User login
Welcome to Current Psychiatry, a leading source of information, online and in print, for practitioners of psychiatry and its related subspecialties, including addiction psychiatry, child and adolescent psychiatry, and geriatric psychiatry. This Web site contains evidence-based reviews of the prevention, diagnosis, and treatment of mental illness and psychological disorders; case reports; updates on psychopharmacology; news about the specialty of psychiatry; pearls for practice; and other topics of interest and use to this audience.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-current-psychiatry')]
div[contains(@class, 'pane-pub-home-current-psychiatry')]
div[contains(@class, 'pane-pub-topic-current-psychiatry')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Medication-induced rhabdomyolysis
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Ms. A, age 32, has a history of anxiety, bipolar disorder, and borderline personality disorder. She is undergoing treatment with lamotrigine 200 mg/d at bedtime, aripiprazole 5 mg/d, trazodone 100 mg/d at bedtime, clonazepam 0.5 mg twice a day, and hydroxyzine 25 mg twice a day. She presents to the emergency department with myalgia, left upper and lower extremity numbness, and weakness. These symptoms started at approximately 3
Ms. A’s vital signs are hemodynamically stable, but her pulse is 113 bpm. On examination, she appears anxious and has decreased sensation in her upper and lower extremities, with 3/5 strength on the left side. Her laboratory results indicate mild leukocytosis, hyponatremia (129 mmol/L; reference range 136 to 145 mmol/L), and elevations in serum creatinine (3.7 mg/dL; reference range 0.6 to 1.2 mg/dL), aspartate aminotransferase (654 U/L; reference range 10 to 42 U/L), alanine transaminase (234 U/L; reference range 10 to 60 U/L), and troponin (2.11 ng/mL; reference range 0 to 0.04 ng/mL). A urinalysis reveals darkly colored urine with large red blood cells.
Neurology and Cardiology consultations are requested to rule out stroke and acute coronary syndromes. A computed tomography scan of the head shows no acute intracranial findings. Her creatinine kinase (CK) level is elevated (>42,670 U/L; reference range 22 to 232 U/L), which prompts a search for causes of rhabdomyolysis, a breakdown of muscle tissue that releases muscle fiber contents into the blood. Ms. A reports no history of recent trauma or strenuous exercise. Infectious, endocrine, and other workups are negative. After a consult to Psychiatry, the treating clinicians suspect that the most likely cause for rhabdomyolysis is aripiprazole.
Ms. A is treated with IV isotonic fluids. Aripiprazole is stopped and her CK levels are closely monitored. CK levels continue to trend down, and by Day 6 of hospitalization her CK level is 1,648 U/L. Her transaminase levels also improve; these elevations are considered likely secondary to rhabdomyolysis. Because there is notable improvement in CK and transaminase levels after stopping aripiprazole, Ms. A is discharged and instructed to follow up with a psychiatrist for further management.
Aripiprazole and rhabdomyolysis
According to the National Institute of Mental Health, an estimated 2.8% of the US population has bipolar disorder and 0.24% to 0.64% has schizophrenia.1,2 Antipsychotics are often used to treat these disorders. The prevalence of antipsychotic use in the general adult population is 1.6%.3 The use of second-generation antipsychotics (SGAs) has increased over recent years with the availability of a variety of formulations, such as immediate-release injectable, long-acting injectable, and orally disintegrating tablets in addition to the customary oral tablets. SGAs can cause several adverse effects, including weight gain, hyperlipidemia, diabetes, QTc prolongation, extrapyramidal side effects, myocarditis, agranulocytosis, cataracts, and sexual adverse effects.4
Antipsychotic use is more commonly associated with serotonin syndrome and neuroleptic malignant syndrome than it is with rhabdomyolysis. Rhabdomyolysis as an adverse effect of antipsychotic use has not been well understood or reported. One study found the prevalence of rhabdomyolysis was approximately 10% among patients who received an antipsychotic medication.5 There have been 4 case reports of clozapine use, 6 of olanzapine use, and 3 of aripiprazole use associated with rhabdomyolysis.6-8 Therefore, this would be the fourth case report to describe aripiprazole-associated rhabdomyolysis.
Aripiprazole is FDA-approved for the treatment of schizophrenia. In this case report, we found that aripiprazole could have led to rhabdomyolysis. Aripiprazole is a quinoline derivative that acts by binding to the 5-HT1A and 5-HT2A receptors.9,10 It acts as a partial agonist at 5-HT1A receptors, an antagonist at 5-HT2A receptors, and a partial agonist and stabilizer at the D2 receptor. By binding to the dopamine receptor in its G protein–coupled state, aripiprazole blocks the receptor in the presence of excessive dopamine.11-13 The mechanism of how aripiprazole could cause rhabdomyolysis is unclear. One proposed mechanism is that it can increase the permeability of skeletal muscle by 5-HT2A antagonism. This leads to a decrease in glucose reuptake in the cell and increases the permeability of the cell membrane, leading to elevations in CK levels.14 Another proposed mechanism is that dopamine blockade in the nigrostriatal pathway can result in muscle stiffness, rigidity, parkinsonian-like symptoms, and akathisia, which can result in elevated CK levels.15 There are only 3 other published cases of aripiprazole-induced rhabdomyolysis; we hope this case report will add value to the available literature. More evidence is needed to establish the safety profile of aripiprazole.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
1. National Institute of Mental Health. Prevalence of bipolar disorder among adults. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/bipolar-disorder#part_2605
2. National Institute of Mental Health. Schizophrenia. Accessed December 21, 2022. https://www.nimh.nih.gov/health/statistics/schizophrenia#part_2543
3. Dennis JA, Gittner LS, Payne JD, et al. Characteristics of U.S. adults taking prescription antipsychotic medications, National Health and Nutrition Examination Survey 2013-2018. BMC Psychiatry. 2020;20(1):483. doi: 10.1186/s12888-020-02895-4
4. Willner K, Vasan S, Abdijadid S. Atypical antipsychotic agents. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated May 2, 2022. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK448156/
5. Packard K, Price P, Hanson A. Antipsychotic use and the risk of rhabdomyolysis. J Pharm Pract 2014;27(5):501-512. doi: 10.1177/0897190013516509
6. Wu YF, Chang KY. Aripiprazole-associated rhabdomyolysis in a patient with schizophrenia. J Neuropsychiatry Clin Neurosci. 2011;23(3):E51.
7. Marzetti E, Bocchino L, Teramo S, et al. Rhabdomyolysis in a patient on aripiprazole with traumatic hip prosthesis luxation. J Neuropsychiatry Clin Neurosci. 2012;24(4):E40-E41.
8. Zhu X, Hu J, Deng S, et al. Rhabdomyolysis and elevated liver enzymes after rapid correction of hyponatremia due to pneumonia and concurrent use of aripiprazole: a case report. Aust N Z J Psychiatry. 2018;52(2):206. doi:10.1177/0004867417743342
9. Stahl SM. Essential Psychopharmacology: Neuroscientific Basis and Practical Application. 2nd ed. Cambridge University Press; 2000.
10. Stahl SM. “Hit-and-run” actions at dopamine receptors, part 1: mechanism of action of atypical antipsychotics. J Clin Psychiatry. 2001;62(9):670-671.
11. Leysen JE, Janssen PM, Schotte A, et al. Interaction of antipsychotic drugs with neurotransmitter receptor sites in vitro and in vivo in relation to pharmacological and clinical effects: role of 5HT2 receptors. Psychopharmacology (Berl). 1993;112(1 Suppl):S40-S54.
12. Millan MJ. Improving the treatment of schizophrenia: focus on serotonin (5-HT)(1A) receptors. J Pharmacol Exp Ther. 2000;295(3):853-861.
13. Millan MJ. The neurobiology and control of anxious states. Prog Neurobiol. 2003;70(2):83-244.
14. Meltzer HY, Cola PA, Parsa M. Marked elevations of serum creatine kinase activity associated with antipsychotic drug treatment. Neuropsychopharmacology. 1996;15(4):395-405.
15. Devarajan S, Dursun SM. Antipsychotic drugs, serum creatine kinase (CPK) and possible mechanisms. Psychopharmacology (Berl). 2000;152(1):122.
Subtle cognitive decline in a patient with depression and anxiety
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
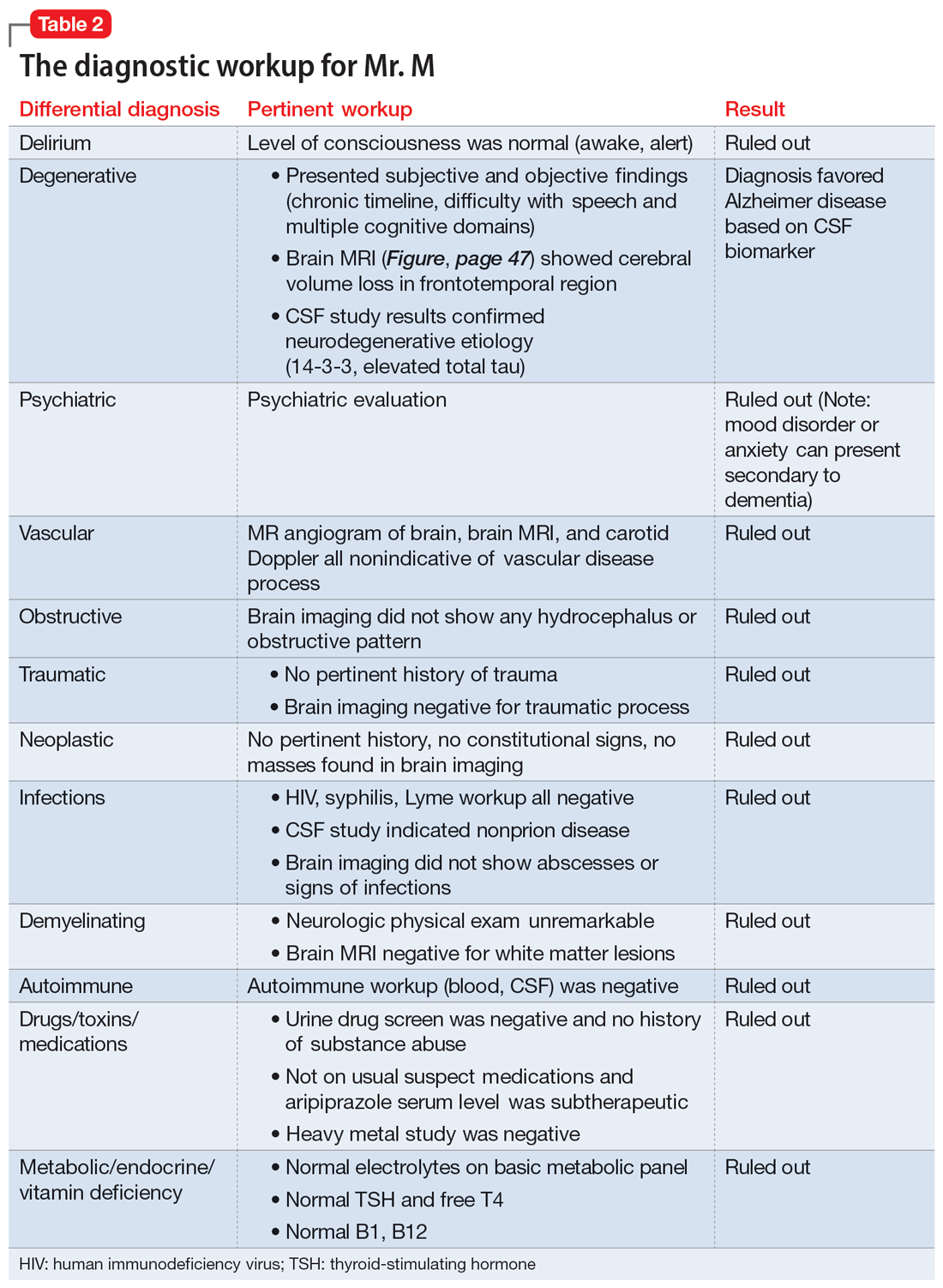
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.
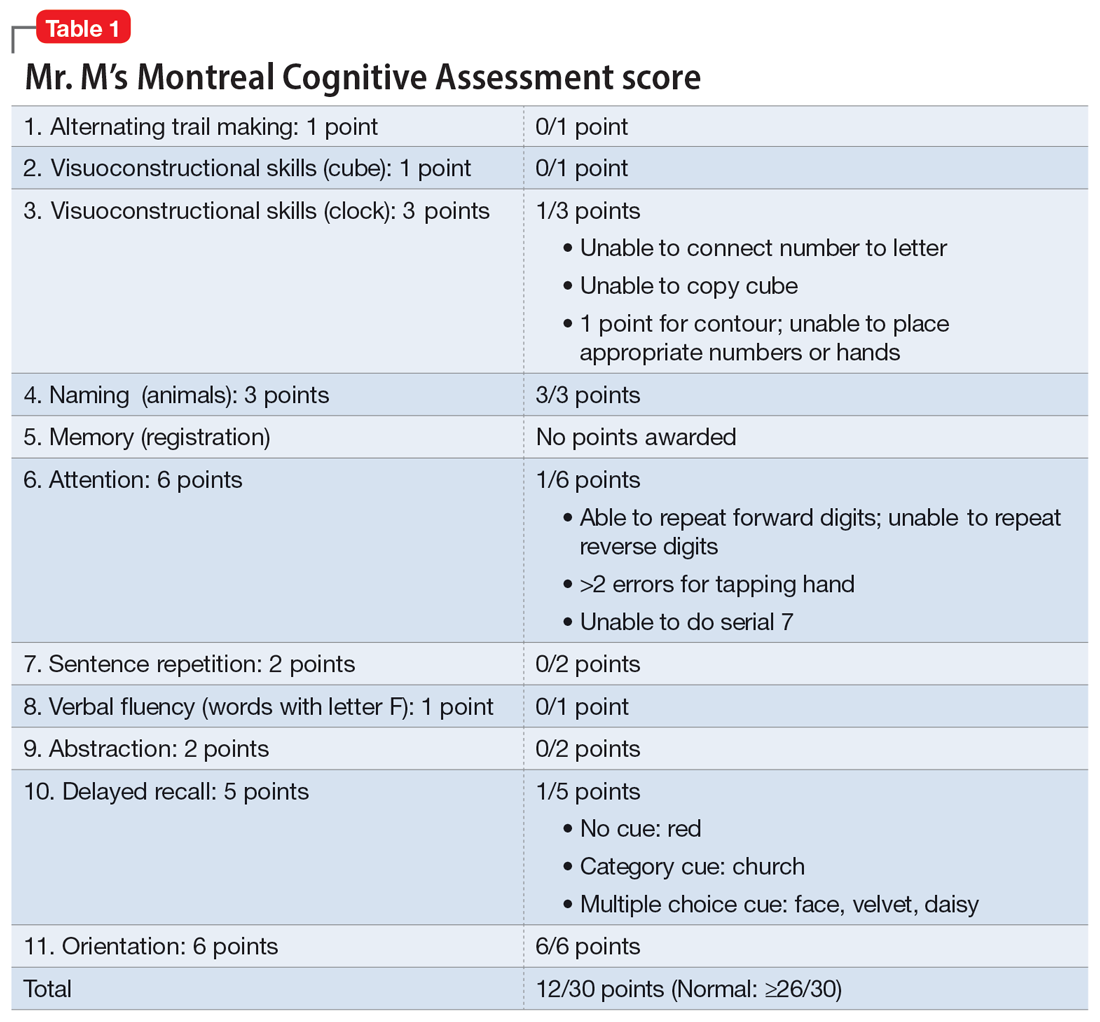
When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.
