Cosmetic Dermatology
Devices and Topical Agents for Rosacea Management
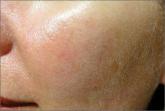
Rosacea is a chronic inflammatory disease that predominantly affects facial skin in light-skinned individuals and can be divided into 4 subtypes....
Zoe Diana Draelos, MD; Boni E. Elewski, MD; Julie C. Harper, MD; Meike Sand, MSc; Gerald Staedtler, MSc; Richard Nkulikiyinka, MD; Kaweh Shakery, MD
Dr. Draelos is from Dermatology Consulting Services, High Point, North Carolina. Dr. Elewski is from the University of Alabama, Birmingham. Dr. Harper is from the Dermatology and Skin Care Center of Birmingham, Alabama. Mr. Sand, Mr. Staedtler, and Drs. Nkulikiyinka and Shakery are from Global Development, Bayer Pharma AG, Berlin, Germany.
Dr. Draelos received a research grant from Bayer HealthCare Pharmaceuticals Inc. Dr. Elewski is an advisory board member, consultant, and investigator for Bayer HealthCare Pharmaceuticals Inc, and she is an investigator for Galderma Laboratories, LP. Dr. Harper is a consultant, researcher, and speaker for Bayer HealthCare Pharmaceuticals Inc. Mr. Sand, Mr. Staedtler, and Drs. Nkulikiyinka and Shakery are employees of Bayer Pharma AG. Mr. Staedtler also holds a patent for the vehicle formulation.
This study was registered on March 13, 2012, at www.clinicaltrials.gov with the identifier NCT01555463.
Additional methodology and the eFigure are available in the Appendix.
Correspondence: Zoe Diana Draelos, MD, 2444 N Main St, High Point, NC 27262 (zdraelos@northstate.net).

Comment
Overall, the results from this phase 3 trial demonstrate that the new foam formulation of AzA was efficacious and safe in a 12-week, twice-daily course of treatment for moderate to severe PPR. The AzA foam formulation was significantly superior to vehicle (P<.001) for both primary efficacy end points. Participants in the AzA foam group achieved therapeutic success at a higher rate than the vehicle group, and the change in nominal ILC at EoT was significantly greater for participants treated with AzA foam than for those treated with vehicle (P<.001). Differences between the 2 treatment groups for the coprimary end point measures arose early in the study, demonstrating that symptoms were rapidly controlled. Between weeks 8 and 12 (EoT), the rate of increase of beneficial effects in the AzA foam group remained high, while the vehicle group showed a notable slowing. There was no indication of any rebound effect in overall disease severity subsequent to EoT. After 4 weeks of follow-up, there was still a beneficial treatment effect present in favor of the AzA foam group, as indicated by the persistence of improvements in both coprimary end point measures throughout the follow-up period.
Analyses of alternative populations and secondary end points (data not shown) supported the efficacy results reported here. There was no indication of irregular study center effects, and the sensitivity analyses demonstrated robustness of the data for the observed treatment effects.
The use of vehicle foam alone appeared to be beneficial in reducing ILC and improving IGA rating, which suggests that the properties of the new foam formulation are favorable for the inflamed lesional skin of rosacea. Of note, other dermatology studies, including trials in rosacea, have reported therapeutic effects of vehicle treatment that may be attributable to the positive effects of skin care with certain formulations.20
Azelaic acid foam was well tolerated in the current study. More than 93% of AEs in either treatment group were of mild or moderate severity. The incidence of drug-related AEs was low in both groups and mainly occurred at the application site. There were no drug-related severe or serious AEs. The low incidence of reported drug-related noncutaneous AEs in the AzA foam group (dysgeusia in 1 patient and headache in 2 patients) supports the known favorable systemic tolerance profile of AzA.
Although most drug-related AEs occurred at the application site, they were generally transient, with the majority of events in the AzA foam group lasting no more than 1 hour. Most cutaneous AEs developed early in the study. In the AzA foam group, the prevalence of drug-related cutaneous AEs dropped at every time interval as the study progressed (eFigure). Very few AEs of any type persisted through the end of the study. These safety results were accompanied by a high compliance rate and a high participation rate throughout the course of the study. Taken together, the available data for this AzA foam formulation support a favorable tolerability profile. The results of this study are consistent with and expand on data from an earlier investigation of similar design.8
Conclusion
The development of an AzA foam formulation with higher lipid content was intended to expand the treatment options available to physicians and patients who are managing rosacea. Most topical dermatologic treatments are currently delivered in classical formulations such as creams or gels, but patients who use topical therapies have rated messiness and ease of application among the most important characteristics affecting quality of life.17,21 Foam formulations may offer improvements in this regard; ease of application may minimize unnecessary manipulation of inflamed skin and contribute to a high level of user satisfaction.22 However, the design of the current study was limited to evaluating only the AzA foam formulation versus a foam vehicle, and direct comparisons of clinical efficacy and tolerability to other AzA topical preparations were not performed. Nonetheless, patients have previously reported that they would be more likely to comply with a recommended course of dermatologic foam therapy than other topical formulations.18 The proposed foam formulation was designed to attend to the specific needs of the dry and sensitive skin in rosacea by combining the demonstrated efficacy properties exhibited by AzA gel 15% with the good tolerability and acceptability of a lipid-containing foam formulation. Development of this formulation was targeted to obtain a product that would be highly spreadable, dry quickly, and be easy to apply. The available data for this AzA foam formulation support the value of this option in the topical treatment of rosacea. The success in reduction of overall disease severity, lack of any rebound after EoT, and the observed tolerability and high adherence rates suggest that this novel formulation is a useful addition to current treatment options for rosacea.
Addendum
After release of the study data for unblinding and statistical evaluation, the following inconsistency regarding patient distribution was noted: 1 participant was incorrectly evaluated as part of the AzA foam analysis group when in fact this patient was randomized to vehicle and was treated throughout the study with vehicle. This participant did not experience any AE and did not show any IGA improvement at the EoT. As this single case did not have an impact on the statistical conclusions or interpretation of the results, the released study data have not been changed. This deviation was described as a database erratum in the study report.
Acknowledgement—Editorial support through inVentiv Medical Communications, New York, New York, was provided by Bayer HealthCare Pharmaceuticals Inc.

Rosacea is a chronic inflammatory disease that predominantly affects facial skin in light-skinned individuals and can be divided into 4 subtypes....
Rosacea is a common chronic disorder that predominantly manifests as facial erythema, telangiectasia, and flushing. Other primary clinical...
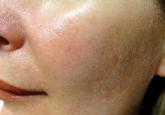
Rosacea is a commonly encountered chronic inflammatory skin disease with a predilection for highly visible areas of the skin such as the face. The...
