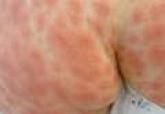
Dabigatran is a direct thrombin inhibitor used to reduce the risk for stroke and systemic embolism in patients with nonvalvular atrial fibrillation. Based on results of the RE-LY (Randomization Evaluation of Long-term Anticoagulation Therapy) trial published in 2009, dabigatran 150 mg twice daily significantly reduced the risk for stroke and systemic emboli in patients with atrial fibrillation compared to warfarin (annual risk, 1.11% vs 1.69%; relative risk, 0.66; 95% CI, 0.53-0.82; P<.001) with the advantage of not requiring frequent monitoring of the international normalized ratio.2 The most common adverse effect of dabigatran in this trial was dyspepsia (11.3% vs 5.8%). Drug hypersensitivity, allergic edema, and anaphylaxis were reported in less than 0.1% of patients taking dabigatran.2
According to a PubMed search of articles indexed for MEDLINE using the search terms dabigatran cutaneous reaction and dabigatran rash, 4 case reports of cutaneous eruption due to dabigatran were identified. In one report, a 20-year-old man with atrial fibrillation developed an eruption similar to our patient on the thigh and forearm after 2 weeks of taking oral dabigatran 150 mg twice daily. It resolved without complication after topical corticosteroid use and discontinuation of dabigatran.3 In another report, a 78-year-old man presented to the emergency department after taking two 150-mg doses of dabigatran with a diffuse, full-body, pruritic rash that resolved with oral diphenhydramine and discontinuation of dabigatran.4 A third case described a 59-year-old man who was taking 150 mg dabigatran twice daily for 5 days before developing a rash.5 The fourth case involved a 74-year-old woman who developed leukocytoclastic vasculitis 1 week after taking dabigatran 150 mg twice daily.6
It is important to monitor for and report hypersensitivity reactions in patients taking dabigatran. Drug exanthems may cause discomfort or even herald more serious hypersensitivity reactions. Patients experiencing these reactions may discontinue therapy without notifying a physician and consequently place themselves at risk for embolism or stroke.