Primary progressive aphasia
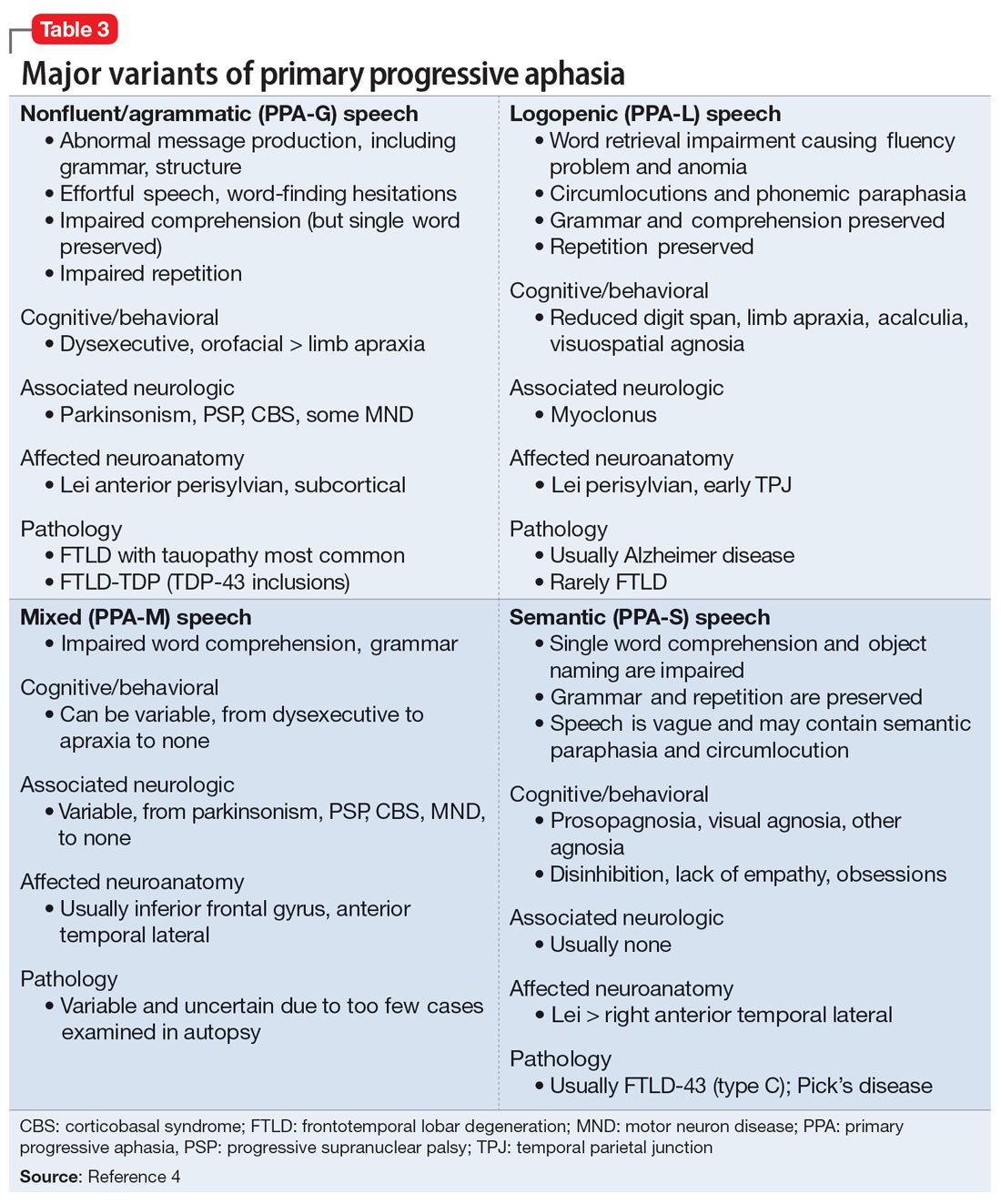
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.
The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
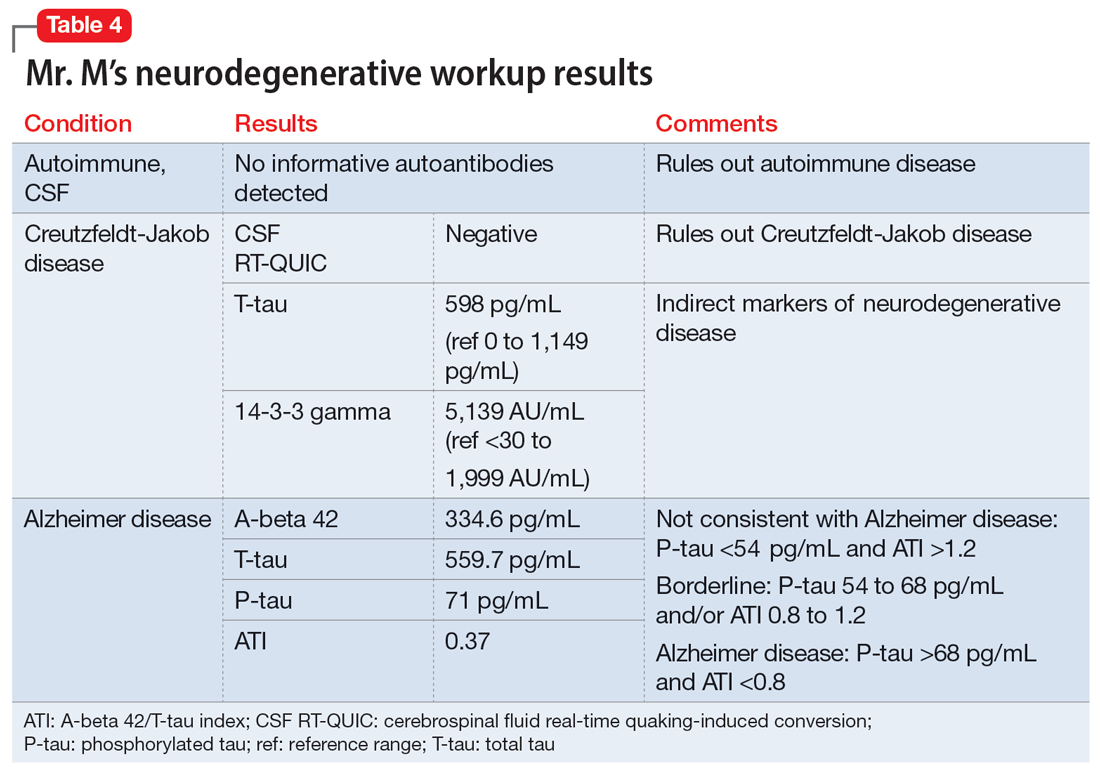
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.
[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7
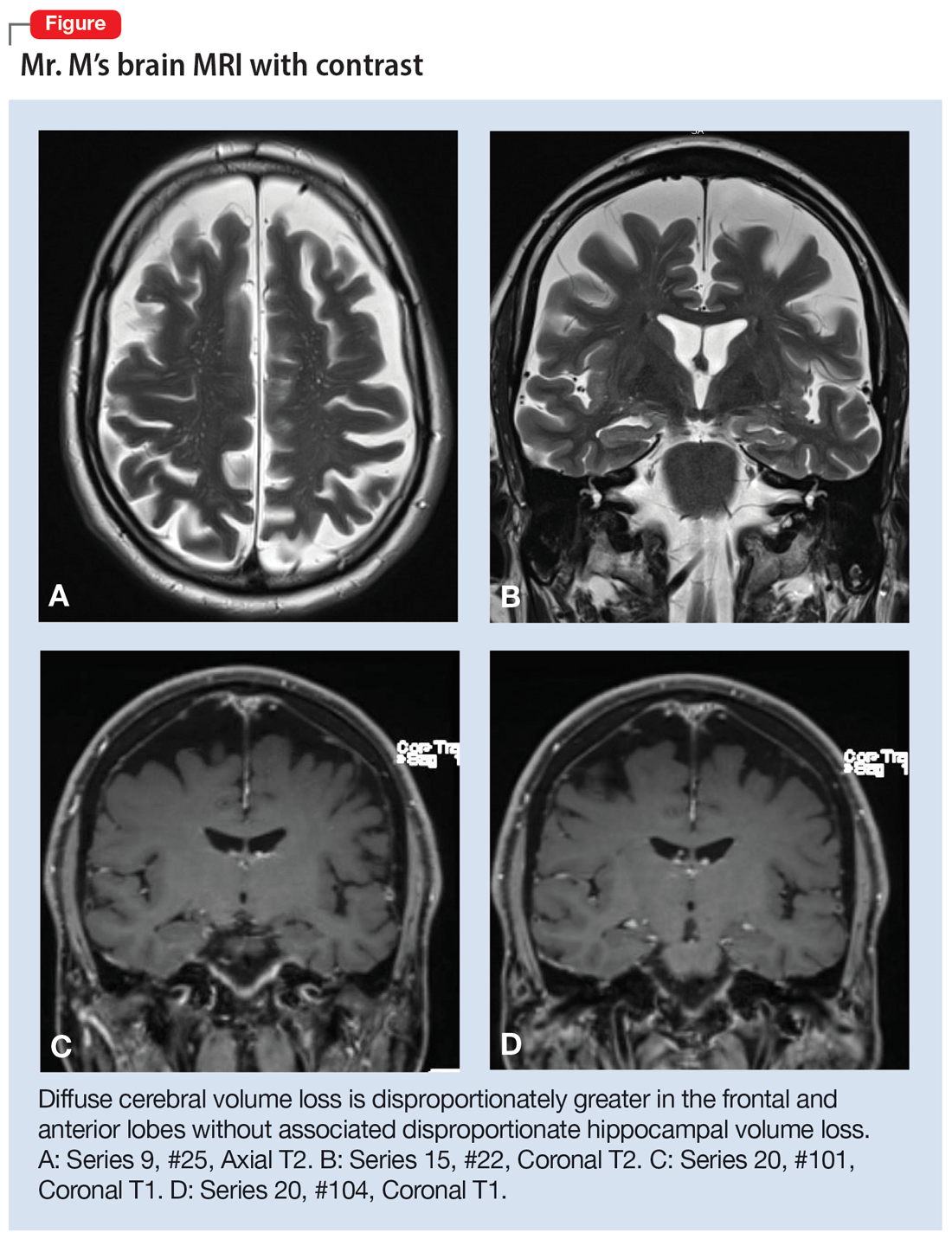
A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
More on psilocybin
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
I would like to remark on “Psychedelics for treating psychiatric disorders: Are they safe?” (
The Oregon Psilocybin Services that will begin in 2023 are not specific to therapeutic use; this is a common misconception. These are specifically referred to as “psilocybin services” in the Oregon Administrative Rules (OAR), and psilocybin facilitators are required to limit their scope such that they are not practicing psychotherapy or other interventions, even if they do have a medical or psychotherapy background. The intention of the Oregon Psilocybin Services rollout was that these services would not be of the medical model. In the spirit of this, services do not require a medical diagnosis or referral, and services are not a medical or clinical treatment (OAR 333-333-5040). Additionally, services cannot be provided in a health care facility (OAR 441). Facilitators receive robust training as defined by Oregon law, and licensed facilitators provide this information during preparation for services. When discussing this model on a large public scale, I have noticed substantial misconceptions; it is imperative that we refer to these services as they are defined so that individuals with mental health conditions who seek them are aware that such services are different from psilocybin-assisted psychotherapy. Instead, Oregon Psilocybin Services might be better categorized as supported psilocybin use.
Lithium toxicity: Lessons learned
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).
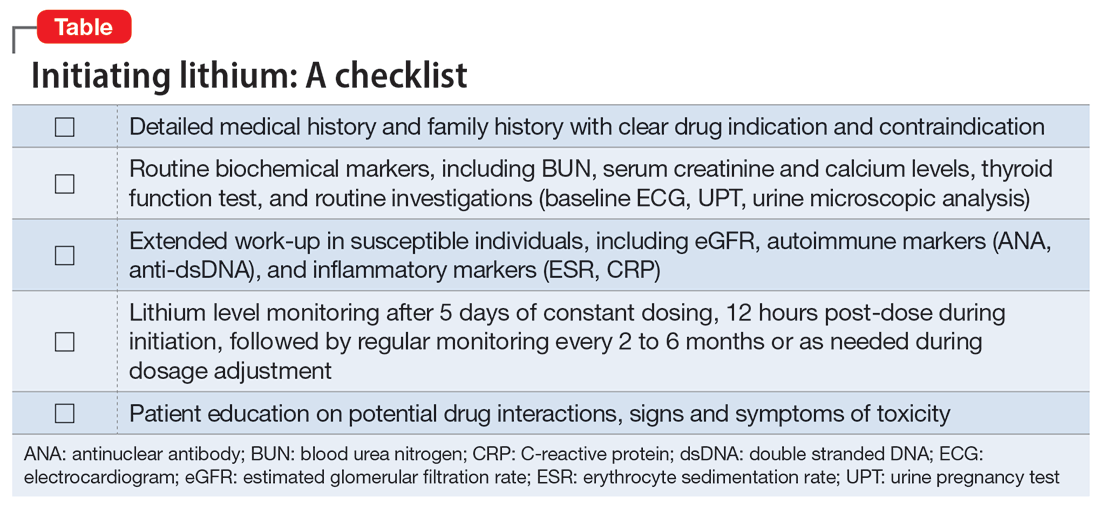
Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).

Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
Lithium carbonate is a mood stabilizer that is effective in the treatment of bipolar disorder, particularly in controlling mania.1 Lithium can reduce the risk of suicide,2 treat aggression and self-mutilating behavior,3 and prevent steroid-induced psychosis.4 It also can raise the white cell count in patients with clozapine-induced leukopenia.5
To prevent or lower the risk of relapse, the therapeutic plasma level of lithium should be regularly monitored to ensure an optimal concentration in the CNS. The highest tolerable level of lithium in the plasma is 0.6 to 0.8 mmol/L, with the optimal level ranging up to 1.2 mmol/L.6 Regular monitoring of renal function is also required to prevent renal toxicity, particularly if the plasma level exceeds 0.8 mmol/L.7 Because of lithium’s relatively narrow therapeutic index, its interaction with other medications, such as angiotensin-converting enzyme inhibitors, diuretics, nonsteroidal anti-inflammatory drugs (NSAIDs), and carbamazepine, can also precipitate lithium toxicity.8 We describe a lesson learned from a case of lithium toxicity in an otherwise healthy patient with bipolar disorder.
Case report
An otherwise healthy 39-year-old woman diagnosed with bipolar type I disorder was receiving valproate sodium 600 mg/d and olanzapine 10 mg/d. Despite improvement in her mood, she gained 11.6 kg following 6 months of treatment. As a result, olanzapine was switched to aripiprazole 10 mg/d that was later increased to 15 mg/d, and sodium valproate was gradually optimized up to 1,000 mg/d. She later complained of hair thinning and hair loss so she self-adjusted her medication dosages, which resulted in frequent relapses. Her mood stabilizer was changed from sodium valproate to lithium 600 mg/d.
Unfortunately, after taking lithium for 15 days, she returned to us with fever associated with reduced oral intake, poor sleep, bilateral upper limb rigidity, and bilateral hand tremor. She also complained of extreme thirst and fatigue but no vomiting or diarrhea. She had difficulty falling asleep and slept for only 1 to 2 hours a day. Her symptoms worsened when a general practitioner prescribed NSAIDs for her fever and body ache. Her tremors were later generalized, which made it difficult for her to take her oral medications and disturbed her speech and movement.
On evaluation, our patient appeared comfortable and not agitated. She was orientated to time, place, and person. Her blood pressure was 139/89 mmHg, heart rate was 104 bpm, and she was afebrile. She was dehydrated with minimal urine output. She had coarse tremor in her upper and lower limbs, which were hypertonic but did not display hyperreflexia or clonus. There was no nystagmus or ataxia. A mental state examination showed no signs of manic, hypomanic, or depressive symptoms. She had slurred speech, and her affect was restricted.
Blood investigation revealed a suprathreshold lithium level of 1.70 mmol/L (normal: 0.8 to 1.2 mmol/L). Biochemical parameters showed evidence of acute kidney injury (urea: 6.1 mmol/L; creatinine: 0.140 mmol/L), with no electrolyte imbalance. There was no evidence of hypothyroidism (thyroid-stimulating hormone: 14.9 mIU/L; free thyroxine: 9.9 pmol/L), hyperparathyroidism, or hypercalcemia. Autoimmune markers were positive for antinuclear antibody (titre 1:320) and anti-double stranded DNA (76.8 IU/mL). Apart from hair loss, she denied other symptoms associated with autoimmune disease, such as joint pain, butterfly rash, or persistent fatigue. Other routine blood investigations were within normal limits. Her urine protein throughout admission had shown persistent proteinuria ranging from 3+ to 4+. Electrocardiogram (ECG) showed normal sinus rhythm with no T wave inversion or QT prolongation.
Continue to: A detailed family history...
A detailed family history later confirmed a strong family history of renal disease: her mother had lupus nephritis with nephrotic syndrome, and her brother had died from complications of a rapidly progressive glomerulonephritis. Her renal function prior to lithium initiation was within normal limits (urea: 4.0 mmol/L; serum creatinine: 78 µmol/L).
In the ward, lithium and aripiprazole were discontinued, and she was hydrated. Combined care with the psychiatric and medical teams was established early to safeguard against potential CNS deterioration. She showed marked clinical improvement by Day 3, with the resolution of coarse tremor and rigidity as well as normalization of blood parameters. Her lithium level returned to a therapeutic level by Day 4 after lithium discontinuation, and her renal profile gradually normalized. She was restarted on aripiprazole 10 mg/d for her bipolar illness and responded well. She was discharged on Day 5 with a referral to the nephrology team for further intervention.
