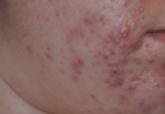
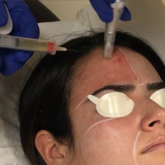
Facial acne vulgaris is a common skin disease among teenagers and adolescents that may negatively affect self-esteem, perceived facial attractiveness, and social participation.1 Treatments for acne often are multimodal and require the utmost adherence. For these reasons, acne treatments have been challenging to clinicians and patients alike, as patient compliance in maintaining the use of prescribed topical and oral medications remains essential to attain improvement in quality of life (QOL).
Salicylic acid is a popular medicament for acne treatment that frequently is used as monotherapy or as an adjuvant for other acne treatments, especially in patients with oily skin.2 Salicylic acid has a keratolytic effect, causing corneocyte discohesion in clogged pores or congested follicles,2 and it is effective in treating both inflammatory and noninflammatory acne.3,4
Light therapy, particularly with visible light, has been demonstrated to improve acne outcomes.5 Pneumatic broadband light (PBBL) is a therapeutic light treatment in the broadband range (400–1200 nm) that is combined with vacuum suction, which creates a mechanical lysis of thin-walled pustules and dislodges pore impaction. Additionally, the blue light portion of the PBBL spectrum targets endogenous porphyrins in Propionibacterium acnes, resulting in bacterial destruction.6-8
The purpose of this study was to compare the efficacy, tolerability, and safety of salicylic acid 30% peel versus PBBL in the treatment of mild to moderately severe facial acne vulgaris.
METHODS
Study Design
This single-blind, randomized, split-face pilot study was approved by the institutional review board of the University of Pennsylvania (Philadelphia, Pennsylvania). All patients provided informed consent before entering the study. The single-blind evaluation was performed by one dermatologist (C.T.) who examined the participants on every visit prior to PBBL treatment.
Before the study started, participants were randomized for which side of the face was to be treated with PBBL using a number assigned to each participant. Participants received both treatments—salicylic acid 30% peel on one side of the face and PBBL treatment on the other side of the face—once weekly for a total of 6 treatments. They were then asked to return for 2 follow-up evaluations at weeks 3 and 6 following the last treatment session and were instructed not to use any topical or oral acne medications during these follow-up periods.
Inclusion and Exclusion Criteria
Patients aged 18 years and older of any race and sex with noninflammatory papules, some inflammatory papules, and no more than 1 nodule (considered as mild to moderately severe facial acne) were included in the study. Participants had not been on any topical acne medications for at least 1 month and/or oral retinoids for at least 1 year prior to the study period. All women completed urine pregnancy tests prior to the study and were advised to utilize birth control during the study period.
Study Treatments
Salicylic Acid 30% Peel
The participant’s face was cleansed thoroughly before application of salicylic acid 30% (1.5 g/2.5 mL) to half of the face and left on for 5 minutes before being carefully rinsed off by spraying with spring water. Prior to initiating PBBL therapy, the peeled side of the participant’s face was covered with a towel.
Pneumatic Broadband Light
On the other side of the face, PBBL was performed to deliver broadband light within the spectrum range of 400 to 1200 nm at a setting approximately equivalent to a fluence of 4 to 6 J/cm2 and a vacuum setting approximately equivalent to a negative pressure of 3 lb/in2. The power setting was increased on each subsequent visit depending on each participant’s tolerability.
Participants were required to apply a moisturizer and sunscreen to the face and avoid excessive sun exposure between study visits.
Efficacy Evaluation
A comparison of the efficacy of the treatments was determined by clinical evaluation and examining the results of the outcome measurements with the modified Global Acne Grading Score (mGAGS) and Acne QOL Scale during each treatment visit. Facial photographs were taken at each visit.
Modified Global Acne Grading Score
The mGAGS is a modification of the Global Acne Grading Scale (GAGS) that has been used to evaluate acne severity in many studies.9-11 The GAGS considers 6 locations on the face with a grading factor for each location. The local score is obtained by multiplying the factor rated by location with the factor of clinical assessment: local score = factor rated by location × factor rated by clinical assessment. The total score is the sum of the individual local scores (Table 1).
Although the original GAGS incorporated the type and location of the lesions in its calculation, we felt that the number of lesions also was important to add to our grading score. Therefore, we modified the GAGS by adding a factor rated by the number of lesions to improve the accuracy of the test. Accordingly, the local mGAGS scores were calculated by multiplying the location factor by the lesion type and number of lesions factors: local score = location factor × lesion type factor × number of lesions factor.
Acne QOL Questionnaire
Acne QOL was assessed during each visit to demonstrate if the treatment results affected participants’ socialization due to appearance.12 Participants were asked to complete the questionnaire, which consisted of 9 questions with 4 rating answers (0=not affected; 1=mildly affected; 2=moderately affected; 3=markedly affected). A total score of 9 or higher (high score) indicated that acne had a substantial negative impact on the participant, while a total score below 9 (low score) meant acne scarcely impacted social aspects and daily activities of the patient.
Safety Evaluation
The safety of the treatments was evaluated by clinical inspection and by comparing the results of the Wong-Baker FACES Pain Rating Scale (WBPRS)13 after treatment. The WBPRS is used worldwide among researchers to assess pain, particularly in children.14,15 It is composed of 6 faces expressing pain with word descriptions with a corresponding number range reflecting pain severity from 0 to 5 (0=no hurt; 1=hurts little bit; 2=hurts little more; 3=hurts even more; 4=hurts whole lot; 5=hurts worst).13
Statistical Analysis
All variables were presented as the median (range). A Wilcoxon signed rank test was used to compare clinical responses between the salicylic acid 30% peel and PBBL therapies. SPSS software version 12.0 was used for all statistical analysis. A 2-tailed P value of ≤.05 was considered statistically significant.