent to continue beta-blockers in patients already using them. Start them as far in advance of surgery as possible in patients with high-risk features (such as a positive stress test). After surgery, pay close attention to volume status, pain, signs of sepsis, or other noncardiac complications.
Back to the Case
As per the 2007 ACC/AHA guidelines, this patient with one clinical risk factor (diabetes) and good functional capacity can proceed to the operating room without further intervention. While it is likely a patient with diabetes and hyperlipidemia has some degree of CAD, including possible vulnerable plaques, the best medical evidence offers little to decrease her operative cardiac risk. Perioperative beta blockade is not indicated at her level of risk (RCRI of one) given the inconsistent benefits and possible harm to patients like this seen in trials to date.
If she were limited in terms of functional capacity (i.e., less than four METS), the 2007 ACC/AHA algorithm suggests preoperative noninvasive testing “if it would change management.”
How might a positive stress test change management in this case? Revascularization with stenting in close proximity to noncardiac surgery is not safe, and there appears to be no benefit to preoperative revascularization before high-risk vascular surgery. However, ischemia on preoperative testing is an indication for a beta-blocker. A brief delay in her surgery to allow dose titration and use of telemetry monitoring after surgery would increase the safety of beta-blockers after surgery. How long to continue beta-blockers is an open question, but at least 30 days would seem adequate, tapering rather than abruptly discontinuing the dose. TH
Dr. Carter is an assistant professor of medicine at the University of Colorado Denver in the Section of Hospital Medicine, where he directs the Medicine Consult Service. Dr. Auerbach is an associate professor of medicine in residence, associate director of the general medicine research fellowship, director of quality improvement for the UCSF Department of Medicine, and director of the surgical care Improvement program at UCSF. His research interests include perioperative medicine and quality improvement.
References
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. Circulation 2002;105:1257-1267.
- Fleischer LA, Beckman JA, Brown KA, et al. ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for noncardiac surgery: executive summary. Circulation. 2007;116:1971-1996.
- Salerno SM, Carlson DW, Soh EK, et al. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763-1769.
- Wilson, SH, Fasseas P, Orford JL, et al. Clinical outcomes of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
- Grines, CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007; 115:813-818.
- Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. Am J Coll Cardiol. 2000;35(5):1288-1294.
- Schouten O, Bax JJ, Damen J, et al. Coronary stent placement immediately before non cardiac surgery: a potential risk? Anesthesiology 106(5);2007:1067.
- Leibowitz D, Cohen M, Planer D, et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
- Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006;113:1361-1376.
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335(23):1713-1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341(24):1789-1794.
- Feringa HH, Bax JJ, Boersma E, et al. High dose b-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. 2006;114(supp):I344.
- Lindenauer PK Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative ß blockade in patients with diabetes undergoing major non-cardiac surgery: randomized placebo controlled, blinded multicentre trial. BMJ. 2006 June;332:1482.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative ß blockers in noncardiac surgery? Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;331:313.
- Devereaux PJ. POISE Abstract. American Heart Association Annual Scientific Session, Orlando, Fla., November 2007.
Case
The orthopedic service asks you to evaluate a 76-year-old woman with a hip fracture. She has diabetes, hypertension, and hyperlipidemia but no known coronary artery disease (CAD). She says she can carry a bag of groceries up one flight of stairs without chest symptoms.
Her physical exam is significant only for a shortened, internally rotated right hip. Her blood pressure is 160/88 mm/hg, her pulse is 75 beats per minute, and her respiratory rate is 16 breaths a minute with an oxygen saturation of 95% on one liter. Her creatinine is 1.2 mg/dL, and her fasting glucose is 106 mg/dL. An electrocardiogram reveals normal sinus rhythm without evidence of prior myocardial infarction (MI).
Her medications are lisinopril, atorvastatin, aspirin, fluoxetine, and diazepam. She is scheduled for the operating room tomorrow. What is the best strategy to evaluate and minimize her perioperative cardiac risk, and does it include a beta-blocker?
Overview
There are many ways to identify patients at risk for perioperative cardiac complications—but few simple, safe, evidence-based means of mitigating risk.1
Over the past 10 years, the general approach has been that preoperative revascularization is beneficial in a limited number of clinical scenarios. Further, beta-blockers reduce risk in nearly all other high- and intermediate-risk patients. Unfortunately, routine perioperative administration of beta-blockers to intermediate-risk patients is not supported by trial evidence and may expose these patients to increased risk of adverse outcomes—including death and stroke.
Review of the Data
Intermediate-risk patients: Inter-mediate risk patients have recently been redefined as patients with a Revised Cardiac Risk Index (RCRI) score of two or one (See Table 1, p. 27).2,3 Older guidelines suggested noninvasive testing for such patients if they had poor functional capacity (less than four metabolic equivalents [METS]) and were undergoing intermediate-risk surgery, including orthopedic, peritoneal, and thoracic procedures.