Lessons learned
This case highlights the issue of lithium safety in susceptible individuals and the importance of risk stratification in this group of patients. Lithium is an effective treatment for bipolar I disorder and has also been used as adjunctive treatment for major depressive disorder, schizoaffective disorder, treatment-resistant schizophrenia, anorexia nervosa and bulimia nervosa, and the control of chronic aggression.9 Lithium is completely absorbed by the gastrointestinal tract following ingestion, is not metabolized, and is eliminated almost entirely by the kidneys (though trace amounts may be found in feces and perspiration).
In our case, a detailed family history of renal disease was not adequately explored until our patient presented with signs suggestive of lithium toxicity. Our patient had been prescribed lithium 600 mg/d as a maintenance therapy. Upon starting lithium, her baseline biochemical parameters were within normal limits, and renal issues were not suspected. The hair thinning and hair loss she experienced could have been an adverse effect of valproate sodium or a manifestation of an underlying autoimmune disease. Coupled with the use of NSAIDs that could have precipitated acute kidney injury, her poor oral intake and dehydration during the acute illness further impaired lithium excretion, leading to a suprathreshold plasma level despite a low dose of lithium. Therefore, before prescribing lithium, a thorough medical and family history is needed, supplemented by an evaluation of renal function, serum electrolytes, and thyroid function to determine the starting dosage of lithium. Routine vital sign assessment and ECG should also be conducted, and concurrent medications and pregnancy status should be confirmed before prescribing lithium. Regular lithium level monitoring is essential.
Measuring a patient’s estimated glomerular filtration rate (eGFR) is recommended to validate renal status10 and classify and stage kidney disease.11 Combining eGFR with blood urea nitrogen, serum creatinine, and urine microscopic analysis further improves the prediction of renal disease in early stages. We recommend considering a blood test for autoimmune markers in patients with clinical suspicion of autoimmune disease, in the presence of suggestive signs and symptoms, and/or in patients with a positive family history (Table).

Before starting lithium, in addition to conducting a detailed clinical evaluation, information about symptoms and the risk of lithium toxicity should be discussed with patients.12 Our case serves as a timely reminder that the lack of suggestive biochemical parameters of renal disease should not rule out an underlying renal disease, and a strong family history of renal disease should warrant suspicion of a possible autoimmune origin.
We suggest that future studies evaluate the risks of lithium toxicity in susceptible groups of patients, such as those with family history of renal disease.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
1. Goodwin GM, Haddad PM, Ferrier IN, et al. Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2016;30(6):495-553.
2. Cipriani A, Hawton K, Stockton S, et al. Lithium in the prevention of suicide in mood disorders: updated systematic review and meta-analysis. BMJ. 2013; 346:f3646.
3. Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92-107.
4. Abou-Saleh MT, Müller-Oerlinghausen B, Coppen AJ. Lithium in the episode and suicide prophylaxis and in augmenting strategies in patients with unipolar depression. Int J Bipolar Disord. 2017;5(1):11.
5. Aydin M, Ilhan BC, Calisir S, et al. Continuing clozapine treatment with lithium in schizophrenic patients with neutropenia or leukopenia: brief review of literature with case reports. Ther Adv Psychopharmacol. 2016;6(1):33-38.
6. Nolen WA, Weisler RH. The association of the effect of lithium in the maintenance treatment of bipolar disorder with lithium plasma levels: a post hoc analysis of a double-blind study comparing switching to lithium or placebo in patients who responded to quetiapine (Trial 144). Bipolar Disord. 2013;15(1):100-109.
7. Aiff H, Attman P, Aurell M, et al. Effects of 10 to 30 years of lithium treatment on kidney function. J Psychopharmacol. 2015;29(5):608-614.
8. Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines in Psychiatry. 13th ed. Wiley-Blackwell; 2018.
9. Sadock BJ, Sadock VA. Kaplan & Sadock’s Synopsis of Psychiatry: Behavioral Sciences/Clinical Psychiatry. 9th ed. Lippincot Williams & Wilkins; 2002.
10. Lopez-Giacoman S, Madero M. Biomarkers in chronic kidney disease, from kidney function to kidney damage. World J Nephrol. 2015;4(1):57-73.
11. McCance RA, Robinson JR. Evaluation of renal clearances. Proc R Soc Med. 1949;42(7):475-480.
12. Gerret D, Lamont T, Paton C, et al. Prescribing and monitoring lithium therapy: summary of a safety report from the National Patient Safety Agency. BMJ. 2010;341:c6258.
Suicidality in an older patient with chronic kidney disease
CASE Depressed, anxious, and suicidal
Mr. J, age 72, is brought to the emergency department by law enforcement at his wife’s request due to worsening suicidal thoughts and anxiety. He has a history of major depressive disorder (MDD) and chronic kidney disease (CKD). Mr. J has been compliant with his medications, but they seem to no longer be effective. He is admitted to the geriatric psychiatry unit.
HISTORY Increased debilitation
Over the past several years, Mr. J has experienced increasing debilitation at home, including difficulty walking and an inability to perform activities of daily life. Recently, he has begun to ask for multiple pills in an attempt to take his own life.
Mr. J has been previously treated in a psychiatric clinic with duloxetine 60 mg/d, mirtazapine 30 mg/d at bedtime, buspirone 15 mg 3 times a day, and trazodone 50 mg/d at bedtime. He is also taking amlodipine 5 mg twice daily for hypertension, lisinopril 2.5 mg/d for hypertension, furosemide 20 mg/d orally for CKD, and potassium chloride 10 mEq/d for hypokalemia secondary to CKD and furosemide use. Over the past year, his psychiatric medications have been steadily increased to target his MDD and anxiety.
EVALUATION Disorientation and Stage 3A CKD
In the psychiatric unit, Mr. J describes panic, feelings of impending doom, and profound anxiety. He states he has increasing anxiety related to “being a burden” on his family and wife. Additionally, he describes decreased appetite, difficulty sleeping, low energy, difficulty concentrating, no interest in outside activities, and feelings of hopelessness.
Mr. J’s temperature is 39.2o C; heart rate is 109 beats per minute; respiratory rate is 18 breaths per minute; blood pressure is 157/83 mm Hg; and pulse oximetry is 97%. Laboratory screening indicates a red blood cell count of 3.57, hemoglobin 11.2, hematocrit 33.8, red blood cell distribution width 17.5, blood urea nitrogen 45, creatinine 1.5 with no known baseline, and an estimated glomerular filtration rate (GFR) of 46 mL/min, indicating Stage 3A CKD (Table 11). Additional testing rules out other potential causes of delirium and psychosis.
A physical exam reveals Mr. J has a fine tremor, myoclonus, muscle rigidity, and hyperreflexia. He is oriented to name, but not to date, place, or situation, and is easily confused. Mr. J uses a walker but has significant tremors while walking and immediately asks for assistance due to profound anxiety related to a fear of falling. Mr. J’s mood and affect are labile with tearful and anxious episodes. His anxiety focuses on overvalued thoughts of minor or irrelevant concerns. Additionally, he has poor insight and judgment. When asked about the cause of his anxiety, Mr. J says, “I don’t know why I’m anxious; I’m just a worrywart.” His memory is impaired, and he does not know why he is in the hospital. Mr. J scores 24 on the Montreal Cognitive Assessment, which indicates mild impairment.
Mr. J continues to endorse suicidal ideation but denies homicidal thoughts. Based on these symptoms, the differential diagnosis includes serotonin syndrome, MDD with suicidal ideation, generalized anxiety disorder, and panic disorder.
Continue to: The authors' observations
[polldaddy:11273789]
The authors’ observations
GFR is used to determine the level of renal impairment. Mr. J’s GFR of 46 mL/min indicates Stage 3A CKD (Table 11 ). Additionally, he displayed anemia and increased creatinine due to CKD. Twenty percent of patients with CKD also experience MDD.2 In a prospective observational cohort study, Hedayati et al3 found that Stage 2 to Stage 5 CKD with MDD leads to an increased risk of death, hospitalization, or progression to dialysis. It is important to properly manage Mr. J’s MDD and CKD to prevent future comorbidities. Renal impairment is common in people age >65.4 Even when GFR is normal, it is recommended to decrease dosing of medications in older adults due to age-related decreased renal excretion. As kidneys decrease in function, their ability to excrete normal amounts of medications also decreases, leading to increased serum levels and potential toxicity.
A combination of 4 serotonergic psychotropic medications may not be unusual to address treatment-resistant depression in a healthy, nongeriatric adult. However, Mr. J displayed signs of serotonin toxicity, such as hyperthermia, tachycardia, increased blood pressure, increased tremors, myoclonus, hyperreflexia, and muscle rigidity. These are classic signs of serotonin toxicity. For Mr. J, serotonin toxicity can be treated with the removal of serotonergic medications and lorazepam for symptom relief. If symptoms persist, cyproheptadine, a serotonin antagonist, can be used. Mr. J’s psychotropic medications were increased in an outpatient setting and he was unable to renally excrete higher doses of these serotonergic agents, which lead to chronic serotonin toxicity.
It is important to rule out other causes of psychosis or delirium in geriatric patients. A study by Marcantonio et al5 found that >40% of patients referred to a consulting psychiatrist for depression ultimately had delirium, and this was more likely in geriatric patients.
TREATMENT Adjustments to the medication regimen
The treatment team decides to taper and discontinue duloxetine, buspirone, and trazodone and reduce mirtazapine to 15 mg/d at bedtime. Additionally, oral lorazepam 1 mg as needed is prescribed to alleviate agitation and correct vital signs. Mr. J’s vital signs improve, with decreased temperature and normal cardiac and respiratory rhythms.
Mr. J’s Stage 3A CKD is treated with oral fluids, and his hypertension is managed with an increase of lisinopril from 2.5 mg/d to 10 mg/d. After 10 days on the psychiatric unit, he shows improvement, decreased anxiety, and remission of suicidal ideation.
Continue to: The authors' observations
[polldaddy:11273790]
The authors’ observations
In 2019, the American Geriatric Society (AGS) updated the Beers Criteria for potentially inappropriate medication use in older adults.4 The Beers Criteria were created to educate clinicians about the use of potentially inappropriate medications that have an unfavorable balance of benefits and risks compared to alternative treatments. The AGS lists medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Duloxetine is one of the medications listed with the recommendation to avoid for patients with a creatinine clearance <30 mL/min. Creatinine clearance is an estimation of GFR.
Although duloxetine is mentioned in the Beers Criteria, many other antidepressants have metabolites excreted by the kidneys.6 Potential adverse effects include increased bleeding, nausea, vomiting, and serotonin toxicity symptoms.7 Mr. J has Stage 3A CKD and takes 4 psychotropics, which will additively increase the serum concentration of serotonergic medications. In terms of treatment for serotonin toxicity, it is important to remove the causative medications. After discontinuing serotonergic medications, lorazepam can be administered as needed. If a patient continues to have symptoms, cyproheptadine is an option.
For patients with impaired renal function, adding nonpharmacologic options should be considered, such as cognitive-behavioral therapy, electroconvulsive therapy, and transcranial magnetic stimulation. Table 24,8-18 lists the minimum effective doses for well-known medications for treating MDD.
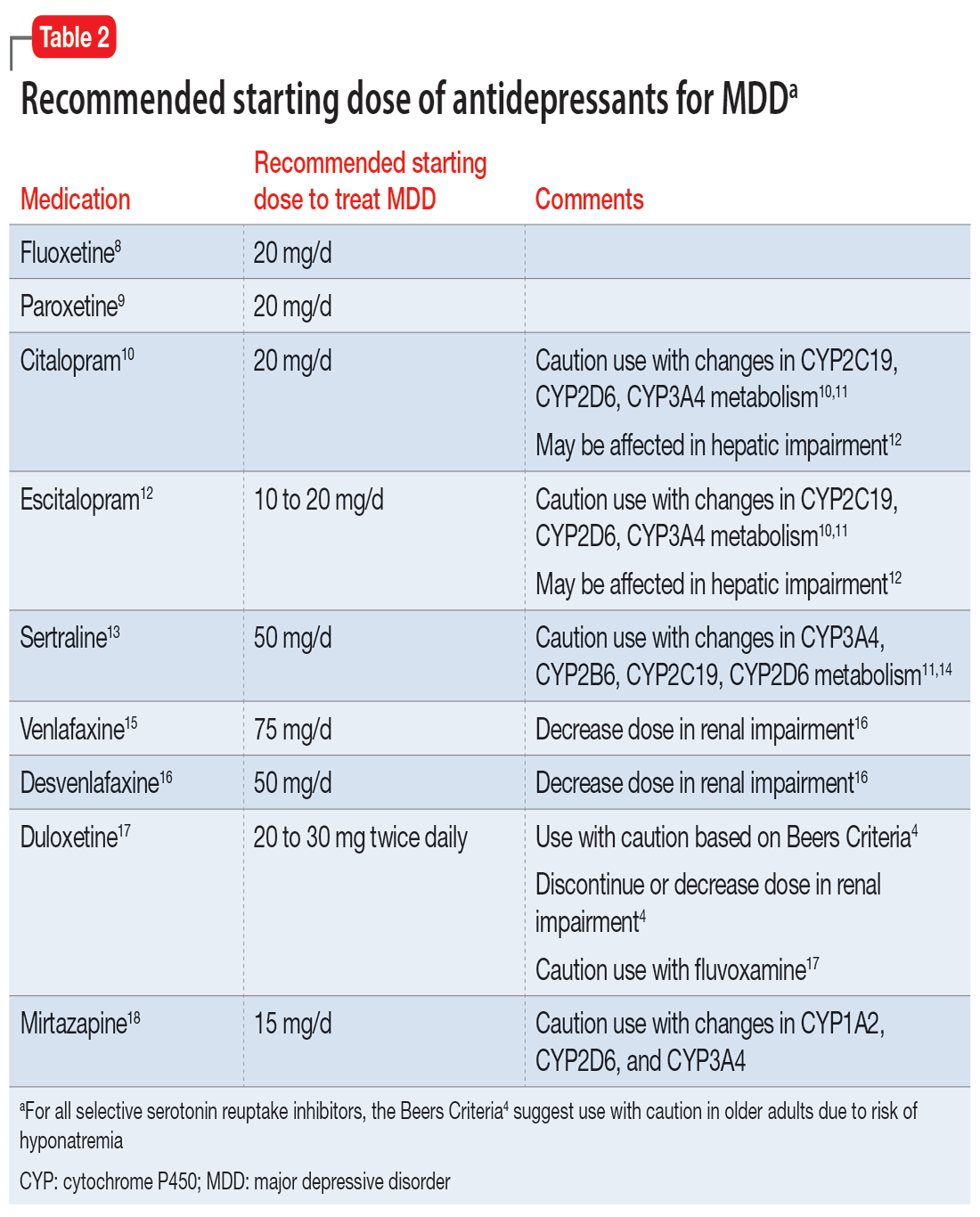
OUTCOME Improvement and discharge
Mr. J’s confusion improves, his heart rate decreases, and his feelings of panic and doom improve. He continues to have depressive symptoms, but his suicidal ideation stops. At discharge, Mr. J is receiving mirtazapine 15 mg/d, potassium chloride 10 mEq/d orally, lisinopril 20 mg/d orally at bedtime, furosemide 20 mg/d orally, and amlodipine 5 mg orally twice a day. Additionally, the treatment team recommends psychotherapy to Mr. J to address his anxiety and depression.
Bottom Line
Older patients are more sensitive to psychotropic medications, regardless of any comorbidities. It is important to review each patient’s glomerular filtration rate to better understand their renal function and adjust medications accordingly.
Related Resources
- Whittaker P, Vordenberg SE, Coe AB. Deprescribing in older adults: an overview. Current Psychiatry. 2022;21(5):40-43. doi:10.12788/cp.0246
- Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
- Barr R, Miskle B, Thomas C. Management of major depressive disorder with psychotic features. Current Psychiatry. 2021;20(2):30-33. doi:10.12788/cp.0092
Drug Brand Names
Amlodipine • Norvasc
Buspirone • BuSpar
Citalopram • Celexa
Cyproheptadine • Periactin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Furosemide • Lasix
Lisinopril • Zestril
Lorazepam • Ativan
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Desyrel
Venlafaxine • Effexor
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266.
2. Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2(1):94-107.
3. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953.
4. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
5. Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850-857.
6. Cukor D, Cohen, SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042-3055.
7. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332-1342.
8. Sommi RW, Crismon ML, Bowden CL. Fluoxetine: a serotonin-specific, second-generation antidepressant. Pharmacotherapy. 1987;7(1):1-15.
9. Jenner PN. Paroxetine: an overview of dosage, tolerability, and safety. Int Clin Psychopharmacol. 1992;6(Suppl 4):69-80.
10. Montgomery SA. Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol. 1995;10(Suppl 1):23-27.
11. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270-280.
12. Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-290.
13. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129-141.
14. Huddart R, Hicks JK, Ramsey LB, et al. PharmGKB summary: sertraline pathway, pharmacokinetics. Pharmacogenet Genomics. 2020;30(2):26-33.
15. Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601-609.
16. Norman TR, Olver JS. Desvenlafaxine in the treatment of major depression: an updated overview. Expert Opin Pharmacother. 2021;22(9):1087-1097.
17. Knadler MP, Lobo E, Chappell J, et al. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281-294.
18. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
CASE Depressed, anxious, and suicidal
Mr. J, age 72, is brought to the emergency department by law enforcement at his wife’s request due to worsening suicidal thoughts and anxiety. He has a history of major depressive disorder (MDD) and chronic kidney disease (CKD). Mr. J has been compliant with his medications, but they seem to no longer be effective. He is admitted to the geriatric psychiatry unit.
HISTORY Increased debilitation
Over the past several years, Mr. J has experienced increasing debilitation at home, including difficulty walking and an inability to perform activities of daily life. Recently, he has begun to ask for multiple pills in an attempt to take his own life.
Mr. J has been previously treated in a psychiatric clinic with duloxetine 60 mg/d, mirtazapine 30 mg/d at bedtime, buspirone 15 mg 3 times a day, and trazodone 50 mg/d at bedtime. He is also taking amlodipine 5 mg twice daily for hypertension, lisinopril 2.5 mg/d for hypertension, furosemide 20 mg/d orally for CKD, and potassium chloride 10 mEq/d for hypokalemia secondary to CKD and furosemide use. Over the past year, his psychiatric medications have been steadily increased to target his MDD and anxiety.
EVALUATION Disorientation and Stage 3A CKD
In the psychiatric unit, Mr. J describes panic, feelings of impending doom, and profound anxiety. He states he has increasing anxiety related to “being a burden” on his family and wife. Additionally, he describes decreased appetite, difficulty sleeping, low energy, difficulty concentrating, no interest in outside activities, and feelings of hopelessness.
Mr. J’s temperature is 39.2o C; heart rate is 109 beats per minute; respiratory rate is 18 breaths per minute; blood pressure is 157/83 mm Hg; and pulse oximetry is 97%. Laboratory screening indicates a red blood cell count of 3.57, hemoglobin 11.2, hematocrit 33.8, red blood cell distribution width 17.5, blood urea nitrogen 45, creatinine 1.5 with no known baseline, and an estimated glomerular filtration rate (GFR) of 46 mL/min, indicating Stage 3A CKD (Table 11). Additional testing rules out other potential causes of delirium and psychosis.

A physical exam reveals Mr. J has a fine tremor, myoclonus, muscle rigidity, and hyperreflexia. He is oriented to name, but not to date, place, or situation, and is easily confused. Mr. J uses a walker but has significant tremors while walking and immediately asks for assistance due to profound anxiety related to a fear of falling. Mr. J’s mood and affect are labile with tearful and anxious episodes. His anxiety focuses on overvalued thoughts of minor or irrelevant concerns. Additionally, he has poor insight and judgment. When asked about the cause of his anxiety, Mr. J says, “I don’t know why I’m anxious; I’m just a worrywart.” His memory is impaired, and he does not know why he is in the hospital. Mr. J scores 24 on the Montreal Cognitive Assessment, which indicates mild impairment.
Mr. J continues to endorse suicidal ideation but denies homicidal thoughts. Based on these symptoms, the differential diagnosis includes serotonin syndrome, MDD with suicidal ideation, generalized anxiety disorder, and panic disorder.
Continue to: The authors' observations
[polldaddy:11273789]
The authors’ observations
GFR is used to determine the level of renal impairment. Mr. J’s GFR of 46 mL/min indicates Stage 3A CKD (Table 11 ). Additionally, he displayed anemia and increased creatinine due to CKD. Twenty percent of patients with CKD also experience MDD.2 In a prospective observational cohort study, Hedayati et al3 found that Stage 2 to Stage 5 CKD with MDD leads to an increased risk of death, hospitalization, or progression to dialysis. It is important to properly manage Mr. J’s MDD and CKD to prevent future comorbidities. Renal impairment is common in people age >65.4 Even when GFR is normal, it is recommended to decrease dosing of medications in older adults due to age-related decreased renal excretion. As kidneys decrease in function, their ability to excrete normal amounts of medications also decreases, leading to increased serum levels and potential toxicity.
A combination of 4 serotonergic psychotropic medications may not be unusual to address treatment-resistant depression in a healthy, nongeriatric adult. However, Mr. J displayed signs of serotonin toxicity, such as hyperthermia, tachycardia, increased blood pressure, increased tremors, myoclonus, hyperreflexia, and muscle rigidity. These are classic signs of serotonin toxicity. For Mr. J, serotonin toxicity can be treated with the removal of serotonergic medications and lorazepam for symptom relief. If symptoms persist, cyproheptadine, a serotonin antagonist, can be used. Mr. J’s psychotropic medications were increased in an outpatient setting and he was unable to renally excrete higher doses of these serotonergic agents, which lead to chronic serotonin toxicity.
It is important to rule out other causes of psychosis or delirium in geriatric patients. A study by Marcantonio et al5 found that >40% of patients referred to a consulting psychiatrist for depression ultimately had delirium, and this was more likely in geriatric patients.
TREATMENT Adjustments to the medication regimen
The treatment team decides to taper and discontinue duloxetine, buspirone, and trazodone and reduce mirtazapine to 15 mg/d at bedtime. Additionally, oral lorazepam 1 mg as needed is prescribed to alleviate agitation and correct vital signs. Mr. J’s vital signs improve, with decreased temperature and normal cardiac and respiratory rhythms.
Mr. J’s Stage 3A CKD is treated with oral fluids, and his hypertension is managed with an increase of lisinopril from 2.5 mg/d to 10 mg/d. After 10 days on the psychiatric unit, he shows improvement, decreased anxiety, and remission of suicidal ideation.
Continue to: The authors' observations
[polldaddy:11273790]
The authors’ observations
In 2019, the American Geriatric Society (AGS) updated the Beers Criteria for potentially inappropriate medication use in older adults.4 The Beers Criteria were created to educate clinicians about the use of potentially inappropriate medications that have an unfavorable balance of benefits and risks compared to alternative treatments. The AGS lists medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Duloxetine is one of the medications listed with the recommendation to avoid for patients with a creatinine clearance <30 mL/min. Creatinine clearance is an estimation of GFR.
Although duloxetine is mentioned in the Beers Criteria, many other antidepressants have metabolites excreted by the kidneys.6 Potential adverse effects include increased bleeding, nausea, vomiting, and serotonin toxicity symptoms.7 Mr. J has Stage 3A CKD and takes 4 psychotropics, which will additively increase the serum concentration of serotonergic medications. In terms of treatment for serotonin toxicity, it is important to remove the causative medications. After discontinuing serotonergic medications, lorazepam can be administered as needed. If a patient continues to have symptoms, cyproheptadine is an option.
For patients with impaired renal function, adding nonpharmacologic options should be considered, such as cognitive-behavioral therapy, electroconvulsive therapy, and transcranial magnetic stimulation. Table 24,8-18 lists the minimum effective doses for well-known medications for treating MDD.

OUTCOME Improvement and discharge
Mr. J’s confusion improves, his heart rate decreases, and his feelings of panic and doom improve. He continues to have depressive symptoms, but his suicidal ideation stops. At discharge, Mr. J is receiving mirtazapine 15 mg/d, potassium chloride 10 mEq/d orally, lisinopril 20 mg/d orally at bedtime, furosemide 20 mg/d orally, and amlodipine 5 mg orally twice a day. Additionally, the treatment team recommends psychotherapy to Mr. J to address his anxiety and depression.
Bottom Line
Older patients are more sensitive to psychotropic medications, regardless of any comorbidities. It is important to review each patient’s glomerular filtration rate to better understand their renal function and adjust medications accordingly.
Related Resources
- Whittaker P, Vordenberg SE, Coe AB. Deprescribing in older adults: an overview. Current Psychiatry. 2022;21(5):40-43. doi:10.12788/cp.0246
- Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
- Barr R, Miskle B, Thomas C. Management of major depressive disorder with psychotic features. Current Psychiatry. 2021;20(2):30-33. doi:10.12788/cp.0092
Drug Brand Names
Amlodipine • Norvasc
Buspirone • BuSpar
Citalopram • Celexa
Cyproheptadine • Periactin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Furosemide • Lasix
Lisinopril • Zestril
Lorazepam • Ativan
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Desyrel
Venlafaxine • Effexor
CASE Depressed, anxious, and suicidal
Mr. J, age 72, is brought to the emergency department by law enforcement at his wife’s request due to worsening suicidal thoughts and anxiety. He has a history of major depressive disorder (MDD) and chronic kidney disease (CKD). Mr. J has been compliant with his medications, but they seem to no longer be effective. He is admitted to the geriatric psychiatry unit.
HISTORY Increased debilitation
Over the past several years, Mr. J has experienced increasing debilitation at home, including difficulty walking and an inability to perform activities of daily life. Recently, he has begun to ask for multiple pills in an attempt to take his own life.
Mr. J has been previously treated in a psychiatric clinic with duloxetine 60 mg/d, mirtazapine 30 mg/d at bedtime, buspirone 15 mg 3 times a day, and trazodone 50 mg/d at bedtime. He is also taking amlodipine 5 mg twice daily for hypertension, lisinopril 2.5 mg/d for hypertension, furosemide 20 mg/d orally for CKD, and potassium chloride 10 mEq/d for hypokalemia secondary to CKD and furosemide use. Over the past year, his psychiatric medications have been steadily increased to target his MDD and anxiety.
EVALUATION Disorientation and Stage 3A CKD
In the psychiatric unit, Mr. J describes panic, feelings of impending doom, and profound anxiety. He states he has increasing anxiety related to “being a burden” on his family and wife. Additionally, he describes decreased appetite, difficulty sleeping, low energy, difficulty concentrating, no interest in outside activities, and feelings of hopelessness.
Mr. J’s temperature is 39.2o C; heart rate is 109 beats per minute; respiratory rate is 18 breaths per minute; blood pressure is 157/83 mm Hg; and pulse oximetry is 97%. Laboratory screening indicates a red blood cell count of 3.57, hemoglobin 11.2, hematocrit 33.8, red blood cell distribution width 17.5, blood urea nitrogen 45, creatinine 1.5 with no known baseline, and an estimated glomerular filtration rate (GFR) of 46 mL/min, indicating Stage 3A CKD (Table 11). Additional testing rules out other potential causes of delirium and psychosis.

A physical exam reveals Mr. J has a fine tremor, myoclonus, muscle rigidity, and hyperreflexia. He is oriented to name, but not to date, place, or situation, and is easily confused. Mr. J uses a walker but has significant tremors while walking and immediately asks for assistance due to profound anxiety related to a fear of falling. Mr. J’s mood and affect are labile with tearful and anxious episodes. His anxiety focuses on overvalued thoughts of minor or irrelevant concerns. Additionally, he has poor insight and judgment. When asked about the cause of his anxiety, Mr. J says, “I don’t know why I’m anxious; I’m just a worrywart.” His memory is impaired, and he does not know why he is in the hospital. Mr. J scores 24 on the Montreal Cognitive Assessment, which indicates mild impairment.
Mr. J continues to endorse suicidal ideation but denies homicidal thoughts. Based on these symptoms, the differential diagnosis includes serotonin syndrome, MDD with suicidal ideation, generalized anxiety disorder, and panic disorder.
Continue to: The authors' observations
[polldaddy:11273789]
The authors’ observations
GFR is used to determine the level of renal impairment. Mr. J’s GFR of 46 mL/min indicates Stage 3A CKD (Table 11 ). Additionally, he displayed anemia and increased creatinine due to CKD. Twenty percent of patients with CKD also experience MDD.2 In a prospective observational cohort study, Hedayati et al3 found that Stage 2 to Stage 5 CKD with MDD leads to an increased risk of death, hospitalization, or progression to dialysis. It is important to properly manage Mr. J’s MDD and CKD to prevent future comorbidities. Renal impairment is common in people age >65.4 Even when GFR is normal, it is recommended to decrease dosing of medications in older adults due to age-related decreased renal excretion. As kidneys decrease in function, their ability to excrete normal amounts of medications also decreases, leading to increased serum levels and potential toxicity.
A combination of 4 serotonergic psychotropic medications may not be unusual to address treatment-resistant depression in a healthy, nongeriatric adult. However, Mr. J displayed signs of serotonin toxicity, such as hyperthermia, tachycardia, increased blood pressure, increased tremors, myoclonus, hyperreflexia, and muscle rigidity. These are classic signs of serotonin toxicity. For Mr. J, serotonin toxicity can be treated with the removal of serotonergic medications and lorazepam for symptom relief. If symptoms persist, cyproheptadine, a serotonin antagonist, can be used. Mr. J’s psychotropic medications were increased in an outpatient setting and he was unable to renally excrete higher doses of these serotonergic agents, which lead to chronic serotonin toxicity.
It is important to rule out other causes of psychosis or delirium in geriatric patients. A study by Marcantonio et al5 found that >40% of patients referred to a consulting psychiatrist for depression ultimately had delirium, and this was more likely in geriatric patients.
TREATMENT Adjustments to the medication regimen
The treatment team decides to taper and discontinue duloxetine, buspirone, and trazodone and reduce mirtazapine to 15 mg/d at bedtime. Additionally, oral lorazepam 1 mg as needed is prescribed to alleviate agitation and correct vital signs. Mr. J’s vital signs improve, with decreased temperature and normal cardiac and respiratory rhythms.
Mr. J’s Stage 3A CKD is treated with oral fluids, and his hypertension is managed with an increase of lisinopril from 2.5 mg/d to 10 mg/d. After 10 days on the psychiatric unit, he shows improvement, decreased anxiety, and remission of suicidal ideation.
Continue to: The authors' observations
[polldaddy:11273790]
The authors’ observations
In 2019, the American Geriatric Society (AGS) updated the Beers Criteria for potentially inappropriate medication use in older adults.4 The Beers Criteria were created to educate clinicians about the use of potentially inappropriate medications that have an unfavorable balance of benefits and risks compared to alternative treatments. The AGS lists medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Duloxetine is one of the medications listed with the recommendation to avoid for patients with a creatinine clearance <30 mL/min. Creatinine clearance is an estimation of GFR.
Although duloxetine is mentioned in the Beers Criteria, many other antidepressants have metabolites excreted by the kidneys.6 Potential adverse effects include increased bleeding, nausea, vomiting, and serotonin toxicity symptoms.7 Mr. J has Stage 3A CKD and takes 4 psychotropics, which will additively increase the serum concentration of serotonergic medications. In terms of treatment for serotonin toxicity, it is important to remove the causative medications. After discontinuing serotonergic medications, lorazepam can be administered as needed. If a patient continues to have symptoms, cyproheptadine is an option.
For patients with impaired renal function, adding nonpharmacologic options should be considered, such as cognitive-behavioral therapy, electroconvulsive therapy, and transcranial magnetic stimulation. Table 24,8-18 lists the minimum effective doses for well-known medications for treating MDD.

OUTCOME Improvement and discharge
Mr. J’s confusion improves, his heart rate decreases, and his feelings of panic and doom improve. He continues to have depressive symptoms, but his suicidal ideation stops. At discharge, Mr. J is receiving mirtazapine 15 mg/d, potassium chloride 10 mEq/d orally, lisinopril 20 mg/d orally at bedtime, furosemide 20 mg/d orally, and amlodipine 5 mg orally twice a day. Additionally, the treatment team recommends psychotherapy to Mr. J to address his anxiety and depression.
Bottom Line
Older patients are more sensitive to psychotropic medications, regardless of any comorbidities. It is important to review each patient’s glomerular filtration rate to better understand their renal function and adjust medications accordingly.
Related Resources
- Whittaker P, Vordenberg SE, Coe AB. Deprescribing in older adults: an overview. Current Psychiatry. 2022;21(5):40-43. doi:10.12788/cp.0246
- Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
- Barr R, Miskle B, Thomas C. Management of major depressive disorder with psychotic features. Current Psychiatry. 2021;20(2):30-33. doi:10.12788/cp.0092
Drug Brand Names
Amlodipine • Norvasc
Buspirone • BuSpar
Citalopram • Celexa
Cyproheptadine • Periactin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Furosemide • Lasix
Lisinopril • Zestril
Lorazepam • Ativan
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Desyrel
Venlafaxine • Effexor
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266.
2. Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2(1):94-107.
3. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953.
4. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
5. Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850-857.
6. Cukor D, Cohen, SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042-3055.
7. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332-1342.
8. Sommi RW, Crismon ML, Bowden CL. Fluoxetine: a serotonin-specific, second-generation antidepressant. Pharmacotherapy. 1987;7(1):1-15.
9. Jenner PN. Paroxetine: an overview of dosage, tolerability, and safety. Int Clin Psychopharmacol. 1992;6(Suppl 4):69-80.