Unfortunately, this situation is common, leading to frequent testing and unclear benefit to patients. Omission of a noninvasive evaluation in intermediate-risk orthopedic surgery patients is not associated with an increase in perioperative cardiac events.4 Most events occur in patients who did not meet criteria for preoperative testing.
The 2007 ACC/AHA Guidelines for Perioperative Evaluation and Care address this by recommending noninvasive testing only “if it will change management.” But they offer little guidance in unclear clinical situations, such as the urgent hip-fracture repair needed by our patient.
Preoperative revascularization: While it makes intuitive sense that preoperative revascularization of high-risk patients would decrease their risk of perioperative cardiac complications, evidence countering this idea is nearly definitive. In a study by McFalls, revascularization prior to major vascular surgery did not decrease the risk of perioperative MI or 30-day mortality; however, it delayed the surgical procedure, even in patients with high-risk noninvasive test results.5,6 It is generally accepted that if these high-risk patients can safely undergo major vascular surgery without revascularization, a lower-risk patient such as ours can do so at even lower risk.
In these trials, revascularization occurred in addition to medical management of coronary disease, including aspirin, statin, and—particularly in the study by Poldermans,6 where beta-blockers were started and titrated well before surgery—beta-blocker therapy.
Patients with active cardiac symptoms or signs or uncharacterized anginal symptoms should have elective surgery delayed. However, delay is rarely an option for the hospitalist, who is typically asked to address a patient’s risk shortly before urgent or emergent surgery. These difficult situations require one to weigh the cardiac risk of surgery in a patient who is not optimized versus the risk of delaying surgery to address the more urgent cardiac situation.
Timing of perioperative percutaneous intervention: For patients with coronary artery disease (CAD) or coronary lesions, the interval between percutaneous revascularization (via stent or percutaneous transluminal coronary angioplasty [PTCA]) and surgery affects rates of postoperative cardiac events.7
The recommended interval between stent placement and noncardiac surgery for patients receiving bare-metal and drug-eluting stents is six weeks and one year, respectively.8 Surgery within two weeks of stent placement can carry mortality rates as high as 40%, and this risk appears to decrease out to one year.9,10 If a new stent is in place, any potential benefit appears to be offset by the increased risk of in-stent thrombosis with subsequent MI and possible death. PTCA may not be a safe alternative, although some recommend using PTCA if the patient has unstable cardiac symptoms and needs urgent/emergent surgery.11
Perioperative discontinuation of dual antiplatelet agents (e.g., clopidogrel and aspirin) is common and appears to increase thrombosis risk. This presents a challenge when patients with recent stent placement present for urgent surgery. Minimizing the interruption of dual antiplatelet therapy is the most important intervention a hospitalist can perform. Interruption is associated with increased risk of stent thrombosis, MI, and death. If clopidogrel must be discontinued in the perioperative period, continuation of aspirin is recommended and intravenous glycoprotein 2b/3a inhibitors can be considered.12
Perioperative beta-blocker: Studies on the outcomes of perioperative beta blockade strongly suggested benefits initially. But a number of randomized trials in the past three years have not shown a positive effect.
In a landmark study published in 1996, Mangano showed that initiation of beta blockade just prior to surgery reduced perioperative MI and cardiac death in a mixed surgical population.13 Similar findings were seen with initiation of beta-blocker one month prior to vascular surgery.14 Additionally, higher doses of beta-blocker and lower heart rates in the perioperative period seem to be associated with decreased troponin release.15 Finally, perioperative beta blockade was associated with decreased mortality in high-risk patients (RCRI of three or greater), but higher mortality in lower-risk patients (e.g., RCRI of zero or one).16
More recent data reveal less benefit for perioperative beta blockade. Yang, et al., suggested that initiation of beta-blockers just prior to surgery did not decrease postoperative cardiac complications in vascular surgery patients.17 Similar results were found in a cohort of diabetic patients undergoing major surgery.18 A subsequent meta-analysis concluded that, in the aggregate, perioperative beta blockade was neither beneficial nor harmful.19
Further data have shown increased mortality with perioperative beta blockade in low-risk patients. Most recently, an abstract from the largest randomized controlled trial to date, the POISE study, suggested that preoperative beta blockade decreased MI and cardiac death, but increased the risk of stroke and produced higher overall mortality.20
It is challenging to reconcile this newer evidence with the previous data. While it seems intuitive that blunting the catecholamine response would minimize cardiac workload and therefore decrease perioperative infarcts, surgical patients are also at risk for poor pain control, sepsis, hypovolemia, and venous thromboembolism. Beta blockade can obscure their clinical manifestations, delaying diagnosis or complicating therapy. Inconsistencies among studies and published guidelines make them difficult to apply broadly, particularly with the intermediate-risk patient. Finally, perioperative beta blockade is poorly defined in terms of timing of initiation, target heart rate, and duration of postoperative use.
Until more definitive trial data are published, it seems most prudent to continue beta-blockers in patients already using them. Start them as far in advance of surgery as possible in patients with high-risk features (such as a positive stress test). After surgery, pay close attention to volume status, pain, signs of sepsis, or other noncardiac complications.
Back to the Case
As per the 2007 ACC/AHA guidelines, this patient with one clinical risk factor (diabetes) and good functional capacity can proceed to the operating room without further intervention. While it is likely a patient with diabetes and hyperlipidemia has some degree of CAD, including possible vulnerable plaques, the best medical evidence offers little to decrease her operative cardiac risk. Perioperative beta blockade is not indicated at her level of risk (RCRI of one) given the inconsistent benefits and possible harm to patients like this seen in trials to date.
If she were limited in terms of functional capacity (i.e., less than four METS), the 2007 ACC/AHA algorithm suggests preoperative noninvasive testing “if it would change management.”
How might a positive stress test change management in this case? Revascularization with stenting in close proximity to noncardiac surgery is not safe, and there appears to be no benefit to preoperative revascularization before high-risk vascular surgery. However, ischemia on preoperative testing is an indication for a beta-blocker. A brief delay in her surgery to allow dose titration and use of telemetry monitoring after surgery would increase the safety of beta-blockers after surgery. How long to continue beta-blockers is an open question, but at least 30 days would seem adequate, tapering rather than abruptly discontinuing the dose. TH
Dr. Carter is an assistant professor of medicine at the University of Colorado Denver in the Section of Hospital Medicine, where he directs the Medicine Consult Service. Dr. Auerbach is an associate professor of medicine in residence, associate director of the general medicine research fellowship, director of quality improvement for the UCSF Department of Medicine, and director of the surgical care Improvement program at UCSF. His research interests include perioperative medicine and quality improvement.
References
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043-1049.
- Eagle KA, Berger PB, Calkins H, et al. ACC/AHA Guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary. Circulation 2002;105:1257-1267.
- Fleischer LA, Beckman JA, Brown KA, et al. ACC/AHA Guidelines on Perioperative Cardiovascular Evaluation and Care for noncardiac surgery: executive summary. Circulation. 2007;116:1971-1996.
- Salerno SM, Carlson DW, Soh EK, et al. Impact of perioperative cardiac assessment guidelines on management of orthopedic surgery patients. Am J Med. 2007;120(2):185.
- McFalls EO, Ward HB, Moritz TE, et al. Coronary artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795-2804.
- Poldermans D, Schouten O, Vidakovic R, et al. A clinical randomized trial to evaluate the safety of a noninvasive approach in high-risk patients undergoing major vascular surgery: The DECREASE-V Pilot Study. J Am Coll Cardiol. 2007;49(17):1763-1769.
- Wilson, SH, Fasseas P, Orford JL, et al. Clinical outcomes of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42(2):234-240.
- Grines, CL, Bonow RO, Casey DE Jr, et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents. Circulation. 2007; 115:813-818.
- Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. Am J Coll Cardiol. 2000;35(5):1288-1294.
- Schouten O, Bax JJ, Damen J, et al. Coronary stent placement immediately before non cardiac surgery: a potential risk? Anesthesiology 106(5);2007:1067.
- Leibowitz D, Cohen M, Planer D, et al. Comparison of cardiovascular risk of noncardiac surgery following coronary angioplasty with versus without stenting. Am J Cardiol. 2006;97(8):1188-1191.
- Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006;113:1361-1376.
- Mangano DT, Layug EL, Wallace A, et al. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996;335(23):1713-1720.
- Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999;341(24):1789-1794.
- Feringa HH, Bax JJ, Boersma E, et al. High dose b-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation. 2006;114(supp):I344.
- Lindenauer PK Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349-361.
- Yang H, Raymer K, Butler R, et al. The effects of perioperative beta blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J. 2006;152(5):983-990.
- Juul AB, Wetterslev J, Gluud C, et al. Effect of perioperative ß blockade in patients with diabetes undergoing major non-cardiac surgery: randomized placebo controlled, blinded multicentre trial. BMJ. 2006 June;332:1482.
- Devereaux PJ, Beattie WS, Choi PT, et al. How strong is the evidence for the use of perioperative ß blockers in noncardiac surgery? Systematic review and meta-analysis of randomized controlled trials. BMJ. 2005;331:313.
- Devereaux PJ. POISE Abstract. American Heart Association Annual Scientific Session, Orlando, Fla., November 2007.