10. Montgomery SA. Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol. 1995;10(Suppl 1):23-27.
11. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270-280.
12. Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-290.
13. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129-141.
14. Huddart R, Hicks JK, Ramsey LB, et al. PharmGKB summary: sertraline pathway, pharmacokinetics. Pharmacogenet Genomics. 2020;30(2):26-33.
15. Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601-609.
16. Norman TR, Olver JS. Desvenlafaxine in the treatment of major depression: an updated overview. Expert Opin Pharmacother. 2021;22(9):1087-1097.
17. Knadler MP, Lobo E, Chappell J, et al. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281-294.
18. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266.
2. Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2(1):94-107.
3. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953.
4. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
5. Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850-857.
6. Cukor D, Cohen, SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042-3055.
7. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332-1342.
8. Sommi RW, Crismon ML, Bowden CL. Fluoxetine: a serotonin-specific, second-generation antidepressant. Pharmacotherapy. 1987;7(1):1-15.
9. Jenner PN. Paroxetine: an overview of dosage, tolerability, and safety. Int Clin Psychopharmacol. 1992;6(Suppl 4):69-80.
10. Montgomery SA. Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol. 1995;10(Suppl 1):23-27.
11. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270-280.
12. Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-290.
13. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129-141.
14. Huddart R, Hicks JK, Ramsey LB, et al. PharmGKB summary: sertraline pathway, pharmacokinetics. Pharmacogenet Genomics. 2020;30(2):26-33.
15. Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601-609.
16. Norman TR, Olver JS. Desvenlafaxine in the treatment of major depression: an updated overview. Expert Opin Pharmacother. 2021;22(9):1087-1097.
17. Knadler MP, Lobo E, Chappell J, et al. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281-294.
18. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
Treating PTSD: A review of 8 studies
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).
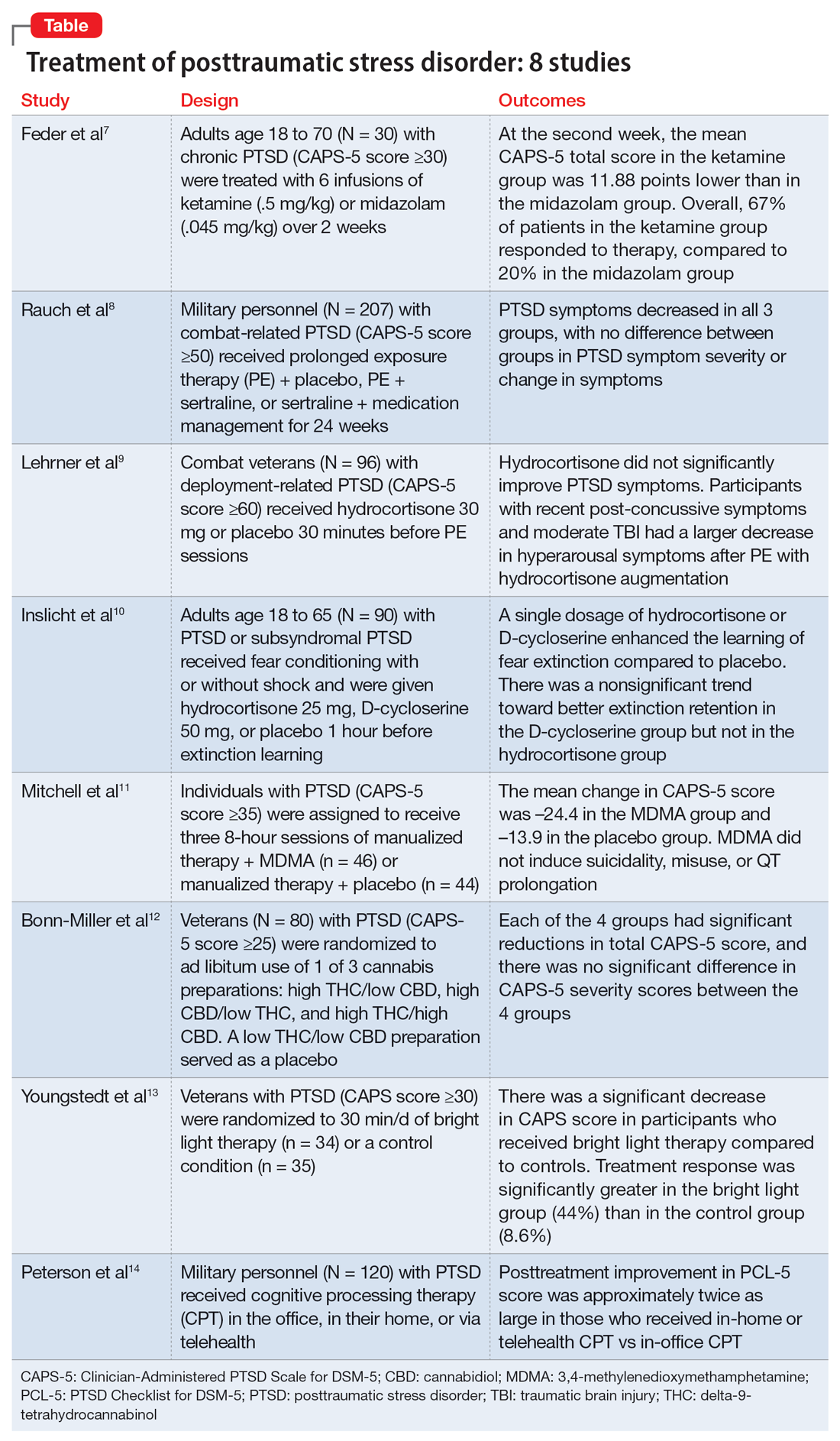
1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
Posttraumatic stress disorder (PTSD) is a chronic and disabling psychiatric disorder. The lifetime prevalence among American adults is 6.8%.1 Management of PTSD includes treating distressing symptoms, reducing avoidant behaviors, treating comorbid conditions (eg, depression, substance use disorders, or mood dysregulation), and improving adaptive functioning, which includes restoring a psychological sense of safety and trust. PTSD can be treated using evidence-based psychotherapies, pharmacotherapy, or a combination of both modalities. For adults, evidence-based treatment guidelines recommend the use of cognitive-behavioral therapy, cognitive processing therapy, cognitive therapy, and prolonged exposure therapy.2 These guidelines also recommend (with some reservations) the use of brief eclectic psychotherapy, eye movement desensitization and reprocessing, and narrative exposure therapy.2 Although the evidence base for the use of medications is not as strong as that for the psychotherapies listed above, the guidelines recommend the use of fluoxetine, paroxetine, sertraline, and venlafaxine.2
Currently available treatments for PTSD have significant limitations. For example, trauma-focused psychotherapies can have significant rates of nonresponse, partial response, or treatment dropout.3,4 Additionally, such therapies are not widely accessible. As for pharmacotherapy, very few available options are supported by evidence, and the efficacy of these options is limited, as shown by the reports that only 60% of patients with PTSD show a response to selective serotonin reuptake inhibitors (SSRIs), and only 20% to 30% achieve complete remission.5 Additionally, it may take months for patients to achieve an acceptable level of improvement with medications. As a result, a substantial proportion of patients who seek treatment continue to remain symptomatic, with impaired levels of functioning. This lack of progress in PTSD treatment has been labeled as a national crisis, calling for an urgent need to find effective pharmacologic treatments for PTSD.6
In this article, we review 8 randomized controlled trials (RCTs) of treatments for PTSD published within the last 5 years (Table7-14).

1. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202
Feder et al had previously found a significant and quick decrease in PTSD symptoms after a single dose of IV ketamine had. This is the first RCT to examine the effectiveness and safety of repeated IV ketamine infusions for the treatment of persistent PTSD.7
Study design
- This randomized, double-blind, parallel-arm controlled trial treated 30 individuals with chronic PTSD with 6 infusions of either ketamine (0.5 mg/kg) or midazolam (0.045 mg/kg) over 2 consecutive weeks.
- Participants were individuals age 18 to 70 with a primary diagnosis of chronic PTSD according to the DSM-5 criteria and determined by The Structure Clinical Interview for DSM-5, with a score ≥30 on the Clinician-Administered PTSD Scale for DSM-5 (CAPS-5).
- Any severe or unstable medical condition, active suicidal or homicidal ideation, lifetime history of psychotic or bipolar disorder, current anorexia nervosa or bulimia, alcohol or substance use disorder within 3 months of screening, history of recreational ketamine or phencyclidine use on more than 1 occasion or any use in the previous 2 years, and ongoing treatment with a long-acting benzodiazepine or opioid medication were all considered exclusion criteria. Individuals who took short-acting benzodiazepines had their morning doses held on infusion days. Marijuana or cannabis derivatives were allowed.
- The primary outcome measure was a change in PTSD symptom severity as measured with CAPS-5. This was administered before the first infusion and weekly thereafter. The Impact of Event Scale-Revised, the Montgomery–Åsberg Depression Rating Scale, and adverse effect measurements were used as secondary outcome measures.
- Treatment response was defined as ≥30% symptom improvement 2 weeks after the first infusion as assessed with CAPS-5.
- Individuals who responded to treatment were followed naturalistically weekly for up to 4 weeks and then monthly until loss of responder status, or up to 6 months if there was no loss of response.
Outcomes
- At the second week, the mean CAPS-5 total score in the ketamine group was 11.88 points (SE = 3.96) lower than in the midazolam group (d = 1.13; 95% CI, 0.36 to 1.91).
- In the ketamine group, 67% of patients responded to therapy, compared to 20% in the midazolam group.
- Following the 2-week course of infusions, the median period until loss of response among ketamine responders was 27.5 days.
- Ketamine infusions showed good tolerability and safety. There were no clinically significant adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Repeated ketamine infusions are effective in reducing symptom severity in individuals with chronic PTSD.
- Limitations to this study include the exclusion of individuals with comorbid bipolar disorder, current alcohol or substance use disorder, or suicidal ideations, the small sample size, and a higher rate of transient dissociative symptoms in the ketamine group.
- Future studies could evaluate the efficacy of repeated ketamine infusions in individuals with treatment-resistant PTSD. Also, further studies are required to assess the efficacy of novel interventions to prevent relapse and evaluate the efficacy, safety, and tolerability of periodic IV ketamine use as maintenance.
- Additional research might determine whether pairing psychotherapy with ketamine administration can lessen the risk of recurrence for PTSD patients after stopping ketamine infusions.
2. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126
Clinical practice recommendations for PTSD have identified trauma-focused psychotherapies and SSRIs as very effective treatments. The few studies that have compared trauma-focused psychotherapy to SSRIs or to a combination of treatments are not generalizable, have significant limitations, or are primarily concerned with refractory disorders or augmentation techniques. This study evaluated the efficacy of prolonged exposure therapy (PE) plus placebo, PE plus sertraline, and sertraline plus enhanced medication management in the treatment of PTSD.8
Study design
- This randomized, 4-site, 24-week clinical trial divided participants into 3 subgroups: PE plus placebo, PE plus sertraline, and sertraline plus enhanced medication management.
- Participants were veterans or service members of the Iraq and/or Afghanistan wars with combat-related PTSD and significant impairment as indicated by a CAPS score ≥50 for at least 3 months. The DSM-IV-TR version of CAPS was used because the DSM-5 version was not available at the time of the study.
- Individuals who had a current, imminent risk of suicide; active psychosis; alcohol or substance dependence in the past 8 weeks; inability to attend weekly appointments for the treatment period; prior intolerance to or failure of an adequate trial of PE or sertraline; medical illness likely to result in hospitalization or contraindication to study treatment; serious cognitive impairment; mild traumatic brain injury; or concurrent use of antidepressants, antipsychotics, benzodiazepines, prazosin, or sleep agents were excluded.
- Participants completed up to thirteen 90-minute sessions of PE.
- The sertraline dosage was titrated during a 10-week period and continued until Week 24. Dosages were adjusted between 50 and 200 mg/d, with the last dose increase at Week 10.
- The primary outcome measure was symptom severity of PTSD in the past month as determined by CAPS score at Week 24.
- The secondary outcome was self-reported symptoms of PTSD (PTSD checklist [PCL] Specific Stressor Version), clinically meaningful change (reduction of ≥20 points or score ≤35 on CAPS), response (reduction of ≥50% in CAPS score), and remission (CAPS score ≤35).
Outcomes
- At Week 24, 149 participants completed the study; 207 were included in the intent-to-treat analysis.
- PTSD symptoms significantly decreased over 24 weeks, according to a modified intent-to-treat analysis utilizing a mixed model of repeated measurements; nevertheless, slopes were similar across therapy groups.
Continue to: Conclusions/limitations
Conclusions/limitations
- Although the severity of PTSD symptoms decreased in all 3 subgroups, there was no difference in PTSD symptom severity or change in symptoms at Week 24 among all 3 subgroups.
- The main limitation of this study was the inclusion of only combat veterans.
- Further research should focus on enhancing treatment retention and should include administering sustained exposure therapy at brief intervals.
3. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924
First-line therapy for PTSD includes cognitive-behavioral therapies such as PE. However, because many people still have major adverse effects after receiving medication, improving treatment efficacy is a concern. Glucocorticoids promote extinction learning, and alterations in glucocorticoid signaling pathways have been associated with PTSD. Lehrner et al previously showed that adding hydrocortisone (HCORT) to PE therapy increased patients’ glucocorticoid sensitivity at baseline, improved treatment retention, and resulted in greater treatment improvements. This study evaluated HCORT in conjunction with PE for combat veterans with PTSD following deployment to Iraq and Afghanistan.9
Study design
- This randomized, double-blind, placebo-controlled trial administered HCORT 30 mg oral or placebo to 96 combat veterans 30 minutes before PE sessions.
- Participants were veterans previously deployed to Afghanistan or Iraq with deployment-related PTSD >6 months with a minimum CAPS score of 60. They were unmedicated or on a stable psychotropic regimen for ≥4 weeks.
- Exclusion criteria included a lifetime history of a primary psychotic disorder (bipolar I disorder or obsessive-compulsive disorder), medical or mental health condition other than PTSD that required immediate clinical attention, moderate to severe traumatic brain injury (TBI), substance abuse or dependence within the past 3 months, medical illness that contraindicated ingestion of hydrocortisone, acute suicide risk, and pregnancy or intent to become pregnant.
- The primary outcome measures included PTSD severity as assessed with CAPS.
- Secondary outcome measures included self-reported PTSD symptoms as assessed with the Posttraumatic Diagnostic Scale (PDS) and depression as assessed with the Beck Depression Inventory-II (BDI). These scales were administered pretreatment, posttreatment, and at 3-months follow-up.
Outcomes
- Out of 96 veterans enrolled, 60 were randomized and 52 completed the treatment.
- Five participants were considered recovered early and completed <12 sessions.
- Of those who completed treatment, 50 completed the 1-week posttreatment evaluations and 49 completed the 3-month follow-up evaluation.
- There was no difference in the proportion of dropouts (13.33%) across the conditions.
- HCORT failed to significantly improve either secondary outcomes or PTSD symptoms, according to an intent-to-treat analysis.
- However, exploratory analyses revealed that veterans with recent post-concussive symptoms and moderate TBI exposure saw a larger decrease in hyperarousal symptoms after PE therapy with HCORT augmentation.
- The reduction in avoidance symptoms with HCORT augmentation was also larger in veterans with higher baseline glucocorticoid sensitivity.
Continue to: Conclusions/limitations
Conclusions/limitations
- HCORT does not improve PTSD symptoms as assessed with the CAPS and PDS, or depression as assessed with the BDI.
- The main limitation of this study is generalizability.
- Further studies are needed to determine whether PE with HCORT could benefit veterans with indicators of enhanced glucocorticoid sensitivity, mild TBI, or postconcussive syndrome.
4. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952
PE, one of the most well-researched therapies for PTSD, is based on fear extinction. Exploring pharmacotherapies that improve fear extinction learning and their potential as supplements to PE is gaining increased attention. Such pharmacotherapies aim to improve the clinical impact of PE on the extent and persistence of symptom reduction. This study evaluated the effects of HCORT and D-cycloserine (DCS), a partial agonist of the N-methyl-D-aspartate (NMDA) receptor, on the learning and consolidation of fear extinction in patients with PTSD.10
Study design
- This double-blind, placebo-controlled, 3-group experimental design evaluated 90 individuals with PTSD who underwent fear conditioning with stimuli that was paired (CS+) or unpaired (CS−) with shock.
- Participants were veterans and civilians age 18 to 65 recruited from VA outpatient and community clinics and internet advertisements who met the criteria for PTSD or subsyndromal PTSD (according to DSM-IV criteria) for at least 3 months.
- Exclusion criteria included schizophrenia, bipolar disorder, substance abuse or dependence, alcohol dependence, previous moderate or severe head injury, seizure or neurological disorder, current infectious illness, systemic illness affecting CNS function, or other conditions known to affect psychophysiological responses. Excluded medications were antipsychotics, mood stabilizers, alpha- and beta-adrenergics, benzodiazepines, anticonvulsants, antihypertensives, sympathomimetics, anticholinergics, and steroids.
- Extinction learning took place 72 hours after extinction, and extinction retention was evaluated 1 week later. Placebo, HCORT 25 mg, or DCS 50 mg was given 1 hour before extinction learning.
- Clinical measures included PTSD diagnosis and symptom levels as determined by interview using CAPS and skin conduction response.
Outcomes
- The mean shock level, mean pre-stimulus skin conductance level (SCL) during habituation, and mean SC orienting response during the habituation phase did not differ between groups and were not associated with differential fear conditioning. Therefore, variations in shock level preference, resting SCL, or SC orienting response magnitude are unlikely to account for differences between groups during extinction learning and retention.
- During extinction learning, the DCS and HCORT groups showed a reduced differential CS+/CS− skin conductance response (SCR) compared to placebo.
- One week later, during the retention testing, there was a nonsignificant trend toward a smaller differential CS+/CS− SCR in the DCS group compared to placebo. HCORT and DCS administered as a single dosage facilitated fear extinction learning in individuals with PTSD symptoms.
Continue to: Conclusions/limitations
Conclusions/limitations
- In traumatized people with PTSD symptoms, a single dosage of HCORT or DCS enhanced the learning of fear extinction compared to placebo. A nonsignificant trend toward better extinction retention in the DCS group but not the HCORT group was also visible.
- These results imply that glucocorticoids and NMDA agonists have the potential to promote extinction learning in PTSD.
- Limitations include a lack of measures of glucocorticoid receptor sensitivity or FKBP5.
- Further studies could evaluate these findings with the addition of blood biomarker measures such as glucocorticoid receptor sensitivity or FKBP5.
5. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
Poor PTSD treatment results are associated with numerous comorbid conditions, such as dissociation, depression, alcohol and substance use disorders, childhood trauma, and suicidal ideation, which frequently leads to treatment resistance. Therefore, it is crucial to find a treatment that works for individuals with PTSD who also have comorbid conditions. In animal models, 3,4-methylenedioxymethamphetamine (MDMA), an empathogen/entactogen with stimulant properties, has been shown to enhance fear memory extinction and modulate fear memory reconsolidation. This study evaluated the efficacy and safety of MDMA-assisted therapy for treating patients with severe PTSD, including those with common comorbidities.11
Study design
- This randomized, double-blind, placebo-controlled, multi-site, phase 3 clinical trial evaluated individuals randomized to receive manualized therapy with MDMA or with placebo, combined with 3 preparatory and 9 integrative therapy sessions.
- Participants were 90 individuals (46 randomized to MDMA and 44 to placebo) with PTSD with a symptom duration ≥6 months and CAPS-5 total severity score ≥35 at baseline.
- Exclusion criteria included primary psychotic disorder, bipolar I disorder, eating disorders with active purging, major depressive disorder with psychotic features, dissociative identity disorder, personality disorders, current alcohol and substance use disorders, lactation or pregnancy, and any condition that could make receiving a sympathomimetic medication dangerous due to hypertension or tachycardia, including uncontrolled hypertension, history of arrhythmia, or marked baseline prolongation of QT and/or QTc interval.
- Three 8-hour experimental sessions of either therapy with MDMA assistance or therapy with a placebo control were given during the treatment period, and they were spaced approximately 4 weeks apart.
- In each session, participants received placebo or a single divided dose of MDMA 80 to 180 mg.
- At baseline and 2 months after the last experimental sessions, PTSD symptoms were measured with CAPS-5, and functional impairment was measured with Sheehan Disability Scale (SDS).
- The primary outcome measure was CAPS-5 total severity score at 18 weeks compared to baseline for MDMA-assisted therapy vs placebo-assisted therapy.
- The secondary outcome measure was clinician-rated functional impairment using the mean difference in SDS total scores from baseline to 18 weeks for MDMA-assisted therapy vs placebo-assisted therapy.
Outcomes
- MDMA was found to induce significant and robust attenuation in CAPS-5 score compared to placebo.
- The mean change in CAPS-5 score in completers was –24.4 in the MDMA group and –13.9 in the placebo group.
- MDMA significantly decreased the SDS total score.
- MDMA did not induce suicidality, misuse, or QT prolongation.
Continue to: Conclusions/limitations
Conclusions/limitations
- MDMA-assisted therapy is significantly more effective than manualized therapy with placebo in treating patients with severe PTSD, and it is also safe and well-tolerated, even in individuals with comorbidities.
- No major safety issues were associated with MDMA-assisted treatment.
- MDMA-assisted therapy should be promptly assessed for clinical usage because it has the potential to significantly transform the way PTSD is treated.
- Limitations of this study include a smaller sample size (due to the COVID-19 pandemic); lack of ethnic and racial diversity; short duration; safety data were collected by site therapist, which limited the blinding; and the blinding of participants was difficult due to the subjective effects of MDMA, which could have resulted in expectation effects.
6. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990
Sertraline and paroxetine are the only FDA-approved medications for treating PTSD. Some evidence suggests cannabis may provide a therapeutic benefit for PTSD.15 This study examined the effects of 3 different preparations of cannabis for treating PTSD symptoms.12
Study design
- This double-blind, randomized, placebo-controlled, crossover trial used 3 active treatment groups of cannabis: high delta-9-tetrahydrocannabinol (THC)/low cannabidiol (CBD), high CBD/low THC, and high THC/high CBD (THC+CBD). A low THC/low CBD preparation was used as a placebo. “High” content contained 9% to 15% concentration by weight of the respective cannabinoid, and “low” content contained <2% concentration by weight.
- Inclusion criteria included being a US military veteran, meeting DSM-5 PTSD criteria for ≥6 months, having moderate symptom severity (CAPS-5 score ≥25), abstaining from cannabis 2 weeks prior to study and agreeing not to use any non-study cannabis during the trial, and being stable on medications/therapy prior to the study.
- Exclusion criteria included women who were pregnant/nursing/child-bearing age and not taking an effective means of birth control; current/past serious mental illness, including psychotic and personality disorders; having a first-degree relative with a psychotic or bipolar disorder; having a high suicide risk based on Columbia-Suicide Severity Rating Scale; meeting DSM-5 criteria for moderate-severe cannabis use disorder; screening positive for illicit substances; or having significant medical disease.
- Participants in Stage 1 (n = 80) were randomized to 1 of the 3 active treatments or placebo for 3 weeks. After a 2-week washout, participants in Stage 2 (n = 74) were randomized to receive for 3 weeks 1 of the 3 active treatments they had not previously received.
- During each stage, participants had ad libitum use for a maximum of 1.8 g/d.
- The primary outcome was change in PTSD symptom severity by the end of Stage 1 as assessed with CAPS-5.
- Secondary outcomes included the PTSD Checklist for DSM-5 (PCL-5), the general depression subscale and anxiety subscale from the self-report Inventory of Depression and Anxiety Symptoms (IDAS), the Inventory of Psychosocial Functioning, and the Insomnia Severity Index.
Outcomes
- Six participants did not continue to Stage 2. Three participants did not finish Stage 2 due to adverse effects, and 7 did not complete outcome measurements. The overall attrition rate was 16.3%.
- There was no significant difference in total grams of smoked cannabis or placebo between the 4 treatment groups in Stage 1 at the end of 3 weeks. In Stage 2, there was a significant difference, with the THC+CBD group using more cannabis compared to the other 2 groups.
- Each of the 4 groups had significant reductions in total CAPS-5 scores at the end of Stage 1, and there was no significant difference in CAPS-5 severity scores between the 4 groups.
- In Stage 1, PCL-5 scores were not significantly different between treatment groups from baseline to the end of stage. There was a significant difference in Stage 2 between the high CBD and THC+CBD groups, with the combined group reporting greater improvement of symptoms.
- In Stage 2, the THC+CBD group reported greater reductions in pre/post IDAS social anxiety scores and IDAS general depression scores, and the high THC group reported greater reductions in pre/post IDAS social anxiety scores.
- In Stage 1, 37 of 60 participants in the active groups reported at least 1 adverse event, and 45 of the 74 Stage 2 participants reported at least 1 adverse event. The most common adverse events were cough, throat irritation, and anxiety. Participants in the Stage 1 high THC group had a significant increase in reported withdrawal symptoms after 1 week of stopping use.
Continue to: Conclusions/limitations
Conclusions/limitations
- This first randomized, placebo-control trial of cannabis in US veterans did not show a significant difference among treatment groups, including placebo, on the primary outcome of CAPS-5 score. All 4 groups had significant reductions in symptom severity on CAPS-5 and showed good tolerability.
- Prior beliefs about the effects of cannabis may have played a role in the reduction of PTSD symptoms in the placebo group.
- Many participants (n =34) were positive for THC during the screening process, so previous cannabis use/chronicity of cannabis use may have contributed.
- One limitation was that participants assigned to the Stage 1 high THC group had Cannabis Use Disorders Identification Test scores (which assesses cannabis use disorder risk) about 2 times greater than participants in other conditions.
- Another limitation was that total cannabis use was lower than expected, as participants in Stage 1 used 8.2 g to 14.6 g over 3 weeks, though they had access to up to 37.8 g.
- There was no placebo in Stage 2.
- Future studies should look at longer treatment periods with more participants.
7. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444
Bright light therapy is an inexpensive treatment approach that may affect serotonergic pathways.16 This study examined bright light therapy for reducing PTSD symptoms and examined if improvement of PTSD is related to a shift in circadian rhythm.13
Study design
- Veterans with combat-related PTSD had to have been stable on treatment for at least 8 weeks or to have not received any other PTSD treatments prior to the study.
- Participants were randomized to active treatment of 30 minutes daily 10,000 lux ultraviolet-filtered white light while sitting within 18 inches (n = 34) or a control condition of 30 minutes daily inactivated negative ion generator (n = 35) for 4 weeks.
- Inclusion criteria included a CAPS score ≥30.
- Exclusion criteria included high suicidality, high probability of alcohol/substance abuse in the past 3 months, bipolar disorder/mania/schizophrenia/psychosis, ophthalmologic deformities, shift work in past 2 months or travel across time zones in past 2 weeks, head trauma, high outdoor light exposure, history of winter depression, history of seizures, or myocardial infarction/stroke/cancer within 3 years.
- Primary outcomes were improvement on CAPS and Clinical Global Impressions-Improvement scale (CGI-IM) score at Week 4.
- Wrist actigraphy recordings measured sleep.
- Other measurements included the Hamilton Depression Rating Scale (HAM-D), Hamilton atypical symptoms (HAM-AS), PCL-Military (PCL-M), Pittsburg Sleep Quality Index (PSQI), BDI, Spielberger State-Trait Anxiety Inventory (STAI Form Y-2), Beck Suicide Scale, and Systematic Assessment for Treatment Emergent Effects questionnaire.
Outcomes
- There was a significant decrease in CAPS score in participants who received bright light therapy compared to controls. Treatment response (defined as ≥33% reduction in score) was significantly greater in the bright light (44%) vs control (8.6%) group. No participants achieved remission.
- There was a significant improvement in CGI-IM scores in the bright light group, but no significant difference in participants who were judged to improve “much” or “very much.”
- PCL-M scores did not change significantly between groups, although a significantly greater proportion of participants had treatment response in the bright light group (33%) vs control (6%).
- There were no significant changes in HAM-D, HAM-AS, STAI, BDI, actigraphic estimates of sleep, or PSQI scores.
- Bright light therapy resulted in phase advancement while control treatment had phase delay.
- There were no significant differences in adverse effects.
Continue to: Conclusions/limitations
Conclusions/limitations
- Bright light therapy may be a treatment option or adjunct for combat-related PTSD as seen by improvement on CAPS and CGI scores, as well as a greater treatment response seen on CAPS and PCL-5 scores in the bright light group.
- There was no significant difference for other measures, including depression, anxiety, and sleep.
- Limitations include excluding patients with a wide variety of medical or psychiatric comorbidities, as well as limited long-term follow up data.
- Other limitations include not knowing the precise amount of time participants stayed in front of the light device and loss of some actigraphic data (data from only 49 of 69 participants).
8. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41 doi:10.1186/s12888-022-03699-4
Cognitive processing therapy (CPT), a type of trauma-focused psychotherapy, is an effective treatment for PTSD in the military population.17,18 However, patients may not be able to or want to participate in such therapy due to barriers such as difficulty arranging transportation, being homebound due to injury, concerns about COVID-19, stigma, familial obligations, and job constraints. This study looked at if CPT delivered face-to-face at the patient’s home or via telehealth in home would be effective and increase accessibility.14
Study design
- Participants (n = 120) were active-duty military and veterans who met DSM-5 criteria for PTSD. They were randomized to receive CPT in the office, in their home, or via telehealth. Participants could choose not to partake in 1 modality but were then randomized to 1 of the other 2.
- Exclusion criteria included suicide/homicide risk needing intervention, items/situations pertaining to danger (ie, aggressive pet or unsafe neighborhood), significant alcohol/substance use, active psychosis, and impaired cognitive functioning.
- The primary outcome measurement was change in PCL-5 and CAPS-5 score over 6 months. The BDI-II was used to assess depressive symptoms.
- Secondary outcomes included the Reliable Change Index (defined as “an improvement of 10 or more points that was sustained at all subsequent assessments”) on the PCL-5 and remission on the CAPS-5.
- CPT was delivered in 60-minute sessions twice a week for 6 weeks. Participants who did not have electronic resources were loaned a telehealth apparatus.
Outcomes
- Overall, 57% of participants opted out of 1 modality, which resulted in fewer participants being placed into the in-home arm (n = 32). Most participants chose not to do in-home treatments (54%), followed by in-office (29%), and telehealth (17%).
- There was a significant posttreatment improvement in PCL-5 scores in all treatment arms, with improvement greater with in-home (d = 2.1) and telehealth (d = 2.0) vs in-office (d=1.3). The in-home and telehealth scores were significantly improved compared to in-office, and the difference between in-home and telehealth PCL-5 scores was minimal.
- At 6 months posttreatment, the differences between the 3 treatment groups on PCL-5 score were negligible.
- CAPS-5 scores were significantly improved in all treatment arms, with improvement largest with in-home treatment; however, the differences between the groups were not significant.
- BDI-II scores improved in all modalities but were larger in the in-home (d = 1.2) and telehealth (d = 1.1) arms than the in-office arm (d = 0.52).
- Therapist time commitment was greater for the in-home and in-office arms (2 hours/session) than the telehealth arm (1 hour/session). This difference was due to commuting time for the patient or therapist.
- The dropout rate was not statistically significant between the groups.
- Adverse events did not significantly differ per group. The most commonly reported ones included nightmares, sleep difficulty, depression, anxiety, and irritability.
Conclusions/limitations
- Patients undergoing CPT had significant improvement in PTSD symptoms, with posttreatment PCL-5 improvement approximately twice as large in those who received the in-home and telehealth modalities vs in-office treatment.
- The group differences were not seen on CAPS-5 scores at posttreatment, or PCL-5 or CAPS-5 scores at 6 months posttreatment.
- In-home CPT was declined the most, which suggests that in-home distractions or the stigma of a mental health clinician being in their home played a role in patients’ decision-making. However, in-home CPT produced the greatest amount of improvement in PTSD symptoms. The authors concluded that in-home therapy should be reserved for those who are homebound or have travel limitations.
- This study shows evidence that telehealth may be a good modality for CPT, as seen by improvement in PTSD symptoms and good acceptability and retention.
- Limitations include more patients opting out of in-home CPT, and reimbursement for travel may not be available in the real-world setting.
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
1. Kessler RC, Berglund P, Delmer O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593-602.
2. Guideline Development Panel for the Treatment of PTSD in Adults, American Psychological Association. Summary of the clinical practice guideline for the treatment of posttraumatic stress disorder (PTSD) in adults. Am Psychol. 2019;74(5):596-607. doi: 10.1037/amp0000473
3. Steenkamp MM, Litz BT, Hoge CW, et al. Psychotherapy for military-related PTSD: a review of randomized clinical trials. JAMA. 2015;314(5):489-500.
4. Steenkamp MM, Litz BT, Marmar CR. First-line psychotherapies for military-related PTSD. JAMA. 2020;323(7):656-657.
5. Berger W, Mendlowicz MV, Marques-Portella C, et al. Pharmacologic alternatives to antidepressants in posttraumatic stress disorder: a systematic review. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(3):169-180.
6. Krystal JH, Davis LL, Neylan TC, et al. It is time to address the crisis in the pharmacotherapy of posttraumatic stress disorder: a consensus statement of the PTSD Psychopharmacology Working Group. Biol Psychiatry. 2017;82(7):e51-e59.
7. Feder A, Costi S, Rutter SB, et al. A randomized controlled trial of repeated ketamine administration for chronic posttraumatic stress disorder. Am J Psychiatry. 2021;178(2):193-202. doi:10.1176/appi.ajp.2020.20050596
8. Rauch SAM, Kim HM, Powell C, et al. Efficacy of prolonged exposure therapy, sertraline hydrochloride, and their combination among combat veterans with posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry. 2019;76(2):117-126. doi:10.1001/jamapsychiatry.2018.3412
9. Lehrner A, Hildebrandt T, Bierer LM, et al. A randomized, double-blind, placebo-controlled trial of hydrocortisone augmentation of prolonged exposure for PTSD in US combat veterans. Behav Res Ther. 2021;144:103924. doi:10.1016/j.brat.2021.103924
10. Inslicht SS, Niles AN, Metzler TJ, et al. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology. 2022;47(11):1945-1952. doi:10.1038/s41386-021-01222-z
11. Mitchell JM, Bogenschutz M, Lilienstein A, et al. MDMA-assisted therapy for severe PTSD: a randomized, double-blind, placebo-controlled phase 3 study. Nat Med. 2021;27(6):1025-1033. doi:10.1038/s41591-021-01336-3
12. Bonn-Miller MO, Sisley S, Riggs P, et al. The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One. 2021;16(3):e0246990. doi:10.1371/journal.pone.0246990
13. Youngstedt SD, Kline CE, Reynolds AM, et al. Bright light treatment of combat-related PTSD: a randomized controlled trial. Milit Med. 2022;187(3-4):e435-e444. doi:10.1093/milmed/usab014
14. Peterson AL, Mintz J, Moring JC, et al. In-office, in-home, and telehealth cognitive processing therapy for posttraumatic stress disorder in veterans: a randomized clinical trial. BMC Psychiatry. 2022;22(1):41. doi:10.1186/s12888-022-03699-4
15. Loflin MJ, Babson KA, Bonn-Miller MO. Cannabinoids as therapeutic for PTSD. Curr Opin Psychol. 2017;14:78-83. doi:10.1016/j.copsyc.2016.12.001
16. Neumeister A, Praschak-Rieder N, Besselmann B, et al. Effects of tryptophan depletion on drug-free patients with seasonal affective disorder during a stable response to bright light therapy. Arch Gen Psychiatry. 1997;54(2):133-138. doi:10.1001/archpsyc.1997.01830140043008
17. Kaysen D, Schumm J, Pedersen ER, et al. Cognitive processing therapy for veterans with comorbid PTSD and alcohol use disorders. Addict Behav. 2014;39(2):420-427. doi:10.1016/j.addbeh.2013.08.016
18. Resick PA, Wachen JS, Mintz J, et al. A randomized clinical trial of group cognitive processing therapy compared with group present-centered therapy for PTSD among active duty military personnel. J Consult Clin Psychol. 2015;83(6):1058-1068. doi:10.1037/ccp0000016
Contemporary psychiatry: A SWOT analysis
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
Editor’s note: This article was adapted with permission from a version originally published in the Ohio Psychiatric Physician Association’s newsletter, Insight Matters, Fall 2022.
Acknowledging and analyzing strengths, weaknesses, opportunities, and threats (SWOT) is an important tactic many organizations use to develop a strategic plan to grow, move forward, and thrive. A SWOT analysis can provide a “big picture” view of the status and the desired future directions not only for companies but for medical disciplines such as psychiatry. So here are my perspectives on psychiatry’s strengths, weaknesses, opportunities, and threats. It is a work in progress, and I welcome (and encourage) you to send additional items or comments to me at henry.nasrallah@currentpsychiatry.com.
Strengths
- The American Psychiatric Association (APA) is the oldest medical professional organization, established in 1844 (3 years before the American Medical Association)1
- Strong organizational structure and governance, and a “big tent” with several tiers of membership
- Effective, member-driven District Branches
- The medical identity at the core of psychiatry—we are psychiatric physicians2
- Escalating number of senior medical students choosing psychiatry as a career, far more than a decade ago
- High demand for psychiatrists in all settings around the country
- Increased compensation for psychiatrists (market forces of supply and demand)
- Psychiatry is continuously evolving and reinventing itself: seismic shifts in etiopathogenesis, disease conceptualization, terminology, and therapies (4 major shifts over the past century)3
- An abundant body of evidence supporting that all psychiatric disorders are brain disorders and transdiagnostic in nature4
- Many vibrant subspecialty societies
- Substantial number of Tier 1, evidence-based treatments
- Novel mechanisms of action and treatment strategies are being introduced on a regular basis for psychotic and mood disorders5,6
- Advances in neuromodulation techniques to treat a wide spectrum of psychiatric disorders, including electroconvulsive therapy, transcranial magnetic stimulation, vagus nerve stimulation, transcranial direct current stimulation, deep brain stimulation, cranial electric stimulation, epidural cortical stimulation, focused ultrasound, low field magnetic stimulation, magnetic seizure therapy, and near infrared light therapy, with mechanisms that are electric, ultrasound, magnetic, or optical7,8
- Psychiatric physicians develop wisdom by practicing psychiatry (ie, they become more empathic, tolerant of ambiguity, prosocial, introspective, aware of one’s strengths and limitations). Neuroplasticity in the frontal cortex is triggered by conducting psychotherapy9
Weaknesses
- Shrinking workforce due to a static number of residency training slots for 40 years10
- High rate of retirement by aging psychiatrists
- Persistent stigma around mental disorders despite massive scientific and medical advances11
- Still no real parity! We need succinct laws with “teeth”12
- Demedicalization in the public sector, referring to psychiatric physicians as “providers” and labeling patients as “clients”2
- Not enough graduating residents choosing to do subspecialty fellowships (especially geriatric, addiction, psychosomatic psychiatry) to meet escalating societal needs
- Very low presence in rural areas (both psychiatrists and psychiatric hospitals)
- Persistent APA member apathy: only 10% to 15% vote in the APA national elections or volunteer to serve on committees
- Widespread member dissatisfaction with maintenance of certification
- Neuroscience advances are not being translated fast enough for practical clinical applications
- Many in the public at large do not realize psychiatric symptoms are generated from anomalous brain circuits or that psychiatric disorders are highly genetic but also have environmental and epigenetic etiologies
- The DSM diagnostic system needs a paradigm shift: it is still based on a menu of clinical signs and symptoms and is devoid of objective diagnostic measures such as biomarkers4
- Neuroscience literacy among busy psychiatric practitioners is insufficient at a time of explosive growth in basic and clinical neuroscience13
- No effective treatment for alcohol or substance use disorders despite their very high morbidity and mortality
- Major psychiatric disorders are still associated with significant disability (schizophrenia, bipolar disorder, major depressive disorder, anxiety disorders, eating disorders, substance use disorders)
- Suicide rate (other than opioid deaths) has continued to rise in the past 3 decades14
Opportunities
- Potentially momentous clinical applications of the neuroscience breakthroughs
- Collaborative care with primary care physicians and increasing colocalization
- Dramatic increase in public awareness about the importance of mental health due to the COVID-19 pandemic15
- Powerful new data management tools, including machine learning, artificial intelligence, super computers, big data, deep learning, nanotechnology, and metabolomics, all of which are expediting neurobiological discoveries16
- The potential of reclassifying psychiatric disorders as neurological disorders, which will improve reimbursement for patient health care and reduce stigma17
- Emergence of new mechanisms of action of disease etiology, such as microbiota, mitochondrial dysfunction, permeable blood-brain barrier, and neuroimmune dysregulation18,19
- The advent and growth of “precision psychiatry”20
- The tremendous potential of molecular genetics and gene therapy for psychiatric disorders, most of which are genetic in etiology
- Expanding applications of neuroimaging techniques, including morphological, spectroscopic, functional, diffusion tensor imaging, and receptor imaging21
- Epigenetic advances in neuropsychiatric disorders
- Remarkably powerful research methods, such as pluripotent cells (producing neurons from skin cells), optogenetics (activating genes with light), gene-wide association studies, CRISPR (clustered regularly interspaced short palindromic repeats, which serve as genetic scissors to remove and replace abnormal genes), and brain connectomics22
- Psychiatry should develop and promote an “annual mental health checkup” for all age groups, similar to an annual physical exam23
- Focus on the social determinants of health
- Address the unmet mental health needs of individuals who are members of minority groups
- Lobby ferociously for a much larger budget for the National Institute of Mental Health to advance funding for research of serious psychiatric brain disorders
- Remind Congress continuously that the cost of mental illness is $700 billion annually and costs can only be reduced by funding neurobiological research1
- Partner with the pharmaceutical industry instead of demonizing them. They are the only entity that develops medication for psychiatry, where 80% of disorders have no FDA-approved drugs.24 Without the pharmaceutical industry and the help of medications, many psychiatric patients would still be institutionalized and unable to lead a normal life. We must recognize the contributions of pharmaceutical companies to the health of our patients, similar to the warp speed development of vaccines for the deadly coronavirus
- Psychiatric clinicians must refer patients to clinical trials because without patients enrolling in FDA studies, no drug developments can take place
- Many “out-of-the-box” therapies are being developed, such as antiapoptotic therapy, microglia inhibition, mitochondrial repair, white matter fiber remyelination, neuroprotection, and reversing N-methyl-
d -aspartate receptor hypofunction25 - The emerging evidence that psychotherapy is in fact a biological treatment that induces brain changes (neuroplasticity) and can modulate the immune system26
- Druggable genes, providing innovative new medications27
- Reposition psychedelics as revolutionary new treatments28
- Emphasize measurement-based care (rating scales), which can upgrade patient care29
- Because psychosis is associated with brain tissue loss, just like heart attacks are associated with myocardium destruction, psychiatrists must act like cardiologists30 and treat psychotic episodes urgently, like a stroke,31 to reduce the duration of untreated psychosis and improve patient outcomes
Threats
- Antipsychiatry cults continue to disparage and attack psychiatry32
- Health delivery systems are replacing psychiatric physicians with nurse practitioners to lower costs, regardless of quality and experience, and they inappropriately lump them together as “providers”2
- Psychologists continue to seek prescribing privileges with absurdly sketchy, predominantly online training supervised by other psychologists33
- Many legislators and policymakers, as well as the public, still don’t understand the difference between psychiatrists and psychologists, and the extensively disparate medical training in quality and quantity
- A dearth of psychiatric physician-scientists because very few residents are pursuing research fellowships after training34
- Disproportionate emphasis on clinical care and generating clinical revenue (relative value units) in academic institutions, with fewer tenure-track faculty members having protected time to write grants for federal or foundation grants to support their salaries and research operations35
- Meager financial support for teaching in psychiatry departments
- Many seriously psychiatrically ill persons do not have access to psychiatric medical care (and often to primary care as well)
- Many in the public falsely believe psychiatric disorders are hopeless and untreatable, which perpetuates stigma
- Long-acting injectable antipsychotic formulations are not used early enough in patients with psychosis, who are known to have a high nonadherence rate with oral medications following discharge from their first hospitalization. This leads to many recurrences with multiple devastating consequences, including progressive brain tissue loss, treatment resistance, disability, incarceration, and suicide36
- Many clinicians do not have full-text access to all studies indexed in PubMed, which is vital for lifelong learning in a rapidly growing medical discipline such as psychiatry
- Psychiatrists are often unable to prescribe medications shortly after they are approved by the FDA due to the insurance companies’ outrageous preauthorization racket that enforces a fail-first policy with cheaper generics, even if generic medications are associated with safety and tolerability problems37
- The continued use of decades-old first-generation antipsychotic medications despite 32 published studies reporting their neurotoxicity and the death of brain cells38
Using this analysis to benefit our patients
Despite its strengths, psychiatry must overcome its weaknesses, fend off its threats, and exploit its many opportunities. The only way to do that is for psychiatrists to unify and for the APA to provide inspired leadership to achieve the aspirational goals of our field. However, we must adopt “moonshot thinking”39 to magnify the Ss, diminish the Ws, exploit the Os, and stave off the Ts of our SWOT, thereby attaining all our cherished and lofty goals. Ultimately, the greatest beneficiaries will be our patients.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
1. Nasrallah HA. 20 reasons to celebrate our APA membership. Current Psychiatry. 2020;19(1):6-9.
2. Nasrallah HA. We are physicians, not providers, and we treat patients, not clients! Current Psychiatry. 2020;19(2):5-8.
3. Nasrallah HA. From bedlam to biomarkers: the transformation of psychiatry’s terminology reflects its 4 conceptual earthquakes. Current Psychiatry. 2015;14(1):5-7.
4. Nasrallah HA. Re-inventing the DSM as a transdiagnostic model: psychiatric disorders are extensively interconnected. Ann Clin Psychiatry. 2021;33(3):148-150.
5. Nasrallah HA. Psychopharmacology 3.0. Current Psychiatry. 2081;17(11):4-7.
6. Nasrallah HA. Reversing depression: a plethora of therapeutic strategies and mechanisms. Current Psychiatry. 2022;21(8):4-6.
7. Rosa MA, Lisanby SH. Somatic treatments for mood disorders. Psychopharmacology. 2012;37(1):102-116.
8. Nasrallah HA. Optimal psychiatric treatment: target the brain and avoid the body. Current Psychiatry. 2022;21(12):3-6.
9. Nasrallah HA. Does psychiatry practice make us wise? Current Psychiatry. 2009;8(10):12-14.
10. Buckley PF, Nasrallah HA. The psychiatry workforce pool is shrinking. What are we doing about it? Current Psychiatry. 2016;15(9):23-24,95.
11. Nasrallah HA. A psychiatric manifesto: stigma is hate speech and a hate crime. Current Psychiatry. 2022;21(6):6-8.
12. Nasrallah HA. The travesty of disparity and non-parity. Current Psychiatry. 2014;13(1):8,19.
13. Nasrallah HA. Advancing clinical neuroscience literacy among psychiatric practitioners. Current Psychiatry. 2017;16(9):17-18.
14. Nasrallah HA. The scourge of societal anosognosia about the mentally ill. Current Psychiatry. 2016;15(6):19-24.
15. Nasrallah HA. 10 silver linings of the COVID-19 pandemic. Insight Matters. 2021;45:3-4.
16. Kalenderian H, Nasrallah HA. Artificial intelligence in psychiatry. Current Psychiatry. 2019:18(8):33-38.
17. Nasrallah HA. Let’s tear down the silos and re-unify psychiatry and neurology! Current Psychiatry. 2013;12(8):8-9.
18. Nasrallah HA. It takes guts to be mentally ill: microbiota and psychopathology. Current Psychiatry. 2018;17(9):4-6.
19. Schrenk DA, Nasrallah HA. Faulty fences: blood-brain barrier dysfunction in schizophrenia. Current Psychiatry. 2022;21(10):28-32.
20. Nasrallah HA. The dawn of precision psychiatry. Current Psychiatry. 2017;16(12):7-8,11.
21. Nasrallah HA. Today’s psychiatric neuroscience advances were science fiction during my residency. Current Psychiatry 2021;20(4):5-7,12,24.
22. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
23. Nasrallah HA. I have a dream…for psychiatry. Current Psychiatry. 2021;20(11):12-14.
24. Devulapalli KK, Nasrallah HA. An analysis of the high psychotropic off-label use in psychiatric disorders: the majority of psychiatric diagnoses have no approved drug. Asian J Psychiatry. 2009;2(1):29-36.
25. Nasrallah HA. Transformative advances are unfolding in psychiatry. Current Psychiatry. 2019;18(9):10-12.
26. Nasrallah HA. Repositioning psychotherapy as a neurobiological intervention. Current Psychiatry. 2013;12(12):18-19.
27. Nasrallah HA. Druggable genes, promiscuous drugs, repurposed medications. Current Psychiatry. 2016;15(5):23,27.
28. Nasrallah HA. Long overdue: measurement-based psychiatric practice. Current Psychiatry. 2009;8(4):14-16.
29. Nasrallah HA. Maddening therapies: how hallucinogens morphed into novel treatments. Current Psychiatry. 2017:16(1):19-21.
30. Nasrallah HA. For first episode psychosis, psychiatrists should behave like cardiologists. Current Psychiatry. 2017;16(8):4-7.
31. Nasrallah HA, Roque A. FAST and RAPID: acronyms to prevent brain damage in stroke and psychosis. Current Psychiatry. 2018;17(8):6-8.
32. Nasrallah HA. The antipsychiatry movement: who and why. Current Psychiatry. 2011;10(12):4,6,53.
33. Nasrallah HA. Prescribing is the culmination of extensive medical training and psychologists do not qualify. Current Psychiatry. 2017;16(6):11-12,14-16.
34. Fenton W, James R, Insel T. Psychiatry residency training, the physician-scientist, and the future of psychiatry. Acad Psychiatry. 2004;28(4):263-266.
35. Balon R, Morreale MK. The precipitous decline of academic medicine in the United States. Ann Clin Psychiatry. 2020;32(4):225-227.
36. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
37. Nasrallah HA. Pre-authorization is illegal, unethical, and adversely disrupts patient care. Current Psychiatry. 2020;19(4):5-11.
38. Nasrallah HA, Chen AT. Multiple neurotoxic effects of haloperidol resulting in neuronal death. Ann Clin Psychiatry. 2017;29(3):195-202.
39. Nasrallah HA. It’s time for moonshot thinking in psychiatry. Current Psychiatry. 2022;21(2):8-10.
From debate to stalemate and hate: An epidemic of intellectual constipation
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
Groupthink is hazardous, especially when perfused with religious fervor. It can lead to adopting irrational thinking1 and aversion to new ideas or facts. Tenaciously clinging to 1 ideology as “the absolute truth” precludes an open-minded, constructive debate with any other point of view.
Three historical examples come to mind:
- The discovery of chlorpromazine in 1952 was a scientifically and clinically seismic and transformational event for the treatment of psychosis, which for centuries had been dogmatically deemed irreversible. Jean Delay, MD, the French psychiatrist and co-discoverer of chlorpromazine, was the first physician to witness the magical and dazzling dissolution of delusions and hallucinations in chronically institutionalized patients with psychosis.2 He published his landmark clinical observations and then traveled to the United States to share the great news and present his findings at a large psychiatric conference, hoping to enthrall American psychiatrists with the historic breakthrough in treating psychosis. This was an era in which psychoanalysis dominated American psychiatry (despite its dearth of empirical evidence). Dr. Delay was shocked when the audience of psychoanalysts booed him for saying that psychosis can be treated with a medication instead of with psychoanalysis (which, in the most intense groupthink in the history of psychiatry, they all believed was the only therapy for psychosis). Deeply disheartened, Dr. Delay returned to France and never returned to the United States. This groupthink was a prime example of intellectual constipation. Since then, not surprisingly, psychopharmacology grew meteorically while psychoanalysis declined precipitously.
- The monoamine hypothesis of depression, first propagated 60 years ago, became a groupthink dogma among psychiatric researchers for the next several decades, stultifying broader antidepressant medication development by focusing only on monoamines (eg, serotonin, norepinephrine, and dopamine). More recently, researchers have become more open-minded, and the monoamine hypothesis has taken a backseat to innovative new models of antidepressant therapy based on advances in the pathophysiology of depression, such as glutamatergic, opioid, and sigma pathways as well as neuroplasticity models.3 The consequence of groupthink in antidepressant research was a half-century delay in the development of effective alternative treatments that could have helped millions of patients recover from a life-threatening brain disorder such as major depressive disorder.
- Peptic ulcer and its serious gastritis were long believed to be due to stress and increased stomach acidity. So the groupthink gastroenterologists mocked 2 Australian researchers, Barry Marshall and Robin Warren, when they proposed that peptic ulcer may be due to an infection with a bacterium called Helicobacter pylori, and published their data demonstrating it.4 Marshall and Warren had the last laugh when they were awarded the 2005 Nobel Prize in Medicine and Physiology. It is ironic that even gastroenterologists are not immune to the affliction of intellectual constipation!
Intellectual constipation’s effects on youth
The principle of a civilized debate of contrarian ideas must be inculcated early, especially during college years. Youth should be mentored about not cowering into an ideological cocoon and shun listening to different or opposing points of view.5 Institutions of higher learning are incubators of future leaders. They must provide their young students with a wide diversity of ideas and philosophies and encourage them to critique those ideas, not “shelter” or isolate them from any ideas. Youth need to recognize that the complex societies in which we all live and work are not placid or unidimensional but a hotbed of clashing ideas and perspectives. An open-minded approach to education will inoculate young minds from developing intellectual constipation in adulthood.
Avoiding or insulating oneself from the ideas of others—no matter how disagreeable—leads to cognitive cowardice and behavioral intolerance. Healthy and vibrant debate is necessary as an inoculation against extremism, hate, paranoia, and, ultimately, violence. Psychiatrists help patients to self-reflect, gain insight, and consider changing their view of themselves and the world to help them grow into mature and resilient individuals. But for the millions of people with intellectual constipation, a potent cerebral enema comprised of a salubrious concoction of insight, common sense, and compromise may be the prescription to forestall lethal intellectual ileus.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
1. Nasrallah HA. Irrational beliefs: a ubiquitous human trait. Current Psychiatry. 2007;6(2):15-16.
2. Ban TA. Fifty years chlorpromazine: a historical perspective. Neuropsychiatr Dis Treat. 2007;3(4):495-500.
3. Boku S, Nakagawa S, Toda H, et al. Neural basis of major depressive disorder: beyond monoamine hypothesis. Psychiatry Clin Neurosci. 2018;72(1):3-12.
4. Warren JR, Marshall B. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet. 1983;1(8336):1273-1275.
5. Lukianoff G, Haidt J. The Coddling of the American Mind: How Good Intentions and Bad Ideas Are Setting Up a Generation for Failure. Penguin Books; 2018.
Positive psychotherapy: Core principles
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.
A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.

A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
In a time of great national and global upheaval, increasing social problems, migration, climate crisis, globalization, and increasingly multicultural societies, our patients and their needs are unique, diverse, and changing. We need a new understanding of mental health to be able to adequately meet the demands of an ever-changing world. Treatment exclusively with psychotropic medications or years of psychoanalysis will not meet these needs.
Psychiatrists and psychotherapists feel (and actually have) a social responsibility, particularly in a multifaceted global society. Psychotherapeutic interventions may contribute to a more peaceful society1 by reducing individuals’ inner stress, solving (unconscious) conflicts, and conveying a humanistic worldview. As an integrative and transcultural method, positive psychotherapy has been applied for more than 45 years in more than 60 countries and is an active force within a “positive mental health movement.”2
The term “positive psychotherapy” describes 2 different approaches3: positive psychotherapy (1977) by Nossrat Peseschkian,4 which is a humanistic psychodynamic approach, and positive psychotherapy (2006) by Martin E.P. Seligman, Tayyab Rashid, and Acacia C. Parks,5 which is a more cognitive-behavioral therapy (CBT)–based approach. This article focuses on the first approach.
Why ‘positive’ psychotherapy?
The term “positive” implies that positive psychotherapy focuses on the patient’s possibilities and capacities. Symptoms and disorders are seen as capacities to react to a conflict. The Latin term “positum” or “positivus” is applied in its original meaning—the factual, the given, the actual. Factual and given are not only the disorder, the symptoms, and the problems but also the capacity to become healthy and/or cope with this situation. This positive meaning confronts the patient (and the therapist) with a lesser-known aspect of the illness, but one that is just as important for the understanding and clinical treatment of the affliction: its function, its meaning, and, consequently, its positive aspects.6
Positive psychotherapy is a humanistic psychodynamic psychotherapy approach developed by Nossrat Peseschkian (1933-2010).4,7 Positive psychotherapy has been developed since the 1970s in the clinical setting with neurotic and psychosomatic patients. It integrates approaches of the 4 main modalities of psychotherapy:
- a humanistic view of human beings
- a systemic approach toward culture, work, and environment
- a psychodynamic understanding of disorders
- a practical, goal-oriented approach with some cognitive-behavioral techniques.
The concept of balance
Based on a humanistic view of human beings and the resources every patient possesses, a key concept of positive psychotherapy is the importance of balance in one’s life. The balance model (Figure) is the core of positive psychotherapy and is applied in clinical and nonclinical settings. This model is based on the concept that there are 4 main areas of life in which a human being lives and functions. These areas influence one’s satisfaction in life, one’s feelings of self-worth, and the way one deals with conflicts and challenges. Although all 4 capacities are latent in every human being, depending on one`s education, environment, and zeitgeist, some will be more developed than others. Our life energies, activities, and reactions belong to these 4 areas of life:
- physical: eating, tenderness, sexuality, sleep, relaxation, sports, appearance, clothing
- achievement: work, job, career, money
- relationships: partner, family, friends, acquaintances and strangers, community life
- meaning and future: existential questions, spirituality, religious practices, future plans, fantasy.

A goal of treatment is to help the patient recognize their own resources and mobilize them with the goal of bringing them into a dynamic equilibrium. This goal places value on a balanced distribution of energy (25% to each area), not of time. According to positive psychotherapy, a person does not become ill because one sphere of life is overemphasized but because of the areas that have been neglected. In the case vignette described in the Box, the problem is not the patient’s work but that his physical health, family and friends, and existential questions are being neglected. That the therapist is not critical from the start of treatment is a constructive experience for the patient and is important and fruitful for building the relationship between the therapist and the patient. Instead of emphasizing the deficits or the disorders, the patient and his family hear that he has neglected other areas of life and not developed them yet.
Box
Mr. M, a 52-year-old manager, is “sent” by his wife to see a psychotherapist. “My wife says I am married to my job, and I should spend more time with her and the children. I understand this, but I love my job. It is no stress for me, but a few minutes at home, and I feel totally stressed out,” he says. During the first interview, the therapist asks Mr. M to draw his energy distribution in the balance model (Figure), and it becomes clear he spends more than 80% of his time and energy on his job.
That is not such a surprise for him. But after some explanation, the therapist tells him that he should continue to do so and that it is an ability to be able to spend so much time every day for his job. Mr. M says, “You are the first person to tell me that it is good that I am working so much. I expected you, like all the others, to tell me I must reduce my working hours immediately, go on vacation, etc.”
Continue to: The balance model...
The balance model also embodies the 4 potential sources of self-esteem. Usually, only 1 or 2 areas provide self-esteem, but in the therapeutic process a patient can learn to uncover the neglected areas so that their self-esteem will have additional pillars of support. By emphasizing how therapy can help to develop one’s self-esteem, many patients can be motivated for the therapeutic process. The balance model, with its concept of devoting 25% of one’s energy to each sphere of life, gives the patient a clear vision about their life and how they can be healthy over the long run by avoiding one-sidedness.8
The transcultural approach
In positive psychotherapy, the term “transcultural” (or cross-cultural) means not only consideration of cultural factors when the therapist and patient come from diverse cultural backgrounds (intercultural psychotherapy or “migrant psychotherapy”) but specifically the consideration of cultural factors in every therapeutic relationship, as a therapeutic attitude and consequently as a sociopolitical dimension of our thinking and behavior. This consideration of the uniqueness of each person, of the relativity of human behavior, and of “unity in diversity” is an essential reason positive psychotherapy is not a “Western” method in the sense of “psychological colonization.”9 Rather, this approach is a culture-sensitive method that can be modified to adapt to particular cultures and life situations.
Transcultural positive psychotherapy begins with answering 2 questions: “How are people different?” and “What do all people have in common?”4 During the therapeutic process, the therapist gives examples from other cultures to the patient to help them relativize their own perspective and broaden their repertoire of behavior.
The use of stories, tales, proverbs, and anecdotes
A special technique of positive psychotherapy is the therapeutic use of stories, tales, proverbs, and anecdotes.10 Often stories from other cultures are used because they offer another perspective when the patient sees none. This has been shown to be highly effective in psychiatric settings, especially in group settings. Psychiatric patients can often easily relate to the images created by stories. In psychiatry and psychotherapy, stories can be a means of changing a patient’s point of view. Such narratives can free up the listener’s feelings and thoughts and often lead to “Aha!” moments. The mirror function of storytelling leads to identification. In the narratives, the reader or listener recognizes themself as well as their needs and situation. They can reflect on the stories without personally becoming the focus of these reflections and remember their own experiences. Stories present solutions that can be models against which one’s own approach can be compared but that also leave room for broader interpretation. Storytelling is particularly useful in bringing about change in patients who are holding fast to old and outworn ideas.
The positive interpretation of disorders
Positive psychotherapy is based on a humanistic view that every human being is good by nature and endowed with unique capacities.11 This positive perspective leads not only to a new quality of relationship between the therapist and patient but also to a new perspective on disorders (Table). Thus, disorders can be “interpreted” in a positive way6: What does the patient unconsciously want to express with their symptoms? What is the function of their disorder? The positive process brings with it a change in perspective to all those concerned: the patient, their family, and the therapist/physician. In this way, one moves from the symptom (which is the disorder and often already has been very thoroughly examined) to the conflict (and the function of the disorder). The positive interpretations are only offered to the patient (“What do you say to this explanation?” “Can you apply this to your own situation?”).

Continue to: This process also helps us...
This process also helps us focus on the “true” patient, who often is not our patient. The patient who comes to us functions as a symptom carrier and can be seen as the “weakest link” in the family chain. The “real patient” is often sitting at home. The positive interpretation of illnesses confronts the patient with the possible function and psychodynamic meaning of their illness for themself and their social milieu, encouraging the patient (and their family) to see their abilities and not merely the pathological aspects.12
Fields of application of positive psychotherapy
As a method positioned between manualized CBT and process-oriented analytical psychotherapy, positive psychotherapy pursues a semi-structured approach in diagnostics (first interview), treatment, posttherapeutic self-help, and training. Positive psychotherapy is applied for the treatment of mood (affective), neurotic, stress-related, and somatoform disorders; behavioral syndromes; and, to some extent, personality disorders. Positive psychotherapy has been employed successfully side-by-side with classical individual therapy as well as in the settings of couple, family, and group therapy.13
What makes positive psychotherapy attractive for mental health professionals?
- As a method that integrates the 4 main modalities of psychotherapy, it does not engage in the conflicts between different schools but combines effective elements into a single approach.
- As an integrative approach, it adjusts to the patient and not vice versa. It gives the therapist the possibility of focusing more on either the actual problems (supportive approach) or the basic conflict (psychodynamic approach).
- It uses vocabulary and terms that can be understood by patients from all strata of society.
- As a culturally sensitive method, it can be applied to patients from different cultures and does not require cultural adaptation.
- As a psychodynamic method, it does not stop after early life conflicts have become more conscious but helps the patient to apply the gained insights using practical techniques.
- It starts with positive affirmations and encouragement but does not later “forget” the unconscious conflicts that have led to disorders. It is not perceived as superficial.
- As a method originally coming from psychiatry and medical practice, it builds a bridge between a scientific basis and psychotherapeutic insights. It favors the biopsychosocial approach.
Bottom Line
Positive psychotherapy combines humanistic, systemic, psychodynamic, and cognitive-behavioral aspects. It is based on a resource-oriented view of human beings in which disorders are interpreted as capacities to react in a specific and unique way to life events and circumstances. Positive psychotherapy can be applied in psychiatry and psychotherapy. This short-term method is easily understood by patients from diverse cultures and social backgrounds.
Related Resources
- Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32. https://doi.org/10.1007/978-3-030-33264-8_2
- Tritt K, Loew T, Meyer M, et al. Positive psychotherapy: effectiveness of an interdisciplinary approach. Eur J Psychiatry. 1999;13(4):231-241.
- World Association for Positive and Transcultural Psychotherapy. http://www.positum.org
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.
1. Mackenthun G. Passt Psychotherapie an ‚die Gesellschaft’ an? Dynamische Psychiatrie. 1991;24(5-6):326-333.
2. Jeste DV. Foreword: positive mental health. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:vii-xiii.
3. Dobiała E, Winkler P. ‘Positive psychotherapy’ according to Seligman and ‘positive psychotherapy’ according to Peseschkian: a comparison. Int J Psychother. 2016;20(3):5-17.
4. Peseschkian N. Positive Psychotherapy: Theory and Practice of a New Method. Springer; 1987.
5. Seligman MEP, Rashid T, Parks AC. Positive psychotherapy. Am Psychol. 2006;61(8):774-788.
6. Peseschkian N. Positive Psychosomatics: Clinical Manual of Positive Psychotherapy. AuthorHouse; 2016.
7. Peseschkian N. Positive psychotherapy. In: Pritz A, ed. Globalized Psychotherapy. Facultas Universitätsverlag; 2002.
8. Peseschkian H, Remmers A. Positive psychotherapy: an introduction. In: Messias E, Peseschkian H, Cagande C, eds. Positive Psychiatry, Psychotherapy and Psychology. Springer; 2020:11-32.
9. Moghaddam FM, Harre R. But is it science? Traditional and alternative approaches to the study of social behavior. World Psychol. 1995;1(4):47-78.
10. Peseschkian N. Oriental Stories as Techniques in Positive Psychotherapy. AuthorHouse; 2016.
11. Cope TA. Positive psychotherapy’s theory of the capacity to know as explication of unconscious contents. J Relig Health. 2009;48(1):79-89.
12. Huebner G. Health-illness from the perspective of positive psychotherapy. Global Psychother. 2021;1(1):57-61.
13. Sinici E. A ‘balance model’ for patients with post-traumatic stress disorder. Int J Psychother. 2015;19(3):13-19.








